

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vitamin D3 receptor (Gallus gallus) | BDBM50145539 ((2S,4R)-2,4-Dihydroxy-6-[2-[(R)-1-((R)-5-hydroxy-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo Curated by ChEMBL | Assay Description In vitro relative binding affinity for chick intestinal vitamin D3 receptor compared to [3H]-1 | Bioorg Med Chem Lett 14: 2579-83 (2004) Article DOI: 10.1016/j.bmcl.2004.02.076 BindingDB Entry DOI: 10.7270/Q20C4V6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM50066441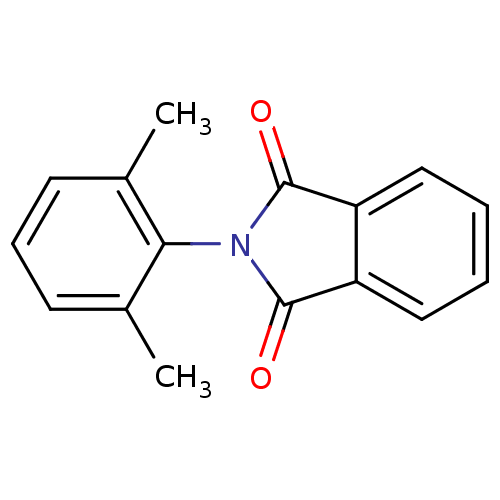 (2-(2,6-Dimethyl-phenyl)-isoindole-1,3-dione | 2-(2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Inhibitory activity (RA2) against Prostaglandin G/H synthase 2 was calculated relative to aspirin | Bioorg Med Chem Lett 12: 1043-6 (2002) BindingDB Entry DOI: 10.7270/Q28K78D9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM50088679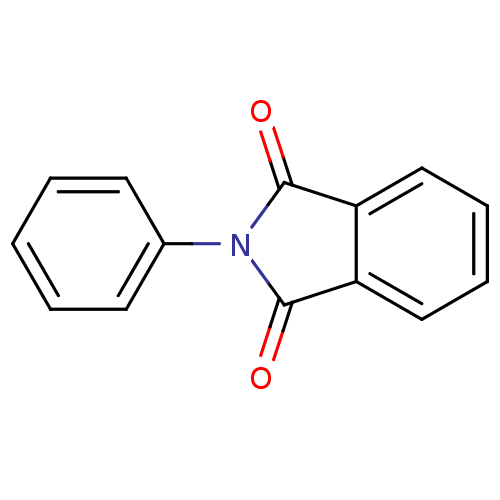 (2-Phenyl-isoindole-1,3-dione | 2-phenyl-1H-isoindo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Inhibitory activity (RA2) against Prostaglandin G/H synthase 2 was calculated relative to aspirin | Bioorg Med Chem Lett 12: 1043-6 (2002) BindingDB Entry DOI: 10.7270/Q28K78D9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50088679 (2-Phenyl-isoindole-1,3-dione | 2-phenyl-1H-isoindo...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Inhibitory activity (RA1) against Prostaglandin G/H synthase 1 was calculated relative to aspirin | Bioorg Med Chem Lett 12: 1043-6 (2002) BindingDB Entry DOI: 10.7270/Q28K78D9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM50219329 (CHEMBL8968) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Inhibitory activity (RA2) against Prostaglandin G/H synthase 2 was calculated relative to aspirin | Bioorg Med Chem Lett 12: 1043-6 (2002) BindingDB Entry DOI: 10.7270/Q28K78D9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50066441 (2-(2,6-Dimethyl-phenyl)-isoindole-1,3-dione | 2-(2...