

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22911 (2-(3H-imidazol-4-yl)ethylsulfanylmethanimidamide |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [125I]iodoproxyfan from human full-length histamine H3 receptor expressed in HEK293 cells after 60 mins | Bioorg Med Chem 19: 2850-8 (2011) Article DOI: 10.1016/j.bmc.2011.03.046 BindingDB Entry DOI: 10.7270/Q2X63N8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM86174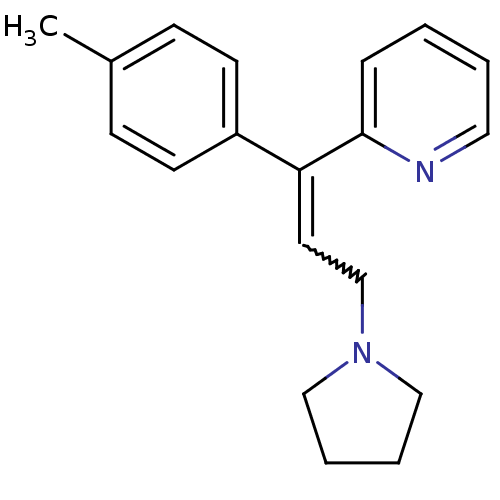 (CAS_486-12-4 | NSC_5282443 | Triprolidine) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 1.15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by PDSP Ki Database | J Pharmacol Exp Ther 305: 1104-15 (2003) Article DOI: 10.1124/jpet.103.049619 BindingDB Entry DOI: 10.7270/Q2HM5708 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM22911 (2-(3H-imidazol-4-yl)ethylsulfanylmethanimidamide |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human full-length histamine H4 receptor expressed in HEK293 cells after 60 mins | Bioorg Med Chem 19: 2850-8 (2011) Article DOI: 10.1016/j.bmc.2011.03.046 BindingDB Entry DOI: 10.7270/Q2X63N8N | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22541 (Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [125I]iodoproxyfan from human full-length histamine H3 receptor expressed in HEK293 cells after 60 mins | Bioorg Med Chem 19: 2850-8 (2011) Article DOI: 10.1016/j.bmc.2011.03.046 BindingDB Entry DOI: 10.7270/Q2X63N8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22541 (Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]iodoproxyfan from human histamine H3 receptor expressed in CHO-K1 cells | Bioorg Med Chem Lett 19: 6682-5 (2009) Article DOI: 10.1016/j.bmcl.2009.10.005 BindingDB Entry DOI: 10.7270/Q2GM87DG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM86174 (CAS_486-12-4 | NSC_5282443 | Triprolidine) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 3.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by PDSP Ki Database | J Pharmacol Exp Ther 305: 1104-15 (2003) Article DOI: 10.1124/jpet.103.049619 BindingDB Entry DOI: 10.7270/Q2HM5708 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM22541 (Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human full-length histamine H4 receptor expressed in HEK293 cells after 