

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50583942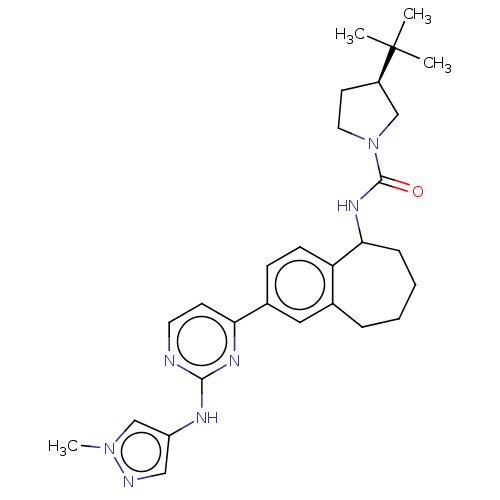 (CHEMBL5088454) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human BTK using fluorescein-labeled polyGAT peptide as substrate incubated for 30 mins by FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00926 BindingDB Entry DOI: 10.7270/Q23B641C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM324380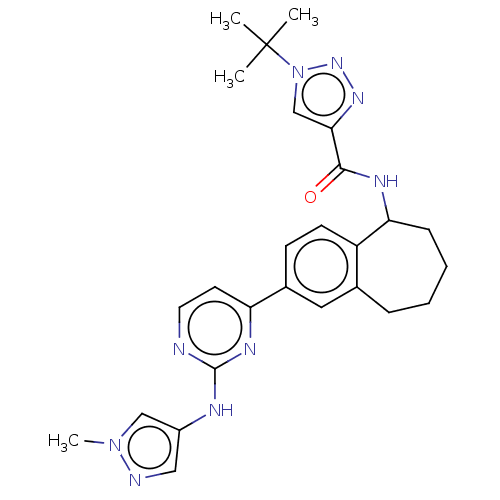 (1-(tert-butyl)-N- (2-(2-((1- methyl-1H-pyrazol-4- ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human BTK using fluorescein-labeled polyGAT peptide as substrate incubated for 30 mins by FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00926 BindingDB Entry DOI: 10.7270/Q23B641C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM324290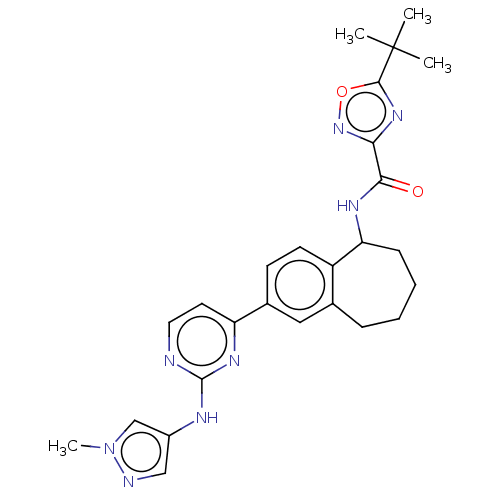 (5-(tert-butyl)-N-(2-(2-((1-methyl-1H-pyrazol-4-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human BTK using fluorescein-labeled polyGAT peptide as substrate incubated for 30 mins by FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00926 BindingDB Entry DOI: 10.7270/Q23B641C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM324294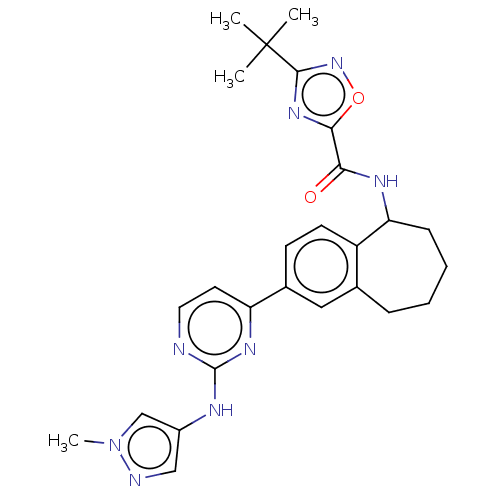 (3-(tert-butyl)-N-(2-(2-((1-methyl-1H-pyrazol-4-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human BTK using fluorescein-labeled polyGAT peptide as substrate incubated for 30 mins by FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00926 BindingDB Entry DOI: 10.7270/Q23B641C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50583944 (CHEMBL5089878) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human BTK using fluorescein-labeled polyGAT peptide as substrate incubated for 30 mins by FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00926 BindingDB Entry DOI: 10.7270/Q23B641C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50583938 (CHEMBL5090290) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human BTK using fluorescein-labeled polyGAT peptide as substrate incubated for 30 mins by FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00926 BindingDB Entry DOI: 10.7270/Q23B641C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM324337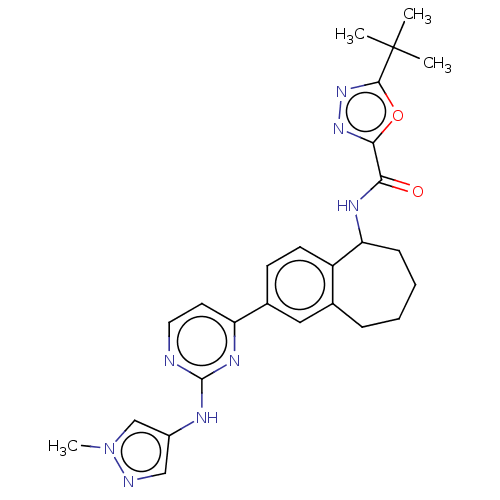 (5-(tert-butyl)-N-(2- (2-((1- methyl-1H-pyrazol-4- ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human BTK using fluorescein-labeled polyGAT peptide as substrate incubated for 30 mins by FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00926 BindingDB Entry DOI: 10.7270/Q23B641C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50583941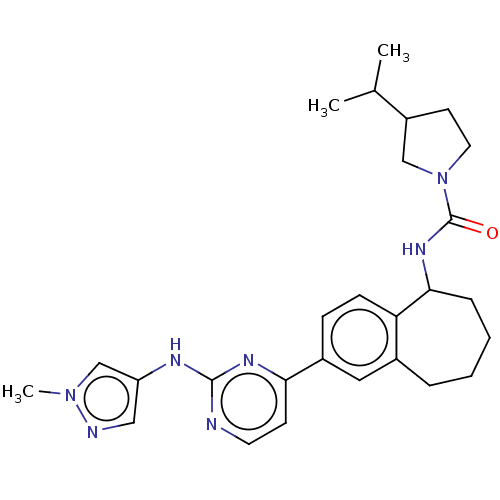 (CHEMBL5087183) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human BTK using fluorescein-labeled polyGAT peptide as substrate incubated for 30 mins by FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00926 BindingDB Entry DOI: 10.7270/Q23B641C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM324288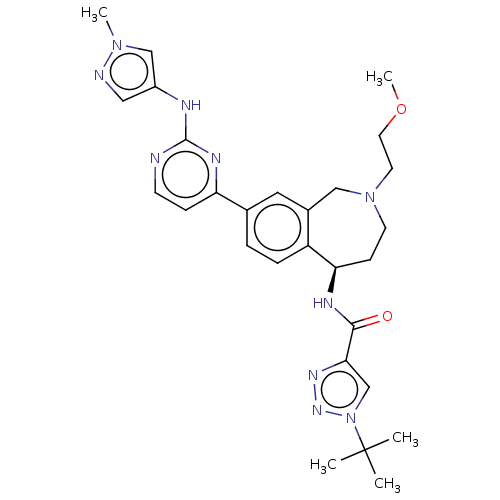 ((R)-1-(tert-butyl)-N-(2-(2-methoxyethyl)-8-(2-((1-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human BTK using fluorescein-labeled polyGAT peptide as substrate incubated for 30 mins by FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00926 BindingDB Entry DOI: 10.7270/Q23B641C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM324284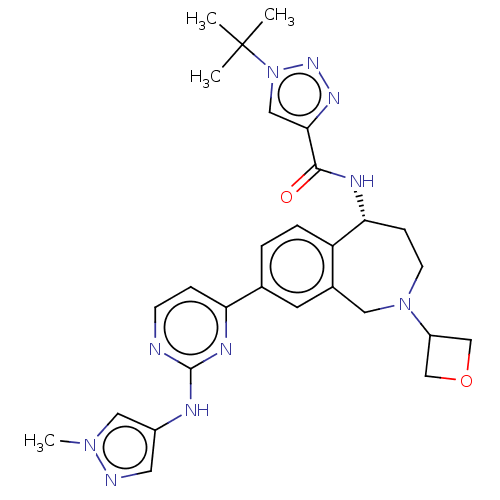 ((R)-1-(tert-butyl)-N-(8-(2-((1-methyl-1H-pyrazol-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human BTK using fluorescein-labeled polyGAT peptide as substrate incubated for 30 mins by FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00926 BindingDB Entry DOI: 10.7270/Q23B641C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM324285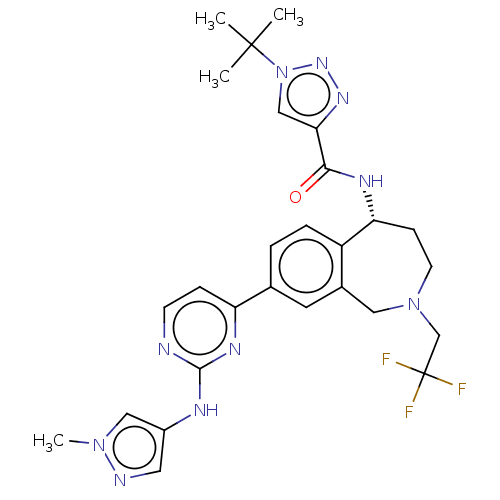 ((R)-1-(tert-butyl)-N-(8-(2-((1-methyl-1H-pyrazol-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human BTK using fluorescein-labeled polyGAT peptide as substrate incubated for 30 mins by FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00926 BindingDB Entry DOI: 10.7270/Q23B641C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50583947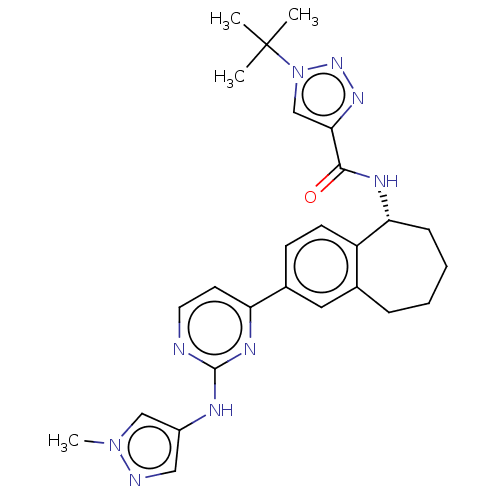 (CHEMBL5085931) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human BTK using fluorescein-labeled polyGAT peptide as substrate incubated for 30 mins by FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00926 BindingDB Entry DOI: 10.7270/Q23B641C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM324293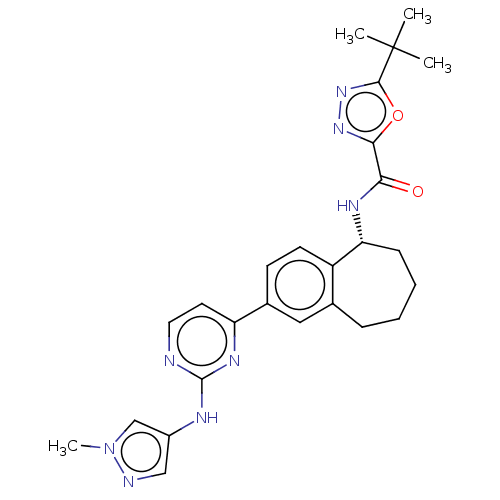 ((R)-5-(tert-butyl)-N-(2-(2-((1-methyl-1H-pyrazol-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human BTK using fluorescein-labeled polyGAT peptide as substrate incubated for 30 mins by FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00926 BindingDB Entry DOI: 10.7270/Q23B641C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50583945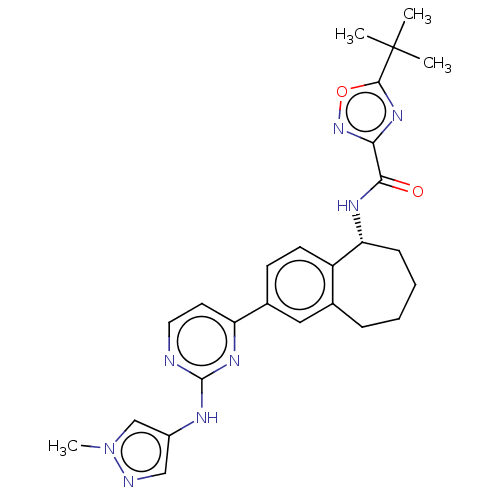 (CHEMBL5080861) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human BTK using fluorescein-labeled polyGAT peptide as substrate incubated for 30 mins by FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00926 BindingDB Entry DOI: 10.7270/Q23B641C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50583946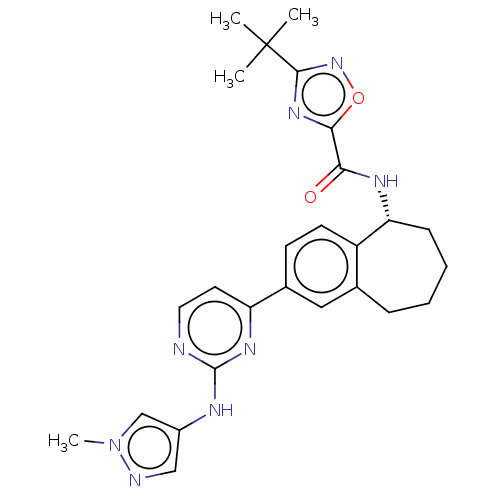 (CHEMBL5076817) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human