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Inhibitory activity (RA1) against Prostaglandin G/H synthase 1 was calculated relative to aspirin | Bioorg Med Chem Lett 12: 1043-6 (2002) BindingDB Entry DOI: 10.7270/Q28K78D9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50219329 (CHEMBL8968) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Inhibitory activity (RA1) against Prostaglandin G/H synthase 1 was calculated relative to aspirin | Bioorg Med Chem Lett 12: 1043-6 (2002) BindingDB Entry DOI: 10.7270/Q28K78D9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Gallus gallus) | BDBM50145538 ((S)-1-Benzyl-5-((R)-2-{(R)-4-[2-[(3S,5R)-3,5-dihyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo Curated by ChEMBL | Assay Description In vitro relative binding affinity for chick intestinal vitamin D3 receptor compared to [3H]-1 | Bioorg Med Chem Lett 14: 2579-83 (2004) Article DOI: 10.1016/j.bmcl.2004.02.076 BindingDB Entry DOI: 10.7270/Q20C4V6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM50111216 (5-Amino-2-((R)-3-methyl-2,6-dioxo-piperidin-3-yl)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Inhibitory activity (RA2) against Prostaglandin G/H synthase 2 was calculated relative to aspirin | Bioorg Med Chem Lett 12: 1043-6 (2002) BindingDB Entry DOI: 10.7270/Q28K78D9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Gallus gallus) | BDBM50145540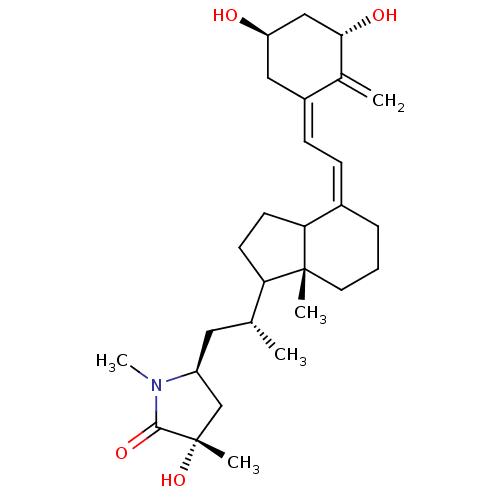 ((S)-5-((R)-2-{(R)-4-[2-[(3S,5R)-3,5-Dihydroxy-2-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo Curated by ChEMBL | Assay Description In vitro relative binding affinity for chick intestinal vitamin D3 receptor compared to [3H]-1 | Bioorg Med Chem Lett 14: 2579-83 (2004) Article DOI: 10.1016/j.bmcl.2004.02.076 BindingDB Entry DOI: 10.7270/Q20C4V6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Gallus gallus) | BDBM50404242 (CHEMBL2114211) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo Curated by ChEMBL | Assay Description In vitro relative binding affinity for chick intestinal vitamin D3 receptor compared to [3H]-1 | Bioorg Med Chem Lett 14: 2579-83 (2004) Article DOI: 10.1016/j.bmcl.2004.02.076 BindingDB Entry DOI: 