60 mins | Bioorg Med Chem 19: 2850-8 (2011) Article DOI: 10.1016/j.bmc.2011.03.046 BindingDB Entry DOI: 10.7270/Q2X63N8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM22541 (Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human histamine H4 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 19: 6682-5 (2009) Article DOI: 10.1016/j.bmcl.2009.10.005 BindingDB Entry DOI: 10.7270/Q2GM87DG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50311769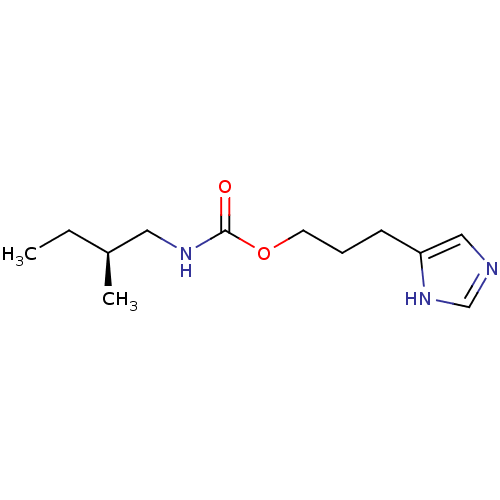 ((S)-3-(1H-imidazol-4-yl)propyl 2-methylbutylcarbam...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]iodoproxyfan from human histamine H3 receptor expressed in CHO-K1 cells | Bioorg Med Chem Lett 19: 6682-5 (2009) Article DOI: 10.1016/j.bmcl.2009.10.005 BindingDB Entry DOI: 10.7270/Q2GM87DG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50074076 ((3-Methyl-pentyl)-carbamic acid 3-(1H-imidazol-4-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]histamine from histamine H3 receptor in rat cerebral cortex | Bioorg Med Chem Lett 19: 6682-5 (2009) Article DOI: 10.1016/j.bmcl.2009.10.005 BindingDB Entry DOI: 10.7270/Q2GM87DG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50074088 ((2-Methyl-pentyl)-carbamic acid 3-(1H-imidazol-4-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]iodoproxyfan from human histamine H3 receptor expressed in CHO-K1 cells | Bioorg Med Chem Lett 19: 6682-5 (2009) Article DOI: 10.1016/j.bmcl.2009.10.005 BindingDB Entry DOI: 10.7270/Q2GM87DG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50074072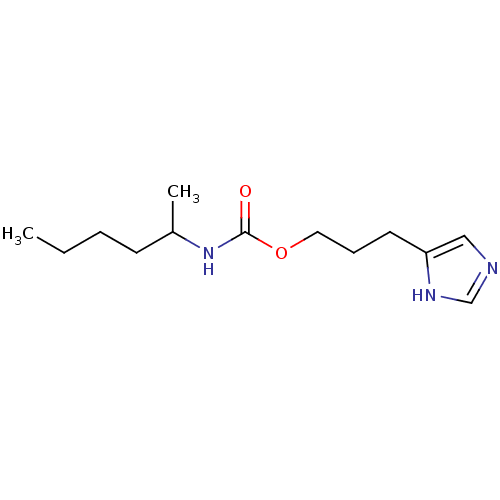 ((1-Methyl-pentyl)-carbamic acid 3-(1H-imidazol-4-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]histamine from histamine H3 receptor in rat cerebral cortex | Bioorg Med Chem Lett 19: 6682-5 (2009) Article DOI: 10.1016/j.bmcl.2009.10.005 BindingDB Entry DOI: 10.7270/Q2GM87DG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Cavia porcellus (domestic guinea pig)) | BDBM86740 (UR-AK24 | UR-AK57) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by PDSP Ki Database | J Pharmacol Exp Ther 317: 1262-8 (2006) Article DOI: 10.1124/jpet.106.102897 BindingDB Entry DOI: 10.7270/Q2X63KHZ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Cavia porcellus (domestic guinea pig)) | BDBM86740 (UR-AK24 | UR-AK57) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by PDSP Ki Database | J Pharmacol Exp Ther 317: 1262-8 (2006) Article DOI: 10.1124/jpet.106.102897 BindingDB Entry DOI: 10.7270/Q2X63KHZ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50311765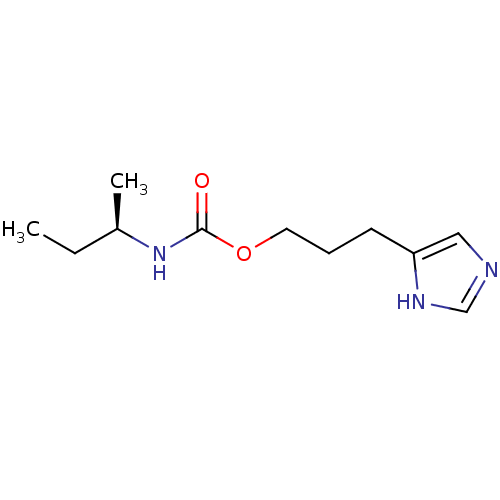 ((R)-3-(1H-imidazol-4-yl)propyl sec-butylcarbamate ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]iodoproxyfan from human histamine H3 receptor expressed in CHO-K1 cells | Bioorg Med Chem Lett 19: 6682-5 (2009) Article DOI: 10.1016/j.bmcl.2009.10.005 BindingDB Entry DOI: 10.7270/Q2GM87DG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50311768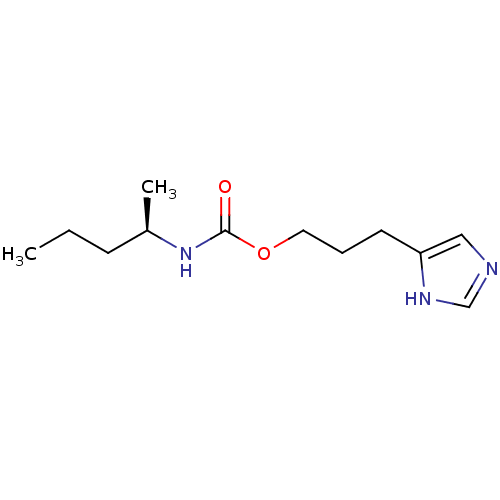 ((R)-3-(1H-imidazol-4-yl)propyl pentan-2-ylcarbamat...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]histamine from histamine H3 receptor in rat cerebral cortex | Bioorg Med Chem Lett 19: 6682-5 (2009) Article DOI: 10.1016/j.bmcl.2009.10.005 BindingDB Entry DOI: 10.7270/Q2GM87DG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50311772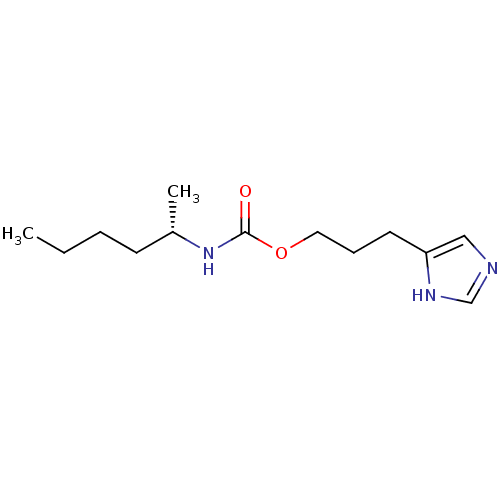 ((S)-3-(1H-imidazol-4-yl)propyl hexan-2-ylcarbamate...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]histamine from histamine H3 receptor in rat cerebral cortex | Bioorg Med Chem Lett 19: 6682-5 (2009) Article DOI: 10.1016/j.bmcl.2009.10.005 BindingDB Entry DOI: 10.7270/Q2GM87DG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50304523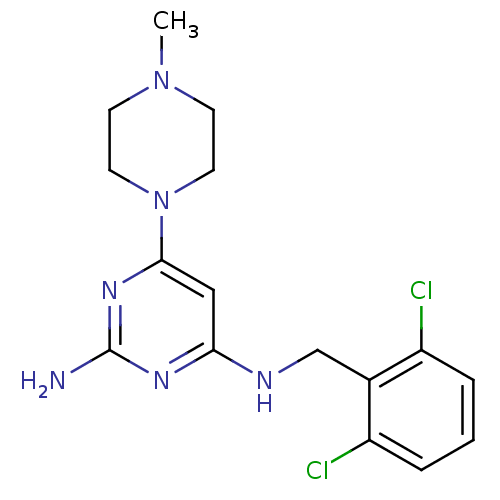 (CHEMBL595180 | N4-(2,6-Dichlorobenzyl)-6-(4-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-University Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human histamine H4 receptor expressed in Sf9 cells co-expressing Galphai2 and Gbeta1gamma2 | Bioorg Med Chem 17: 7186-96 (2009) Article DOI: 10.1016/j.bmc.2009.08.059 BindingDB Entry DOI: 10.7270/Q2416X4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50311780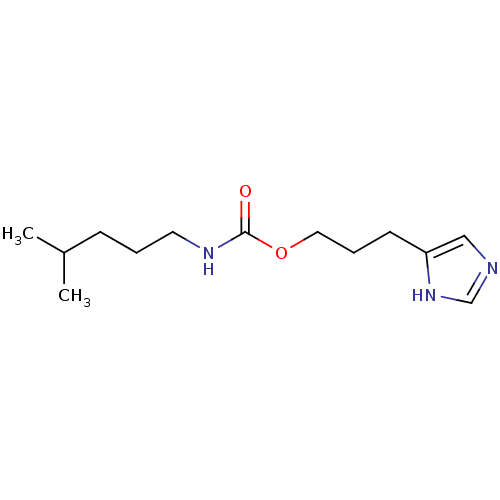 (3-(1H-imidazol-4-yl)propyl 4-methylpentylcarbamate...