BTK using fluorescein-labeled polyGAT peptide as substrate incubated for 30 mins by FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00926 BindingDB Entry DOI: 10.7270/Q23B641C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50583932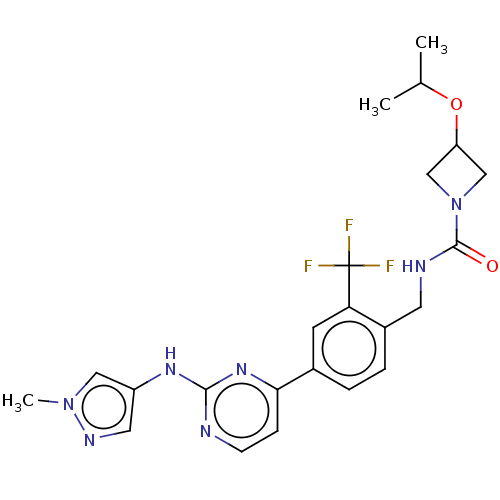 (CHEMBL5073223) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human BTK using fluorescein-labeled polyGAT peptide as substrate incubated for 30 mins by FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00926 BindingDB Entry DOI: 10.7270/Q23B641C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50583937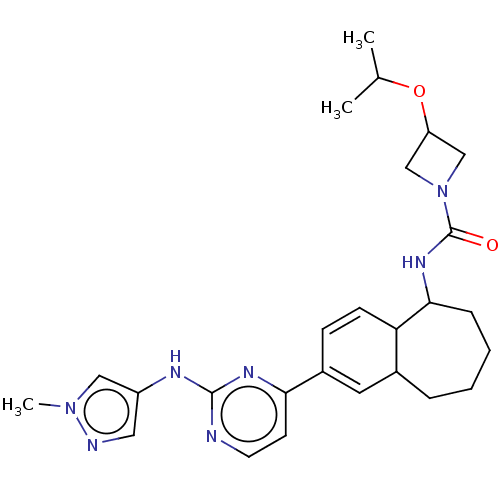 (CHEMBL5091935) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human BTK using fluorescein-labeled polyGAT peptide as substrate incubated for 30 mins by FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00926 BindingDB Entry DOI: 10.7270/Q23B641C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50583950 (CHEMBL5094998) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human BTK using fluorescein-labeled polyGAT peptide as substrate incubated for 30 mins by FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00926 BindingDB Entry DOI: 10.7270/Q23B641C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50583948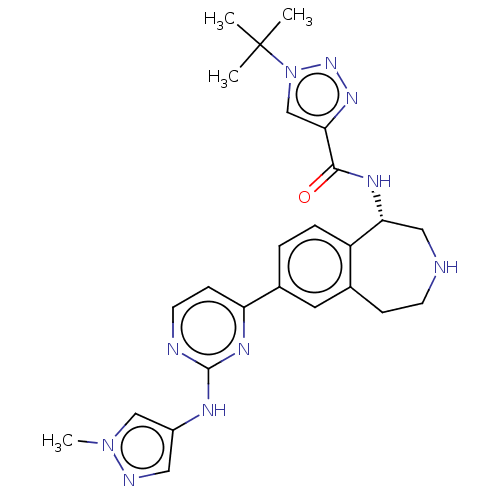 (CHEMBL5081318) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human BTK using fluorescein-labeled polyGAT peptide as substrate incubated for 30 mins by FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00926 BindingDB Entry DOI: 10.7270/Q23B641C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM480005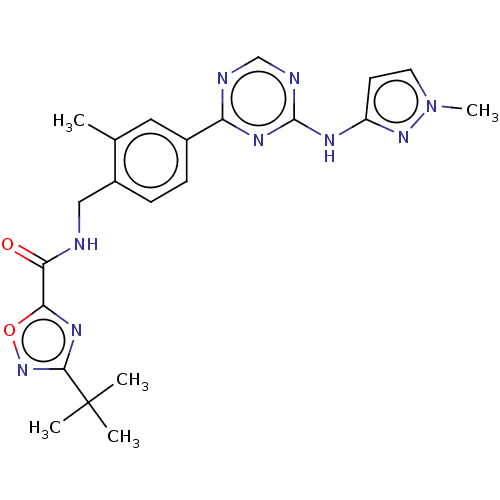 (3-(tert-butyl)-N-(2-methyl-4-(4-((1-methyl-1H-pyra...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
BIOGEN MA INC. US Patent | Assay Description The purpose of the BTK in vitro assay is to determine compound potency against BTK through the measurement of IC50. Compound inhibition is measured a... | US Patent US10899753 (2021) BindingDB Entry DOI: 10.7270/Q2154M4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM480006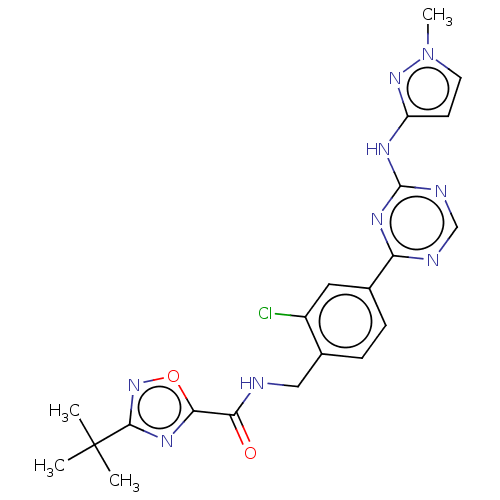 (3-(tert-butyl)-N-(2-chloro-4-(4-((1-methyl-1H-pyra...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
BIOGEN MA INC. US Patent | Assay Description The purpose of the BTK in vitro assay is to determine compound potency against BTK through the measurement of IC50. Compound inhibition is measured a... | US Patent US10899753 (2021) BindingDB Entry DOI: 10.7270/Q2154M4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM480007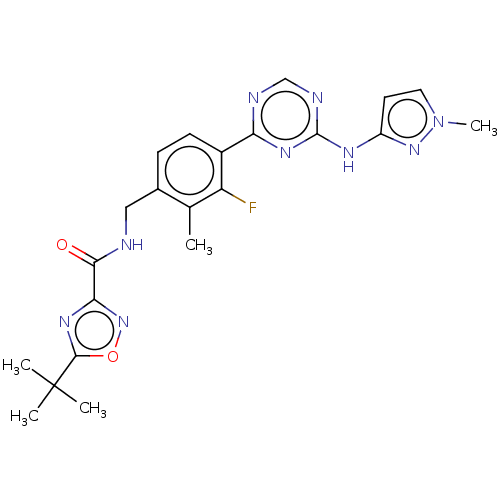 (5-(tert-butyl)-N-(3-fluoro-2-methyl-4-(4-((1-methy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