10.7270/Q20C4V6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Gallus gallus) | BDBM50404243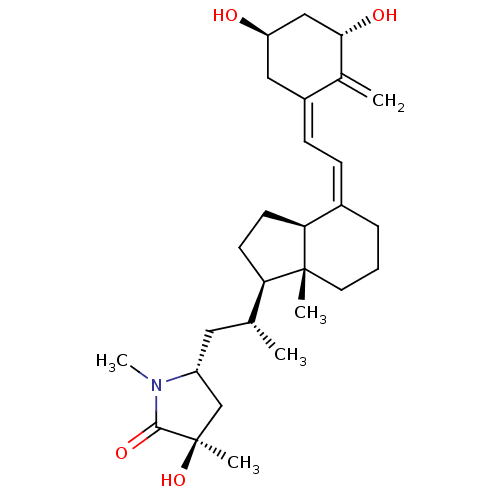 (CHEMBL2114212 | CHEMBL3350688) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo Curated by ChEMBL | Assay Description In vitro relative binding affinity for chick intestinal vitamin D3 receptor compared to [3H]-1 | Bioorg Med Chem Lett 14: 2579-83 (2004) Article DOI: 10.1016/j.bmcl.2004.02.076 BindingDB Entry DOI: 10.7270/Q20C4V6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Gallus gallus) | BDBM50223417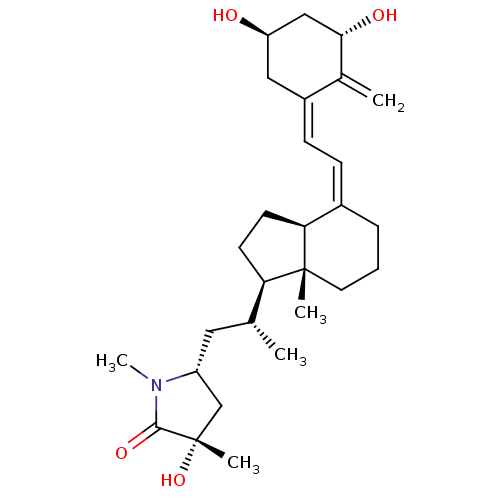 (CHEMBL3350292) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo Curated by ChEMBL | Assay Description In vitro relative binding affinity for chick intestinal vitamin D3 receptor compared to [3H]-1 | Bioorg Med Chem Lett 14: 2579-83 (2004) Article DOI: 10.1016/j.bmcl.2004.02.076 BindingDB Entry DOI: 10.7270/Q20C4V6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM50111221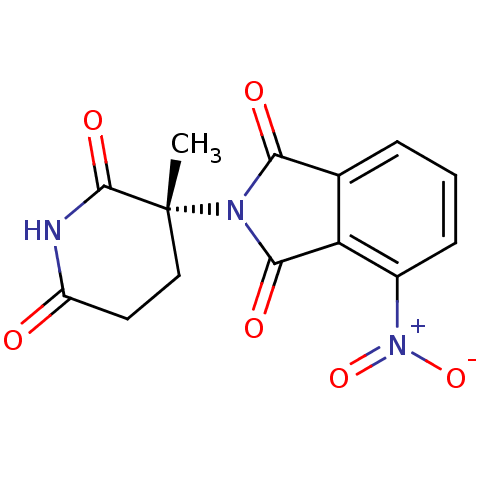 (2-((R)-3-Methyl-2,6-dioxo-piperidin-3-yl)-4-nitro-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Inhibitory activity (RA2) of the compound against Prostaglandin G/H synthase 2 was calculated relative to aspirin | Bioorg Med Chem Lett 12: 1043-6 (2002) BindingDB Entry DOI: 10.7270/Q28K78D9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50111216 (5-Amino-2-((R)-3-methyl-2,6-dioxo-piperidin-3-yl)-...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Inhibitory activity (RA1) of the compound against Prostaglandin G/H synthase 1 was calculated relative to aspirin | Bioorg Med Chem Lett 12: 1043-6 (2002) BindingDB Entry DOI: 10.7270/Q28K78D9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50111222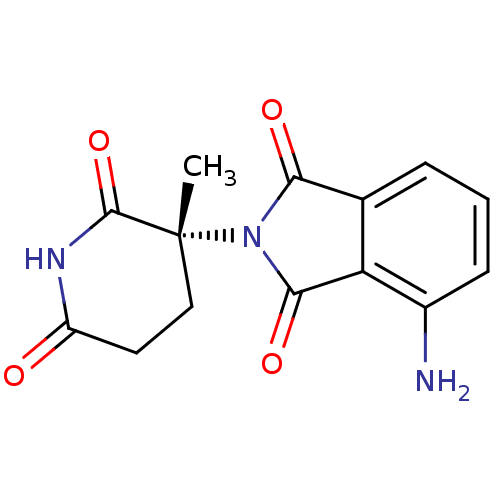 (4-Amino-2-((R)-3-methyl-2,6-dioxo-piperidin-3-yl)-...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Inhibitory activity (RA1) of the compound against Prostaglandin G/H synthase 1 was calculated relative to aspirin | Bioorg Med Chem Lett 12: 1043-6 (2002) BindingDB Entry DOI: 10.7270/Q28K78D9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50111209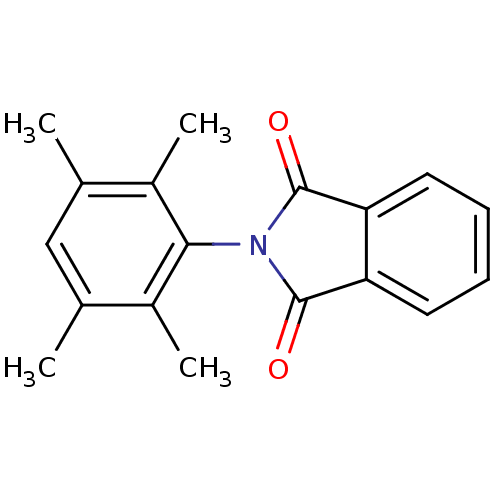 (2-(2,3,5,6-Tetramethyl-phenyl)-isoindole-1,3-dione...