 Found 9 hits Enz. Inhib. hit(s) with Target = 'Tyrosine-protein kinase BTK' and Ligand = 'BDBM324284'
Found 9 hits Enz. Inhib. hit(s) with Target = 'Tyrosine-protein kinase BTK' and Ligand = 'BDBM324284' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM324284
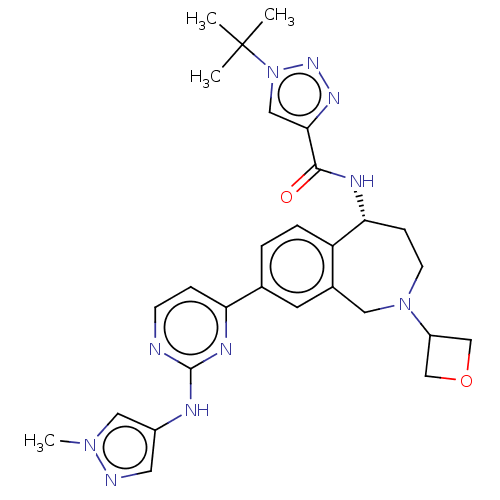
((R)-1-(tert-butyl)-N-(8-(2-((1-methyl-1H-pyrazol-4...)Show SMILES Cn1cc(Nc2nccc(n2)-c2ccc3[C@@H](CCN(Cc3c2)C2COC2)NC(=O)c2cn(nn2)C(C)(C)C)cn1 |r| Show InChI InChI=1S/C28H34N10O2/c1-28(2,3)38-15-25(34-35-38)26(39)32-24-8-10-37(21-16-40-17-21)13-19-11-18(5-6-22(19)24)23-7-9-29-27(33-23)31-20-12-30-36(4)14-20/h5-7,9,11-12,14-15,21,24H,8,10,13,16-17H2,1-4H3,(H,32,39)(H,29,31,33)/t24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human BTK using fluorescein-labeled polyGAT peptide as substrate incubated for 30 mins by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00926
BindingDB Entry DOI: 10.7270/Q23B641C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM324284

((R)-1-(tert-butyl)-N-(8-(2-((1-methyl-1H-pyrazol-4...)Show SMILES Cn1cc(Nc2nccc(n2)-c2ccc3[C@@H](CCN(Cc3c2)C2COC2)NC(=O)c2cn(nn2)C(C)(C)C)cn1 |r| Show InChI InChI=1S/C28H34N10O2/c1-28(2,3)38-15-25(34-35-38)26(39)32-24-8-10-37(21-16-40-17-21)13-19-11-18(5-6-22(19)24)23-7-9-29-27(33-23)31-20-12-30-36(4)14-20/h5-7,9,11-12,14-15,21,24H,8,10,13,16-17H2,1-4H3,(H,32,39)(H,29,31,33)/t24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
US Patent
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
BIOGEN MA INC.
US Patent
| Assay Description
The purpose of the BTK in vitro assay is to determine compound potency against BTK through the measurement of IC50. Compound inhibition is measured a... |
US Patent US10189829 (2019)
BindingDB Entry DOI: 10.7270/Q2V69MP8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM324284

((R)-1-(tert-butyl)-N-(8-(2-((1-methyl-1H-pyrazol-4...)Show SMILES Cn1cc(Nc2nccc(n2)-c2ccc3[C@@H](CCN(Cc3c2)C2COC2)NC(=O)c2cn(nn2)C(C)(C)C)cn1 |r| Show InChI InChI=1S/C28H34N10O2/c1-28(2,3)38-15-25(34-35-38)26(39)32-24-8-10-37(21-16-40-17-21)13-19-11-18(5-6-22(19)24)23-7-9-29-27(33-23)31-20-12-30-36(4)14-20/h5-7,9,11-12,14-15,21,24H,8,10,13,16-17H2,1-4H3,(H,32,39)(H,29,31,33)/t24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
US Patent
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
BIOGEN MA INC.
US Patent
| Assay Description
The purpose of the BTK in vitro assay is to determine compound potency against BTK through the measurement of IC50. Compound inhibition is measured a... |
US Patent US10961237 (2021)
BindingDB Entry DOI: 10.7270/Q2GH9N29 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM324284

((R)-1-(tert-butyl)-N-(8-(2-((1-methyl-1H-pyrazol-4...)Show SMILES Cn1cc(Nc2nccc(n2)-c2ccc3[C@@H](CCN(Cc3c2)C2COC2)NC(=O)c2cn(nn2)C(C)(C)C)cn1 |r| Show InChI InChI=1S/C28H34N10O2/c1-28(2,3)38-15-25(34-35-38)26(39)32-24-8-10-37(21-16-40-17-21)13-19-11-18(5-6-22(19)24)23-7-9-29-27(33-23)31-20-12-30-36(4)14-20/h5-7,9,11-12,14-15,21,24H,8,10,13,16-17H2,1-4H3,(H,32,39)(H,29,31,33)/t24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK in anti-IgM stimulated human PBMC cells assessed as reduction in PLCgamma2 phosphorylation |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00926
BindingDB Entry DOI: 10.7270/Q23B641C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM324284