BIOGEN MA INC. US Patent | Assay Description The purpose of the BTK in vitro assay is to determine compound potency against BTK through the measurement of IC50. Compound inhibition is measured a... | US Patent US10899753 (2021) BindingDB Entry DOI: 10.7270/Q2154M4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM480008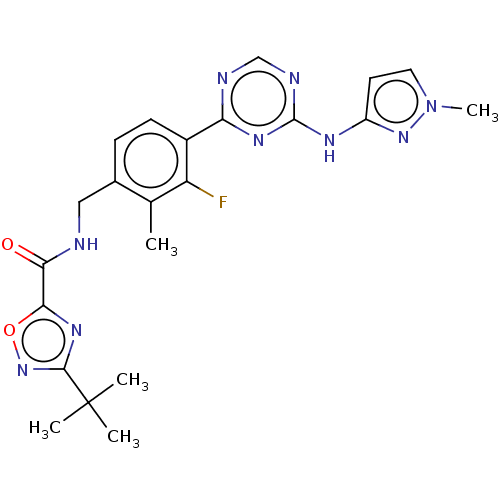 (3-(tert-butyl)-N-(3-fluoro-2-methyl-4-(4-((1-methy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
BIOGEN MA INC. US Patent | Assay Description The purpose of the BTK in vitro assay is to determine compound potency against BTK through the measurement of IC50. Compound inhibition is measured a... | US Patent US10899753 (2021) BindingDB Entry DOI: 10.7270/Q2154M4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM480034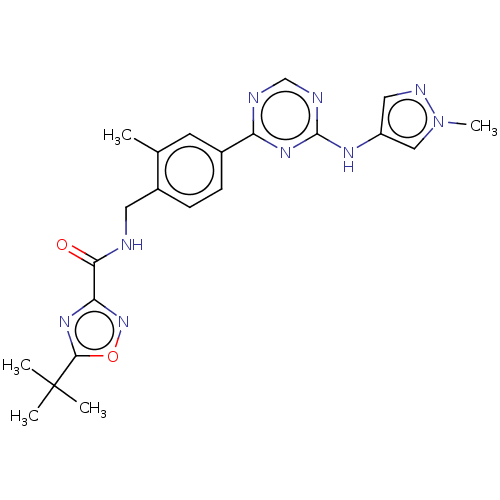 (5-(tert-butyl)-N-(2-methyl-4-(4-((1-methyl-1H-pyra...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
BIOGEN MA INC. US Patent | Assay Description The purpose of the BTK in vitro assay is to determine compound potency against BTK through the measurement of IC50. Compound inhibition is measured a... | US Patent US10899753 (2021) BindingDB Entry DOI: 10.7270/Q2154M4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM480041 (3-(tert-butyl)-N-(2-methyl-4-(4-((1-methyl-1H-pyra...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
BIOGEN MA INC. US Patent | Assay Description The purpose of the BTK in vitro assay is to determine compound potency against BTK through the measurement of IC50. Compound inhibition is measured a... | US Patent US10899753 (2021) BindingDB Entry DOI: 10.7270/Q2154M4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM480062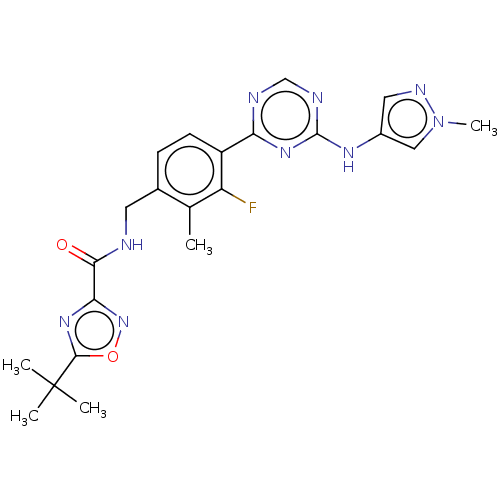 (5-(tert-butyl)-N-(3-fluoro-2-methyl-4-(4-((1-methy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
BIOGEN MA INC. US Patent | Assay Description The purpose of the BTK in vitro assay is to determine compound potency against BTK through the measurement of IC50. Compound inhibition is measured a... | US Patent US10899753 (2021) BindingDB Entry DOI: 10.7270/Q2154M4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM480064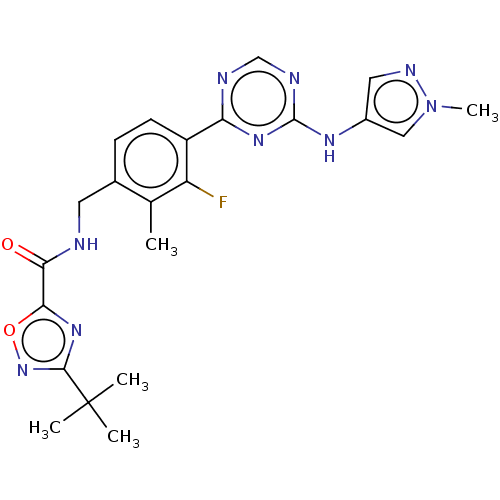 (3-(tert-butyl)-N-(3-fluoro-2-methyl-4-(4-((1-methy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
BIOGEN MA INC. US Patent | Assay Description The purpose of the BTK in vitro assay is to determine compound potency against BTK through the measurement of IC50. Compound inhibition is measured a... | US Patent US10899753 (2021) BindingDB Entry DOI: 10.7270/Q2154M4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM480075 (5-(tert-butyl)-N-(2-chloro-4-(4-((1-methyl-1H-pyra...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