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Inhibitory activity (RA1) of the compound against Prostaglandin G/H synthase 1 was calculated relative to aspirin | Bioorg Med Chem Lett 12: 1043-6 (2002) BindingDB Entry DOI: 10.7270/Q28K78D9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50111217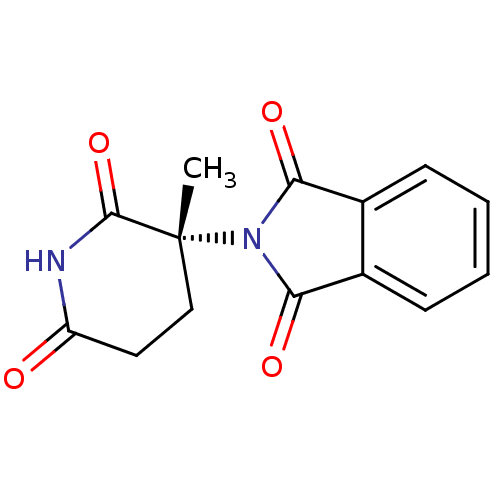 (2-((R)-3-Methyl-2,6-dioxo-piperidin-3-yl)-isoindol...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Inhibitory activity (RA1) of the compound against Prostaglandin G/H synthase 1 was calculated relative to aspirin | Bioorg Med Chem Lett 12: 1043-6 (2002) BindingDB Entry DOI: 10.7270/Q28K78D9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50111213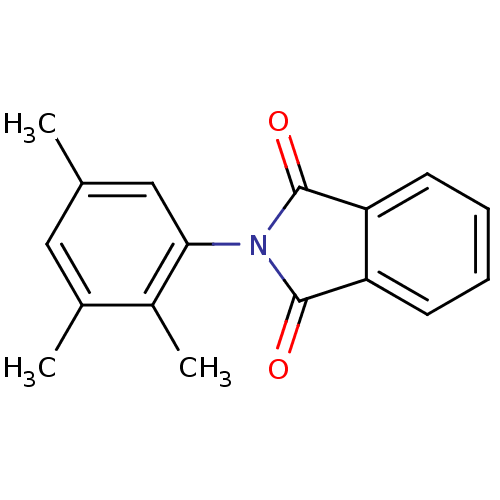 (2-(2,3,5-Trimethyl-phenyl)-isoindole-1,3-dione | C...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Inhibitory activity (RA1) of the compound against Prostaglandin G/H synthase 1 was calculated relative to aspirin | Bioorg Med Chem Lett 12: 1043-6 (2002) BindingDB Entry DOI: 10.7270/Q28K78D9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50070114 ((+/-)-thalidomide | 2-(2,6-Dioxo-piperidin-3-yl)-i...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Inhibitory activity (RA1) of the compound against Prostaglandin G/H synthase 1 was calculated relative to aspirin | Bioorg Med Chem Lett 12: 1043-6 (2002) BindingDB Entry DOI: 10.7270/Q28K78D9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50111221 (2-((R)-3-Methyl-2,6-dioxo-piperidin-3-yl)-4-nitro-...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Inhibitory activity (RA1) of the compound against Prostaglandin G/H synthase 1 was calculated relative to aspirin | Bioorg Med Chem Lett 12: 1043-6 (2002) BindingDB Entry DOI: 10.7270/Q28K78D9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50111220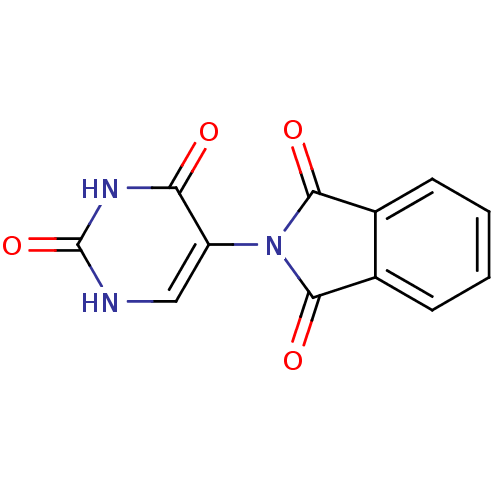 (2-(2,4-Dioxo-1,2,3,4-tetrahydro-pyrimidin-5-yl)-is...