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]iodoproxyfan from human histamine H3 receptor expressed in CHO-K1 cells | Bioorg Med Chem Lett 19: 6682-5 (2009) Article DOI: 10.1016/j.bmcl.2009.10.005 BindingDB Entry DOI: 10.7270/Q2GM87DG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50074076 ((3-Methyl-pentyl)-carbamic acid 3-(1H-imidazol-4-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]iodoproxyfan from human histamine H3 receptor expressed in CHO-K1 cells | Bioorg Med Chem Lett 19: 6682-5 (2009) Article DOI: 10.1016/j.bmcl.2009.10.005 BindingDB Entry DOI: 10.7270/Q2GM87DG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM22566 (5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]-1H-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-University Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human histamine H4 receptor expressed in Sf9 cells co-expressing Galphai2 and Gbeta1gamma2 | Bioorg Med Chem 17: 7186-96 (2009) Article DOI: 10.1016/j.bmc.2009.08.059 BindingDB Entry DOI: 10.7270/Q2416X4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50343837 (3-(1H-imidazol-4-yl)propyl pent-4-enylcarbamate hy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [125I]iodoproxyfan from human histamine H3 receptor expressed in CHO-K1 cells after 60 mins by gamma counting | Bioorg Med Chem 19: 2850-8 (2011) Article DOI: 10.1016/j.bmc.2011.03.046 BindingDB Entry DOI: 10.7270/Q2X63N8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50074088 ((2-Methyl-pentyl)-carbamic acid 3-(1H-imidazol-4-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]histamine from histamine H3 receptor in rat cerebral cortex | Bioorg Med Chem Lett 19: 6682-5 (2009) Article DOI: 10.1016/j.bmcl.2009.10.005 BindingDB Entry DOI: 10.7270/Q2GM87DG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50311768 ((R)-3-(1H-imidazol-4-yl)propyl pentan-2-ylcarbamat...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]iodoproxyfan from human histamine H3 receptor expressed in CHO-K1 cells | Bioorg Med Chem Lett 19: 6682-5 (2009) Article DOI: 10.1016/j.bmcl.2009.10.005 BindingDB Entry DOI: 10.7270/Q2GM87DG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Cavia porcellus (domestic guinea pig)) | BDBM86742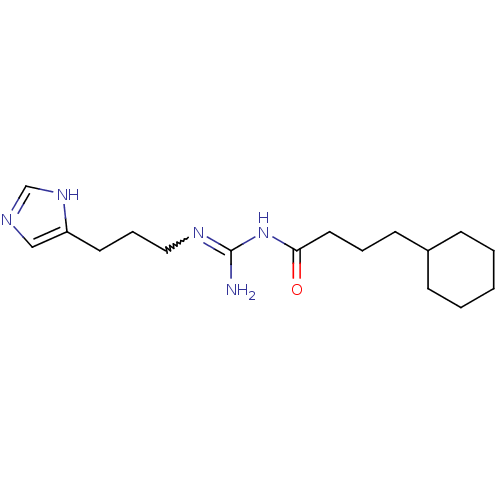 (UR-AK64) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by PDSP Ki Database | J Pharmacol Exp Ther 317: 1262-8 (2006) Article DOI: 10.1124/jpet.106.102897 BindingDB Entry DOI: 10.7270/Q2X63KHZ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50343838 (3-(1H-imidazol-4-yl)propyl 2-cyclopropylethylcarba...