BIOGEN MA INC. US Patent | Assay Description The purpose of the BTK in vitro assay is to determine compound potency against BTK through the measurement of IC50. Compound inhibition is measured a... | US Patent US10899753 (2021) BindingDB Entry DOI: 10.7270/Q2154M4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM480078 (3-(tert-butyl)-N-(2-chloro-4-(4-((1-methyl-1H-pyra...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
BIOGEN MA INC. US Patent | Assay Description The purpose of the BTK in vitro assay is to determine compound potency against BTK through the measurement of IC50. Compound inhibition is measured a... | US Patent US10899753 (2021) BindingDB Entry DOI: 10.7270/Q2154M4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM480079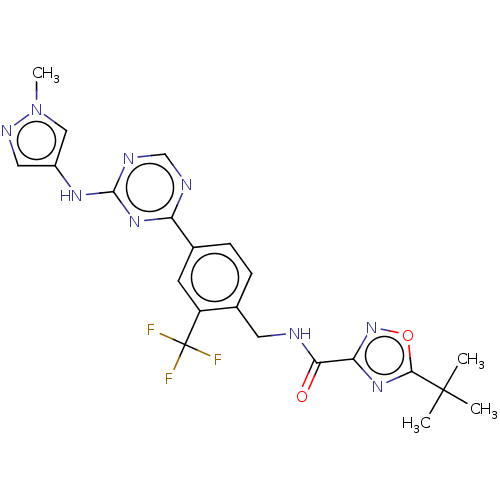 (5-(tert-butyl)-N-(4-(4-((1-methyl-1H-pyrazol-4-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
BIOGEN MA INC. US Patent | Assay Description The purpose of the BTK in vitro assay is to determine compound potency against BTK through the measurement of IC50. Compound inhibition is measured a... | US Patent US10899753 (2021) BindingDB Entry DOI: 10.7270/Q2154M4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM480080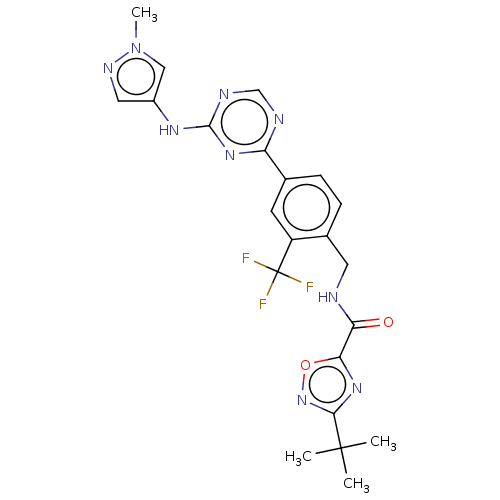 (3-(tert-butyl)-N-(4-(4-((1-methyl-1H-pyrazol-4-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
BIOGEN MA INC. US Patent | Assay Description The purpose of the BTK in vitro assay is to determine compound potency against BTK through the measurement of IC50. Compound inhibition is measured a... | US Patent US10899753 (2021) BindingDB Entry DOI: 10.7270/Q2154M4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM324209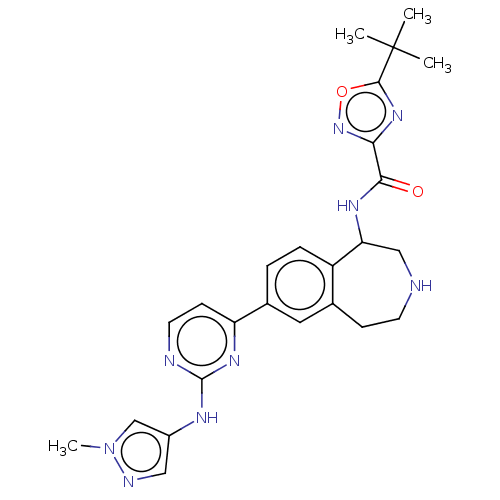 (5-(tert-butyl)-N-(7-(2-((1-methyl-1H-pyrazol-4-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
BIOGEN MA INC. US Patent | Assay Description The purpose of the BTK in vitro assay is to determine compound potency against BTK through the measurement of IC50. Compound inhibition is measured a... | US Patent US10189829 (2019) BindingDB Entry DOI: 10.7270/Q2V69MP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM324234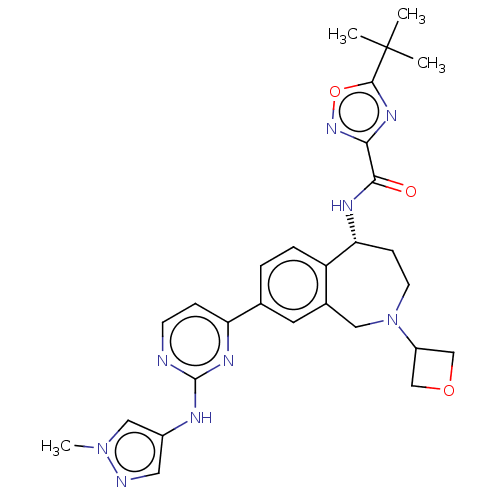 ((R)-5-(tert-butyl)-N-(8-(2-((1-methyl-1H-pyrazol-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
BIOGEN MA INC. US Patent | Assay Description The purpose of the BTK in vitro assay is to determine compound potency against BTK through the measurement of IC50. Compound inhibition is measured a... | US Patent US10189829 (2019) BindingDB Entry DOI: 10.7270/Q2V69MP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM324258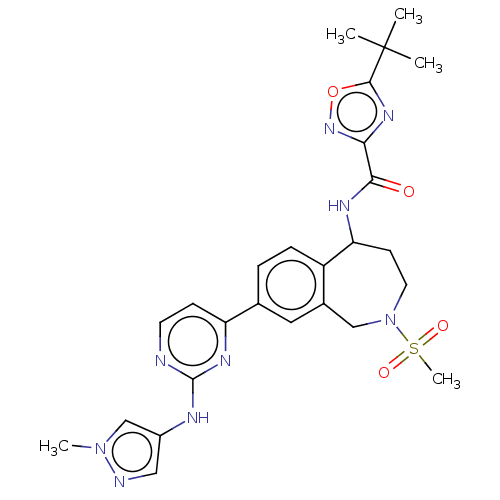 (5-(tert-butyl)-N-(8-(2-((1-methyl-1H-pyrazol-4-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