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Inhibitory activity (RA1) of the compound against Prostaglandin G/H synthase 1 was calculated relative to aspirin | Bioorg Med Chem Lett 12: 1043-6 (2002) BindingDB Entry DOI: 10.7270/Q28K78D9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50147586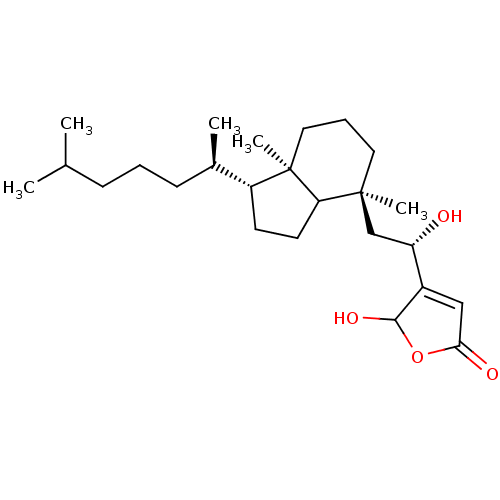 ((S)-4-{2-[(3R,7S)-1-((2R,3R)-1,5-Dimethyl-hexyl)-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Concentration required to inhibit human Cell division cycle 25A activity | Bioorg Med Chem Lett 14: 3291-4 (2004) Article DOI: 10.1016/j.bmcl.2004.03.100 BindingDB Entry DOI: 10.7270/Q23T9GNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50147585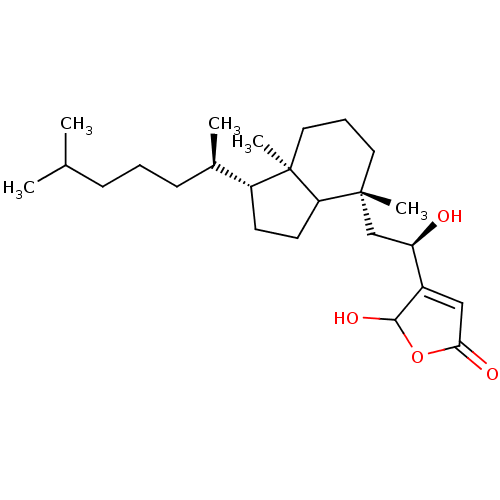 ((R)-4-{2-[(3R,7R)-1-((2R,3R)-1,5-Dimethyl-hexyl)-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Concentration required to inhibit human Cell division cycle 25A activity | Bioorg Med Chem Lett 14: 3291-4 (2004) Article DOI: 10.1016/j.bmcl.2004.03.100 BindingDB Entry DOI: 10.7270/Q23T9GNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM50070114 ((+/-)-thalidomide | 2-(2,6-Dioxo-piperidin-3-yl)-i...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Inhibitory activity (RA2) of the compound against Prostaglandin G/H synthase 2 was calculated relative to aspirin | Bioorg Med Chem Lett 12: 1043-6 (2002) BindingDB Entry DOI: 10.7270/Q28K78D9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50111211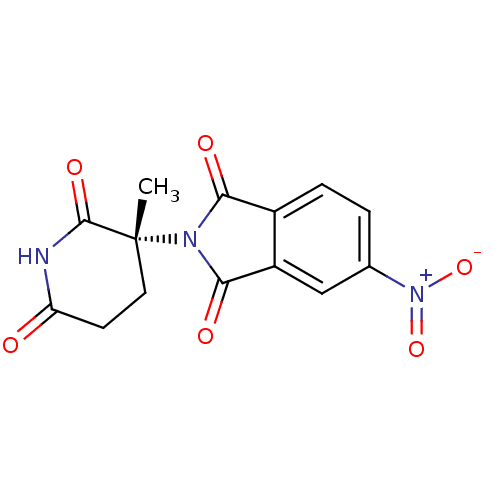 (2-((R)-3-Methyl-2,6-dioxo-piperidin-3-yl)-5-nitro-...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Inhibitory activity (RA1) of the compound against Prostaglandin G/H synthase 1 was calculated relative to aspirin | Bioorg Med Chem Lett 12: 1043-6 (2002) BindingDB Entry DOI: 10.7270/Q28K78D9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50111207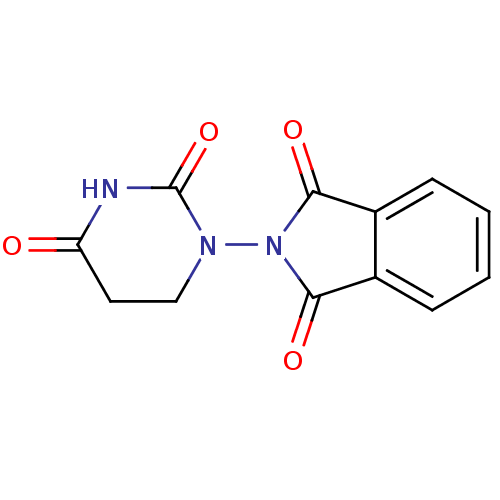 (2-(2,4-Dioxo-tetrahydro-pyrimidin-1-yl)-isoindole-...