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [125I]iodoproxyfan from human histamine H3 receptor expressed in CHO-K1 cells after 60 mins by gamma counting | Bioorg Med Chem 19: 2850-8 (2011) Article DOI: 10.1016/j.bmc.2011.03.046 BindingDB Entry DOI: 10.7270/Q2X63N8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50311777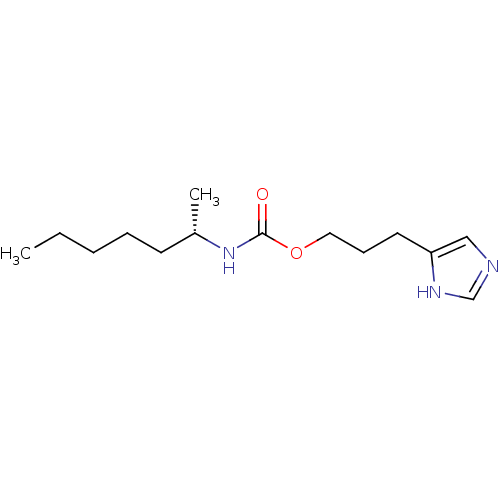 ((S)-3-(1H-imidazol-4-yl)propyl heptan-2-ylcarbamat...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]histamine from histamine H3 receptor in rat cerebral cortex | Bioorg Med Chem Lett 19: 6682-5 (2009) Article DOI: 10.1016/j.bmcl.2009.10.005 BindingDB Entry DOI: 10.7270/Q2GM87DG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50074069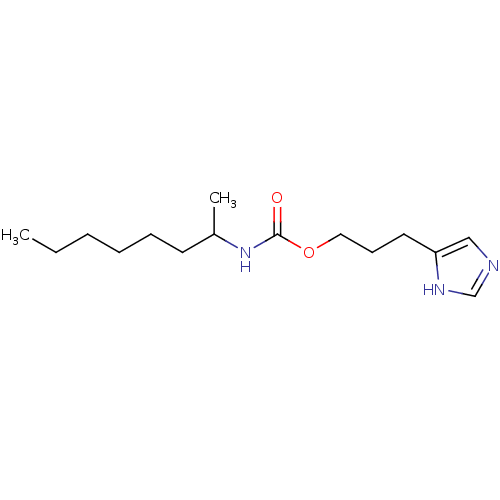 ((1-Methyl-heptyl)-carbamic acid 3-(1H-imidazol-4-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]histamine from histamine H3 receptor in rat cerebral cortex | Bioorg Med Chem Lett 19: 6682-5 (2009) Article DOI: 10.1016/j.bmcl.2009.10.005 BindingDB Entry DOI: 10.7270/Q2GM87DG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50304524 (CHEMBL594721 | N4-(2-Chlorobenzyl)-6-(4-methylpipe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-University Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human histamine H4 receptor expressed in Sf9 cells co-expressing Galphai2 and Gbeta1gamma2 | Bioorg Med Chem 17: 7186-96 (2009) Article DOI: 10.1016/j.bmc.2009.08.059 BindingDB Entry DOI: 10.7270/Q2416X4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50311769 ((S)-3-(1H-imidazol-4-yl)propyl 2-methylbutylcarbam...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]histamine from histamine H3 receptor in rat cerebral cortex | Bioorg Med Chem Lett 19: 6682-5 (2009) Article DOI: 10.1016/j.bmcl.2009.10.005 BindingDB Entry DOI: 10.7270/Q2GM87DG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50074073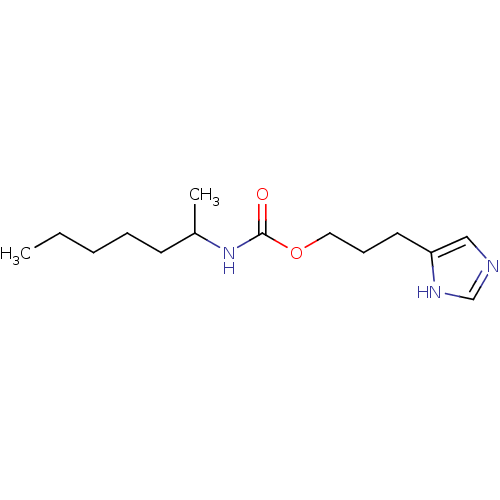 ((1-Methyl-hexyl)-carbamic acid 3-(1H-imidazol-4-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]histamine from histamine H3 receptor in rat cerebral cortex | Bioorg Med Chem Lett 19: 6682-5 (2009) Article DOI: 10.1016/j.bmcl.2009.10.005 BindingDB Entry DOI: 10.7270/Q2GM87DG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50311771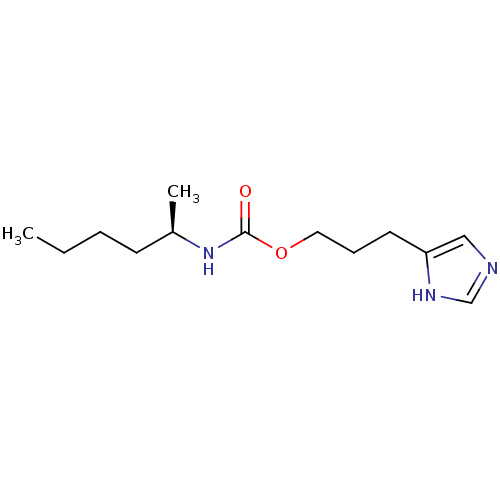 ((R)-3-(1H-imidazol-4-yl)propyl hexan-2-ylcarbamate...