BIOGEN MA INC. US Patent | Assay Description The purpose of the BTK in vitro assay is to determine compound potency against BTK through the measurement of IC50. Compound inhibition is measured a... | US Patent US10189829 (2019) BindingDB Entry DOI: 10.7270/Q2V69MP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM324261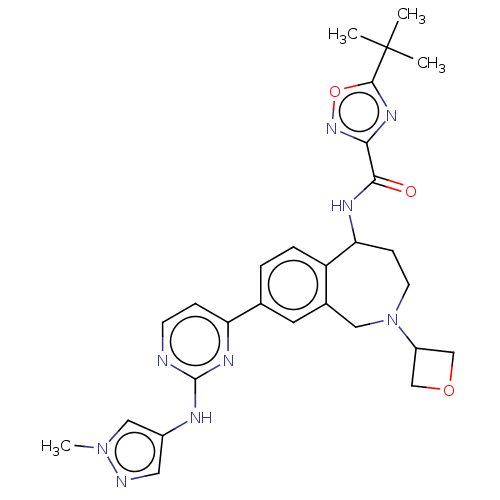 (5-(tert-butyl)-N-(8-(2-((1-methyl-1H-pyrazol-4-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
BIOGEN MA INC. US Patent | Assay Description The purpose of the BTK in vitro assay is to determine compound potency against BTK through the measurement of IC50. Compound inhibition is measured a... | US Patent US10189829 (2019) BindingDB Entry DOI: 10.7270/Q2V69MP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM324262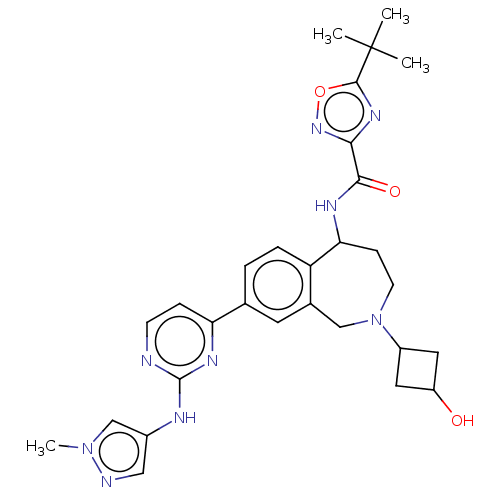 (5-(tert-butyl)-N-(2-(3-hydroxycyclobutyl)-8-(2-((1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
BIOGEN MA INC. US Patent | Assay Description The purpose of the BTK in vitro assay is to determine compound potency against BTK through the measurement of IC50. Compound inhibition is measured a... | US Patent US10189829 (2019) BindingDB Entry DOI: 10.7270/Q2V69MP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM324263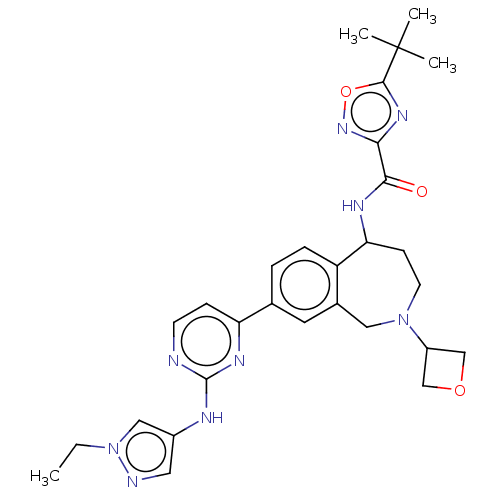 (5-(tert-butyl)-N-(8-(2-((1-ethyl-1H-pyrazol-4-yl)a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
BIOGEN MA INC. US Patent | Assay Description The purpose of the BTK in vitro assay is to determine compound potency against BTK through the measurement of IC50. Compound inhibition is measured a... | US Patent US10189829 (2019) BindingDB Entry DOI: 10.7270/Q2V69MP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM324264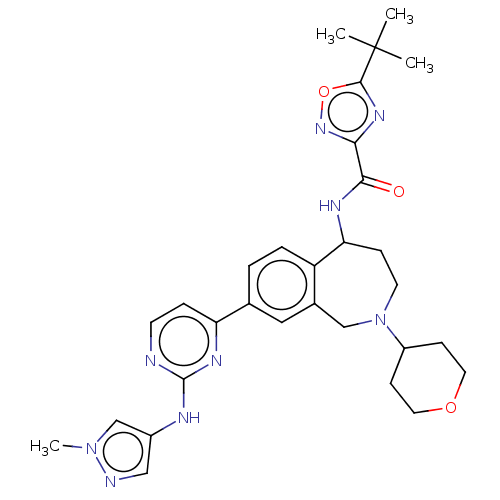 (5-(tert-butyl)-N-(8-(2-((1-methyl-1H-pyrazol-4-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
BIOGEN MA INC. US Patent | Assay Description The purpose of the BTK in vitro assay is to determine compound potency against BTK through the measurement of IC50. Compound inhibition is measured a... | US Patent US10189829 (2019) BindingDB Entry DOI: 10.7270/Q2V69MP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM324265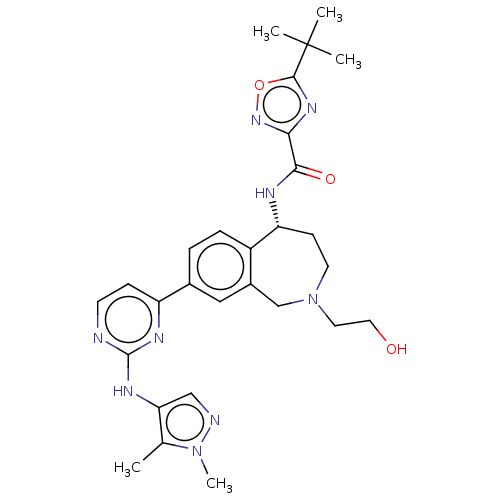 ((R)-5-(tert-butyl)-N-(8-(2-((1,5-dimethyl-1H-pyraz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