((R)-1-(tert-butyl)-N-(8-(2-((1-methyl-1H-pyrazol-4...)Show SMILES Cn1cc(Nc2nccc(n2)-c2ccc3[C@@H](CCN(Cc3c2)C2COC2)NC(=O)c2cn(nn2)C(C)(C)C)cn1 |r| Show InChI InChI=1S/C28H34N10O2/c1-28(2,3)38-15-25(34-35-38)26(39)32-24-8-10-37(21-16-40-17-21)13-19-11-18(5-6-22(19)24)23-7-9-29-27(33-23)31-20-12-30-36(4)14-20/h5-7,9,11-12,14-15,21,24H,8,10,13,16-17H2,1-4H3,(H,32,39)(H,29,31,33)/t24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human BTK assessed as reduction in OVA323-329 specific T cells proliferation |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00926
BindingDB Entry DOI: 10.7270/Q23B641C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM324284

((R)-1-(tert-butyl)-N-(8-(2-((1-methyl-1H-pyrazol-4...)Show SMILES Cn1cc(Nc2nccc(n2)-c2ccc3[C@@H](CCN(Cc3c2)C2COC2)NC(=O)c2cn(nn2)C(C)(C)C)cn1 |r| Show InChI InChI=1S/C28H34N10O2/c1-28(2,3)38-15-25(34-35-38)26(39)32-24-8-10-37(21-16-40-17-21)13-19-11-18(5-6-22(19)24)23-7-9-29-27(33-23)31-20-12-30-36(4)14-20/h5-7,9,11-12,14-15,21,24H,8,10,13,16-17H2,1-4H3,(H,32,39)(H,29,31,33)/t24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK phosphorylation in human whole blood assessed as reduction in BTK phosphorylation incubated for 30 mins |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00926
BindingDB Entry DOI: 10.7270/Q23B641C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM324284

((R)-1-(tert-butyl)-N-(8-(2-((1-methyl-1H-pyrazol-4...)Show SMILES Cn1cc(Nc2nccc(n2)-c2ccc3[C@@H](CCN(Cc3c2)C2COC2)NC(=O)c2cn(nn2)C(C)(C)C)cn1 |r| Show InChI InChI=1S/C28H34N10O2/c1-28(2,3)38-15-25(34-35-38)26(39)32-24-8-10-37(21-16-40-17-21)13-19-11-18(5-6-22(19)24)23-7-9-29-27(33-23)31-20-12-30-36(4)14-20/h5-7,9,11-12,14-15,21,24H,8,10,13,16-17H2,1-4H3,(H,32,39)(H,29,31,33)/t24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
US Patent
| n/a | n/a | 505 | n/a | n/a | n/a | n/a | n/a | n/a |
Neuroscience
| Assay Description
The purpose of the BTK in vitro assay is to determine compound potency against BTK through the measurement of IC50. Compound inhibition is measured a... |
J Med Chem 51: 1730-9 (2008)
BindingDB Entry DOI: 10.7270/Q25M681D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM324284

((R)-1-(tert-butyl)-N-(8-(2-((1-methyl-1H-pyrazol-4...)Show SMILES Cn1cc(Nc2nccc(n2)-c2ccc3[C@@H](CCN(Cc3c2)C2COC2)NC(=O)c2cn(nn2)C(C)(C)C)cn1 |r| Show InChI InChI=1S/C28H34N10O2/c1-28(2,3)38-15-25(34-35-38)26(39)32-24-8-10-37(21-16-40-17-21)13-19-11-18(5-6-22(19)24)23-7-9-29-27(33-23)31-20-12-30-36(4)14-20/h5-7,9,11-12,14-15,21,24H,8,10,13,16-17H2,1-4H3,(H,32,39)(H,29,31,33)/t24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK in human Ramos cells assessed as reduction in PLCgamma2 phosphorylation |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00926
BindingDB Entry DOI: 10.7270/Q23B641C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM324284

((R)-1-(tert-butyl)-N-(8-(2-((1-methyl-1H-pyrazol-4...)Show SMILES Cn1cc(Nc2nccc(n2)-c2ccc3[C@@H](CCN(Cc3c2)C2COC2)NC(=O)c2cn(nn2)C(C)(C)C)cn1 |r| Show InChI InChI=1S/C28H34N10O2/c1-28(2,3)38-15-25(34-35-38)26(39)32-24-8-10-37(21-16-40-17-21)13-19-11-18(5-6-22(19)24)23-7-9-29-27(33-23)31-20-12-30-36(4)14-20/h5-7,9,11-12,14-15,21,24H,8,10,13,16-17H2,1-4H3,(H,32,39)(H,29,31,33)/t24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to wild-type human full length BTK (M1 to S659 residues) expressed in mammalian expression system by Kinomescan method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00926
BindingDB Entry DOI: 10.7270/Q23B641C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data