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]iodoproxyfan from human histamine H3 receptor expressed in CHO-K1 cells | Bioorg Med Chem Lett 19: 6682-5 (2009) Article DOI: 10.1016/j.bmcl.2009.10.005 BindingDB Entry DOI: 10.7270/Q2GM87DG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50311765 ((R)-3-(1H-imidazol-4-yl)propyl sec-butylcarbamate ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]histamine from histamine H3 receptor in rat cerebral cortex | Bioorg Med Chem Lett 19: 6682-5 (2009) Article DOI: 10.1016/j.bmcl.2009.10.005 BindingDB Entry DOI: 10.7270/Q2GM87DG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50311771 ((R)-3-(1H-imidazol-4-yl)propyl hexan-2-ylcarbamate...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]histamine from histamine H3 receptor in rat cerebral cortex | Bioorg Med Chem Lett 19: 6682-5 (2009) Article DOI: 10.1016/j.bmcl.2009.10.005 BindingDB Entry DOI: 10.7270/Q2GM87DG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50074087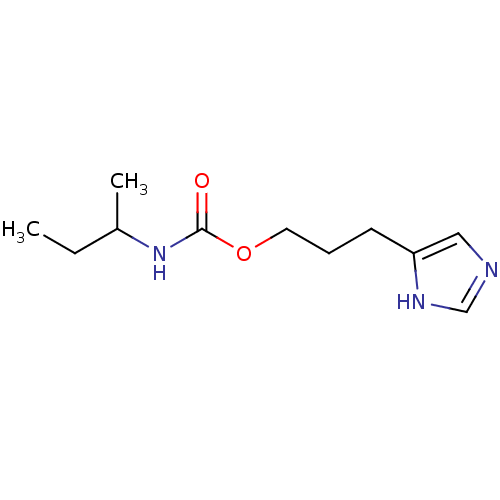 (CHEMBL1076266 | CHEMBL157493 | sec-Butyl-carbamic ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]histamine from histamine H3 receptor in rat cerebral cortex | Bioorg Med Chem Lett 19: 6682-5 (2009) Article DOI: 10.1016/j.bmcl.2009.10.005 BindingDB Entry DOI: 10.7270/Q2GM87DG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50343839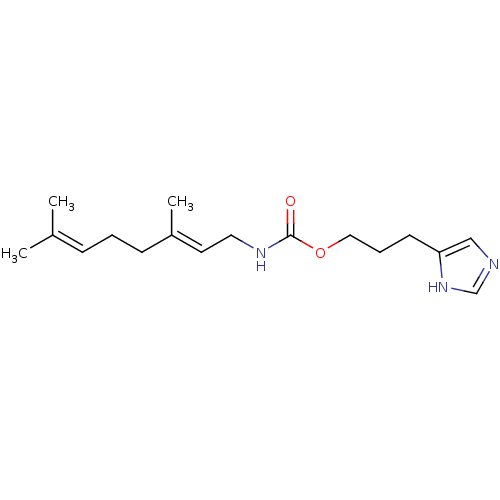 ((E)-3-(1H-Imidazol-4-yl)propyl3,7-dimethylocta-2,6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [125I]iodoproxyfan from human histamine H3 receptor expressed in CHO-K1 cells after 60 mins by gamma counting | Bioorg Med Chem 19: 2850-8 (2011) Article DOI: 10.1016/j.bmc.2011.03.046 BindingDB Entry DOI: 10.7270/Q2X63N8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Cavia porcellus (domestic guinea pig)) | BDBM86747 (UR-AK67) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by PDSP Ki Database | J Pharmacol Exp Ther 317: 1262-8 (2006) Article DOI: 10.1124/jpet.106.102897 BindingDB Entry DOI: 10.7270/Q2X63KHZ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50074062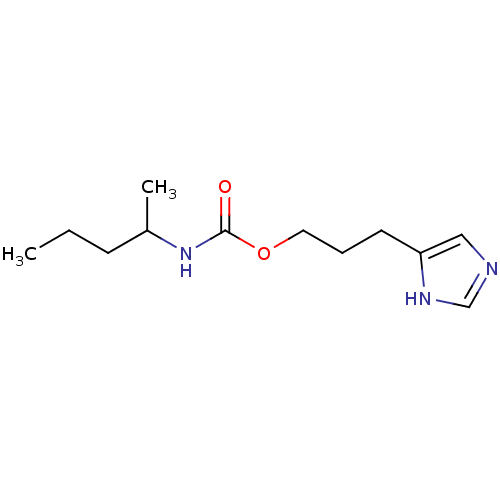 ((1-Methyl-butyl)-carbamic acid 3-(1H-imidazol-4-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]iodoproxyfan