BIOGEN MA INC. US Patent | Assay Description The purpose of the BTK in vitro assay is to determine compound potency against BTK through the measurement of IC50. Compound inhibition is measured a... | US Patent US10189829 (2019) BindingDB Entry DOI: 10.7270/Q2V69MP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM324266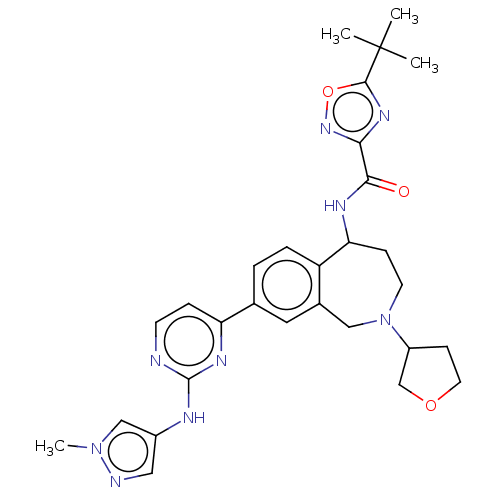 (5-(tert-butyl)-N-(8-(2-((1-methyl-1H-pyrazol-4-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
BIOGEN MA INC. US Patent | Assay Description The purpose of the BTK in vitro assay is to determine compound potency against BTK through the measurement of IC50. Compound inhibition is measured a... | US Patent US10189829 (2019) BindingDB Entry DOI: 10.7270/Q2V69MP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM324267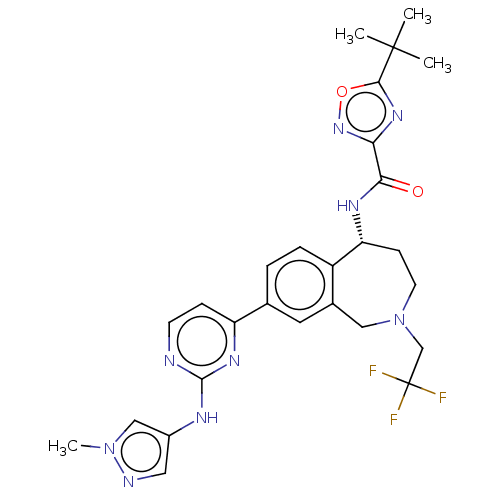 ((R)-5-(tert-butyl)-N-(8-(2-((1-methyl-1H-pyrazol-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
BIOGEN MA INC. US Patent | Assay Description The purpose of the BTK in vitro assay is to determine compound potency against BTK through the measurement of IC50. Compound inhibition is measured a... | US Patent US10189829 (2019) BindingDB Entry DOI: 10.7270/Q2V69MP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM324268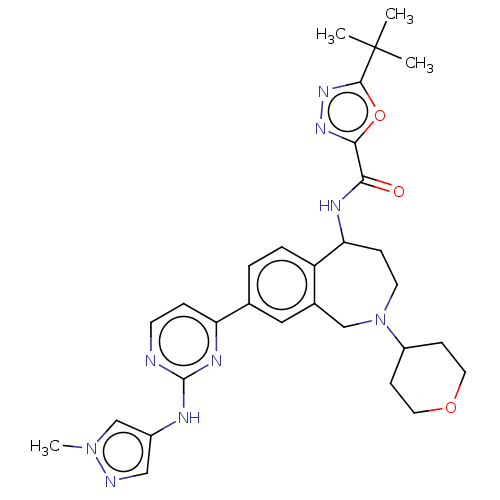 (5-(tert-butyl)-N-(8-(2-((1-methyl-1H-pyrazol-4-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
BIOGEN MA INC. US Patent | Assay Description The purpose of the BTK in vitro assay is to determine compound potency against BTK through the measurement of IC50. Compound inhibition is measured a... | US Patent US10189829 (2019) BindingDB Entry DOI: 10.7270/Q2V69MP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM324269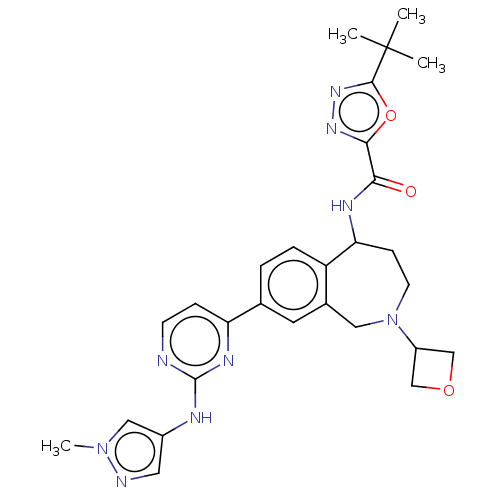 (5-(tert-butyl)-N-(8-(2-((1-methyl-1H-pyrazol-4-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
BIOGEN MA INC. US Patent | Assay Description The purpose of the BTK in vitro assay is to determine compound potency against BTK through the measurement of IC50. Compound inhibition is measured a... | US Patent US10189829 (2019) BindingDB Entry DOI: 10.7270/Q2V69MP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM324234 ((R)-5-(tert-butyl)-N-(8-(2-((1-methyl-1H-pyrazol-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
BIOGEN MA INC. US Patent | Assay Description The purpose of the BTK in vitro assay is to determine compound potency against BTK through the measurement of IC50. Compound inhibition is measured a... | US Patent US10189829 (2019) BindingDB Entry DOI: 10.7270/Q2V69MP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM324271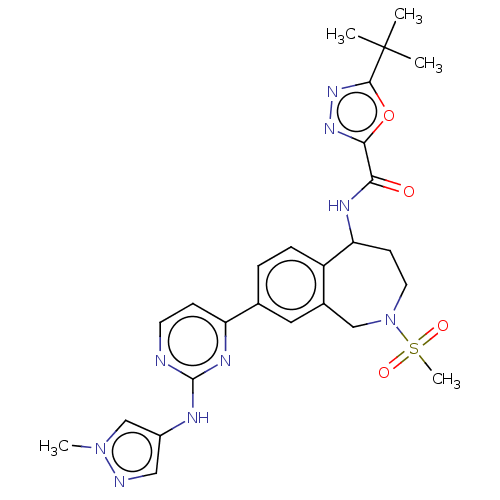 (5-(tert-butyl)-N-(8-(2-((1-methyl-1H-pyrazol-4-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