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Inhibitory activity (RA1) of the compound against Prostaglandin G/H synthase 1 was calculated relative to aspirin | Bioorg Med Chem Lett 12: 1043-6 (2002) BindingDB Entry DOI: 10.7270/Q28K78D9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM50111214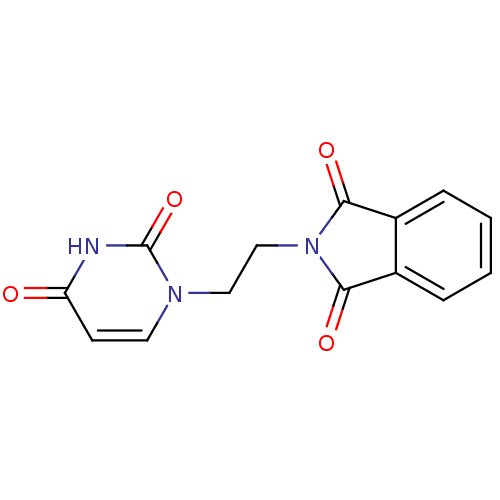 (2-[2-(2,4-Dioxo-3,4-dihydro-2H-pyrimidin-1-yl)-eth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Inhibitory activity (RA2) of the compound against Prostaglandin G/H synthase 2 was calculated relative to aspirin | Bioorg Med Chem Lett 12: 1043-6 (2002) BindingDB Entry DOI: 10.7270/Q28K78D9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM50111209 (2-(2,3,5,6-Tetramethyl-phenyl)-isoindole-1,3-dione...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Inhibitory activity (RA2) of the compound against Prostaglandin G/H synthase 2 was calculated relative to aspirin | Bioorg Med Chem Lett 12: 1043-6 (2002) BindingDB Entry DOI: 10.7270/Q28K78D9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50150613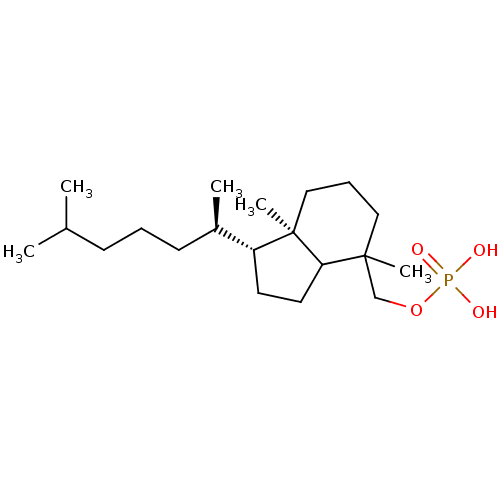 (CHEMBL179992 | CHEMBL182645 | [(1R,4S,7aR)-1-(1,5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Inhibitory concentration againsts dual-specificity phosphatase Cell division cycle (Cdc) 25A | Bioorg Med Chem Lett 14: 4339-42 (2004) Article DOI: 10.1016/j.bmcl.2004.05.083 BindingDB Entry DOI: 10.7270/Q2Q52P37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM50111217 (2-((R)-3-Methyl-2,6-dioxo-piperidin-3-yl)-isoindol...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Inhibitory activity (RA2) of the compound against Prostaglandin G/H synthase 2 was calculated relative to aspirin | Bioorg Med Chem Lett 12: 1043-6 (2002) BindingDB Entry DOI: 10.7270/Q28K78D9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50111219 (2-(2-Oxo-piperidin-3-yl)-isoindole-1,3-dione | CHE...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Inhibitory activity (RA1) of the compound against Prostaglandin G/H synthase 1 was calculated relative to aspirin | Bioorg Med Chem Lett 12: 1043-6 (2002) BindingDB Entry DOI: 10.7270/Q28K78D9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50147582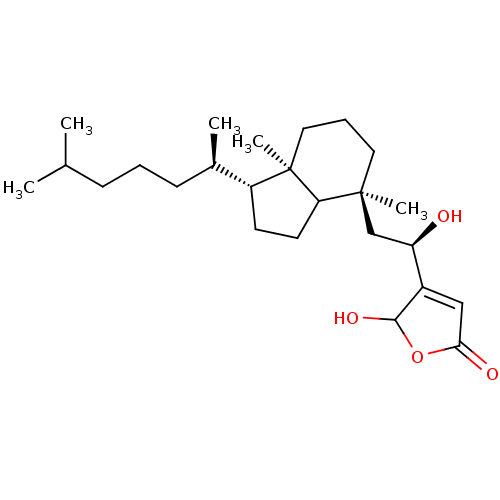 (4-{(R)-2-[(3aR,4S)-1-((1R,2R)-1,5-Dimethyl-hexyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Concentration required to inhibit human Cell division cycle 25A activity | Bioorg Med Chem Lett 14: 3291-4 (2004) Article DOI: 10.1016/j.bmcl.2004.03.100 BindingDB Entry DOI: 10.7270/Q23T9GNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50147583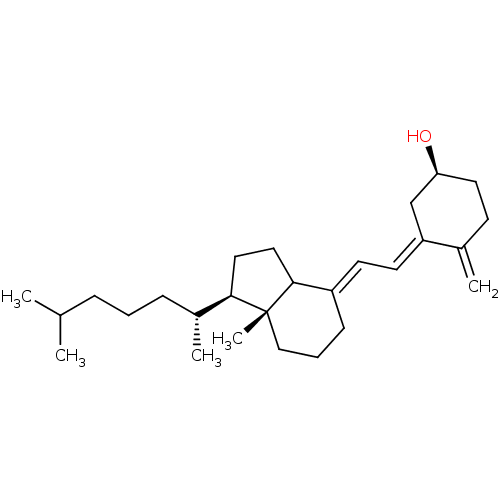 ((S)-4-Methylene-3-{2-[(R)-7a-(R)-methyl-1-((R)-5-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 890 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Concentration required to inhibit human Cell division cycle 25A activity; Value ranges from 0.44 uM to 0.89 uM | Bioorg Med Chem Lett 14: 3291-4 (2004) Article DOI: 10.1016/j.bmcl.2004.03.100 BindingDB Entry DOI: 10.7270/Q23T9GNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50147587 ((S)-4-{2-[(3R,7R)-1-((2R,3R)-1,5-Dimethyl-hexyl)-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 890 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Concentration required to inhibit human Cell division cycle 25A activity | Bioorg Med Chem Lett 14: 3291-4 (2004) Article DOI: 10.1016/j.bmcl.2004.03.100 BindingDB Entry DOI: 10.7270/Q23T9GNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM50111207 (2-(2,4-Dioxo-tetrahydro-pyrimidin-1-yl)-isoindole-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Inhibitory activity (RA2) of the compound against Prostaglandin G/H synthase 2 was calculated relative to aspirin | Bioorg Med Chem Lett 12: 1043-6 (2002) BindingDB Entry DOI: 10.7270/Q28K78D9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50150613 (CHEMBL179992 | CHEMBL182645 | [(1R,4S,7aR)-1-(1,5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Inhibitory concentration againsts dual-specificity phosphatase Cell division cycle (Cdc) 25A | Bioorg Med Chem Lett 14: 4339-42 (2004) Article DOI: 10.1016/j.bmcl.2004.05.083 BindingDB Entry DOI: 10.7270/Q2Q52P37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM50111213 (2-(2,3,5-Trimethyl-phenyl)-isoindole-1,3-dione | C...