from human histamine H3 receptor expressed in CHO-K1 cells | Bioorg Med Chem Lett 19: 6682-5 (2009) Article DOI: 10.1016/j.bmcl.2009.10.005 BindingDB Entry DOI: 10.7270/Q2GM87DG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Cavia porcellus (domestic guinea pig)) | BDBM86738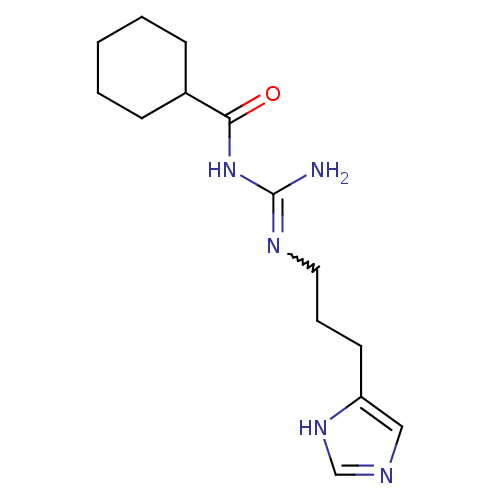 (UR-AK46) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by PDSP Ki Database | J Pharmacol Exp Ther 317: 1262-8 (2006) Article DOI: 10.1124/jpet.106.102897 BindingDB Entry DOI: 10.7270/Q2X63KHZ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Cavia porcellus (domestic guinea pig)) | BDBM86743 (UR-AK49) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by PDSP Ki Database | J Pharmacol Exp Ther 317: 1262-8 (2006) Article DOI: 10.1124/jpet.106.102897 BindingDB Entry DOI: 10.7270/Q2X63KHZ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50311778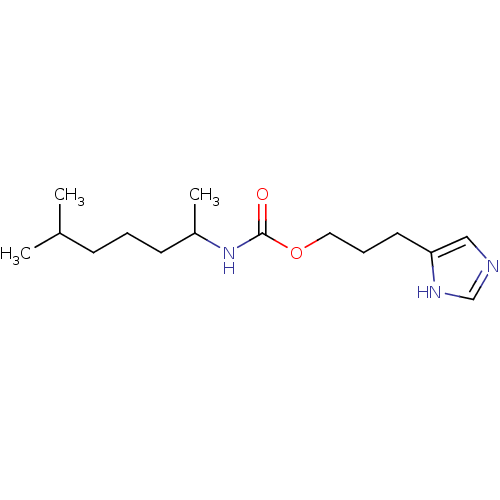 ((R,S)-3-(1H-imidazol-4-yl)propyl 6-methylheptan-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]histamine from histamine H3 receptor in rat cerebral cortex | Bioorg Med Chem Lett 19: 6682-5 (2009) Article DOI: 10.1016/j.bmcl.2009.10.005 BindingDB Entry DOI: 10.7270/Q2GM87DG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM86740 (UR-AK24 | UR-AK57) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by PDSP Ki Database | J Pharmacol Exp Ther 317: 1262-8 (2006) Article DOI: 10.1124/jpet.106.102897 BindingDB Entry DOI: 10.7270/Q2X63KHZ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Cavia porcellus (domestic guinea pig)) | BDBM86744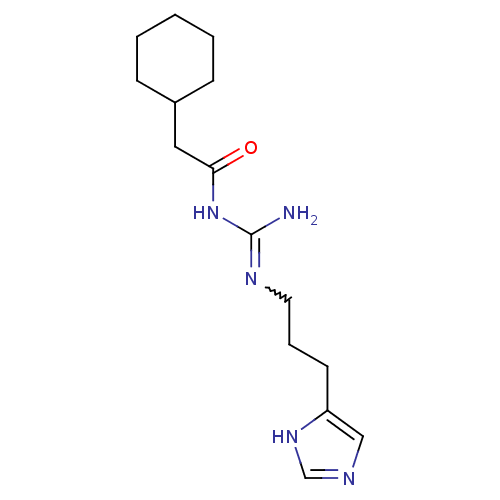 (UR-AK62) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by PDSP Ki Database | J Pharmacol Exp Ther 317: 1262-8 (2006) Article DOI: 10.1124/jpet.106.102897 BindingDB Entry DOI: 10.7270/Q2X63KHZ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50311766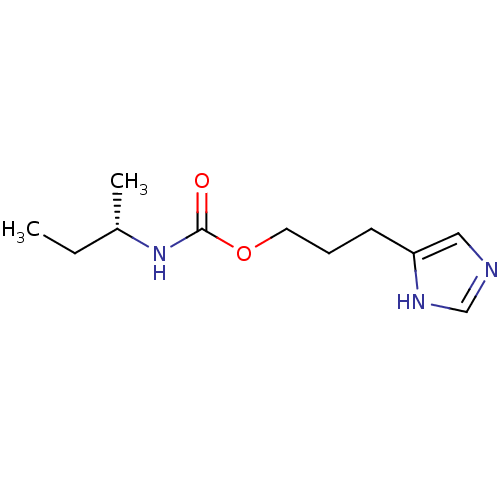 ((S)-3-(1H-imidazol-4-yl)propyl sec-butylcarbamate ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]histamine from histamine H3 receptor in rat cerebral cortex | Bioorg Med Chem Lett 19: 6682-5 (2009) Article DOI: 10.1016/j.bmcl.2009.10.005 BindingDB Entry DOI: 10.7270/Q2GM87DG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM86742 (UR-AK64) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by PDSP Ki Database | J Pharmacol Exp Ther 317: 1262-8 (2006) Article DOI: 10.1124/jpet.106.102897 BindingDB Entry DOI: 10.7270/Q2X63KHZ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Cavia porcellus (domestic guinea pig)) | BDBM86745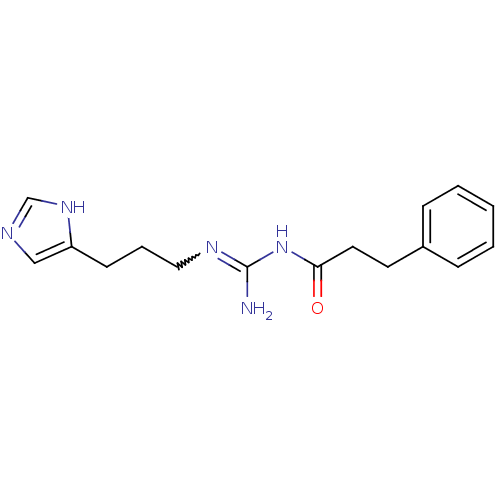 (UR-AK51) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by PDSP Ki Database | J Pharmacol Exp Ther 317: 1262-8 (2006) Article DOI: 10.1124/jpet.106.102897 BindingDB Entry DOI: 10.7270/Q2X63KHZ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50311776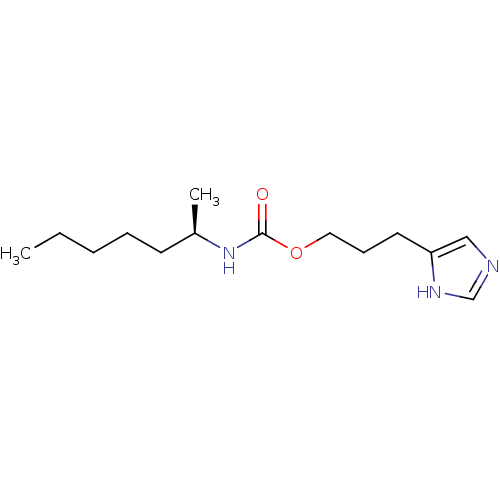 ((R)-3-(1H-imidazol-4-yl)propyl heptan-2-ylcarbamat...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]histamine from histamine H3 receptor in rat cerebral cortex | Bioorg Med Chem Lett 19: 6682-5 (2009) Article DOI: 10.1016/j.bmcl.2009.10.005 BindingDB Entry DOI: 10.7270/Q2GM87DG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50343840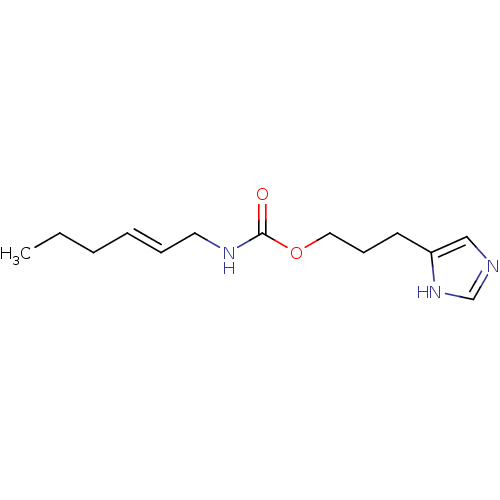 ((E)-3-(1H-Imidazol-4-yl)propylhex-2-enylcarbamateh...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [125I]iodoproxyfan from human histamine H3 receptor expressed in CHO-K1 cells after 60 mins by gamma counting | Bioorg Med Chem 19: 2850-8 (2011) Article DOI: 10.1016/j.bmc.2011.03.046 BindingDB Entry DOI: 10.7270/Q2X63N8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50343841 (3-(1H-Imidazol-4-yl)propyl2-cyclohexylethylcarbama...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [125I]iodoproxyfan from human histamine H3 receptor expressed in CHO-K1 cells after 60 mins by gamma counting | Bioorg Med Chem 19: 2850-8 (2011) Article DOI: 10.1016/j.bmc.2011.03.046 BindingDB Entry DOI: 10.7270/Q2X63N8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50304525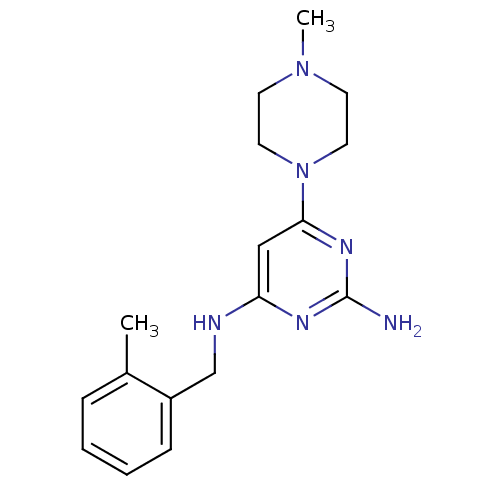 (CHEMBL594731 | N4-(2-Methylbenzyl)-6-(4-methylpipe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-University Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human histamine H4 receptor expressed in Sf9 cells co-expressing Galphai2 and Gbeta1gamma2 | Bioorg Med Chem 17: 7186-96 (2009) Article DOI: 10.1016/j.bmc.2009.08.059 BindingDB Entry DOI: 10.7270/Q2416X4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 510 total ) | Next | Last >> |