BIOGEN MA INC. US Patent | Assay Description The purpose of the BTK in vitro assay is to determine compound potency against BTK through the measurement of IC50. Compound inhibition is measured a... | US Patent US10189829 (2019) BindingDB Entry DOI: 10.7270/Q2V69MP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM324272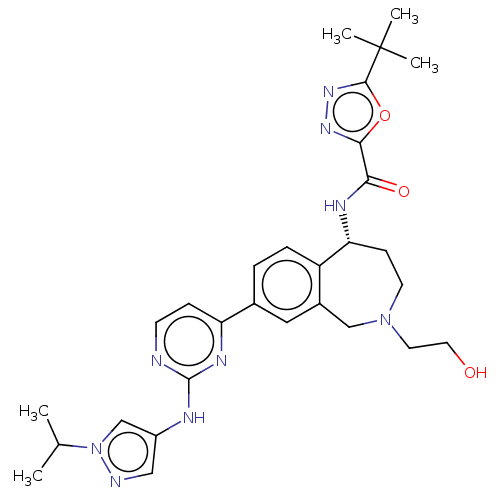 ((R)-5-(tert-butyl)-N-(2-(2-hydroxyethyl)-8-(2-((1-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
BIOGEN MA INC. US Patent | Assay Description The purpose of the BTK in vitro assay is to determine compound potency against BTK through the measurement of IC50. Compound inhibition is measured a... | US Patent US10189829 (2019) BindingDB Entry DOI: 10.7270/Q2V69MP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM324273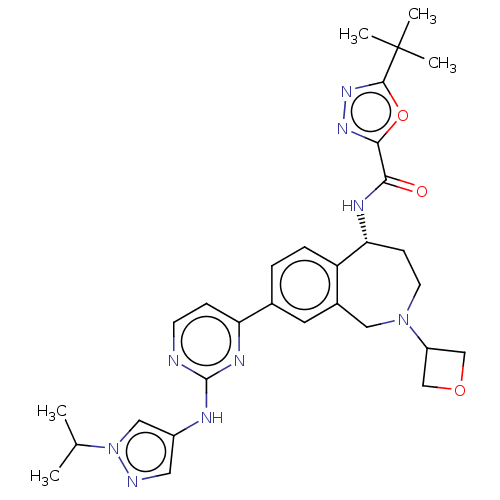 ((R)-5-(tert-butyl)-N-(8-(2-((1-isopropyl-1H-pyrazo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
BIOGEN MA INC. US Patent | Assay Description The purpose of the BTK in vitro assay is to determine compound potency against BTK through the measurement of IC50. Compound inhibition is measured a... | US Patent US10189829 (2019) BindingDB Entry DOI: 10.7270/Q2V69MP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM324274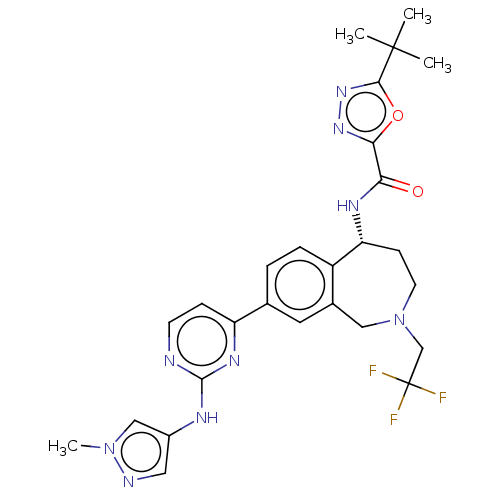 ((R)-5-(tert-butyl)-N-(8-(2-((1-methyl-1H-pyrazol-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
BIOGEN MA INC. US Patent | Assay Description The purpose of the BTK in vitro assay is to determine compound potency against BTK through the measurement of IC50. Compound inhibition is measured a... | US Patent US10189829 (2019) BindingDB Entry DOI: 10.7270/Q2V69MP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM324275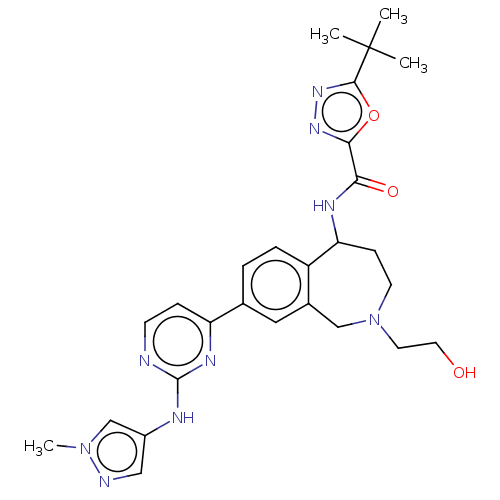 (5-(tert-butyl)-N-(2-(2-hydroxyethyl)-8-(2-((1-meth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
BIOGEN MA INC. US Patent | Assay Description The purpose of the BTK in vitro assay is to determine compound potency against BTK through the measurement of IC50. Compound inhibition is measured a... | US Patent US10189829 (2019) BindingDB Entry DOI: 10.7270/Q2V69MP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM324276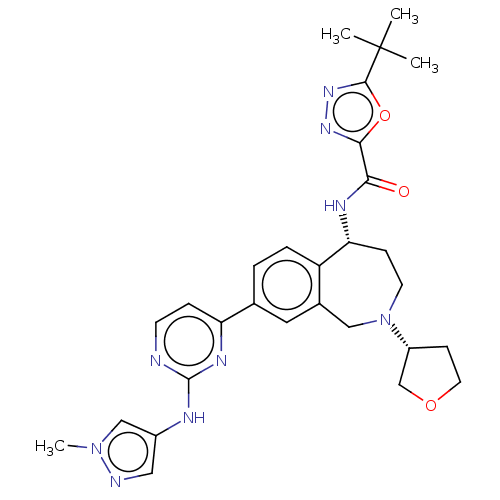 (5-(tert-butyl)-N—((R)-8-(2-((1-methyl-1H-pyra...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
BIOGEN MA INC. US Patent | Assay Description The purpose of the BTK in vitro assay is to determine compound potency against BTK through the measurement of IC50. Compound inhibition is measured a... | US Patent US10189829 (2019) BindingDB Entry DOI: 10.7270/Q2V69MP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1044 total ) | Next | Last >> |