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Inhibitory activity (RA2) of the compound against Prostaglandin G/H synthase 2 was calculated relative to aspirin | Bioorg Med Chem Lett 12: 1043-6 (2002) BindingDB Entry DOI: 10.7270/Q28K78D9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50150612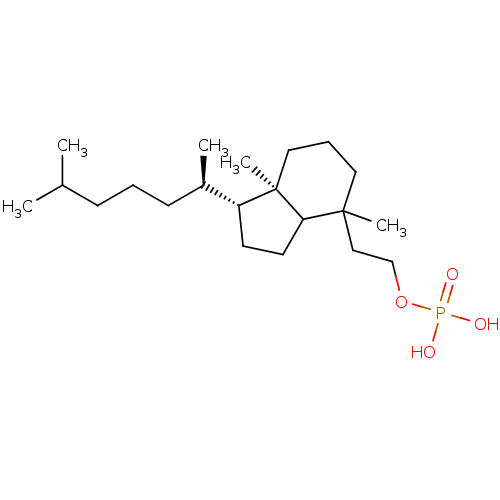 (CHEMBL181402 | CHEMBL182548 | Phosphoric acid mono...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Inhibitory concentration agaunst dual-specificity phosphatase Cell division cycle (Cdc) 25B | Bioorg Med Chem Lett 14: 4339-42 (2004) Article DOI: 10.1016/j.bmcl.2004.05.083 BindingDB Entry DOI: 10.7270/Q2Q52P37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM50111219 (2-(2-Oxo-piperidin-3-yl)-isoindole-1,3-dione | CHE...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Inhibitory activity (RA2) of the compound against Prostaglandin G/H synthase 2 was calculated relative to aspirin | Bioorg Med Chem Lett 12: 1043-6 (2002) BindingDB Entry DOI: 10.7270/Q28K78D9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50111214 (2-[2-(2,4-Dioxo-3,4-dihydro-2H-pyrimidin-1-yl)-eth...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Inhibitory activity (RA1) of the compound against Prostaglandin G/H synthase 1 was calculated relative to aspirin | Bioorg Med Chem Lett 12: 1043-6 (2002) BindingDB Entry DOI: 10.7270/Q28K78D9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50150608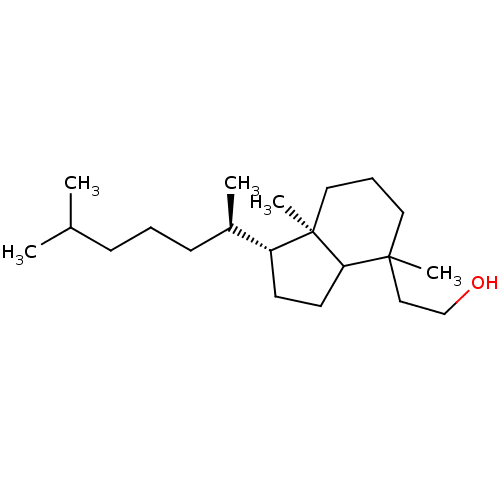 (2-[1-(1,5-dimethylhexyl)-4,7a-dimethylperhydro-4-i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Inhibitory concentration agaunst dual-specificity phosphatase Cell division cycle (Cdc) 25B | Bioorg Med Chem Lett 14: 4339-42 (2004) Article DOI: 10.1016/j.bmcl.2004.05.083 BindingDB Entry DOI: 10.7270/Q2Q52P37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50147585 ((R)-4-{2-[(3R,7R)-1-((2R,3R)-1,5-Dimethyl-hexyl)-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Concentration required to inhibit human Cell division cycle 25B activity | Bioorg Med Chem Lett 14: 3291-4 (2004) Article DOI: 10.1016/j.bmcl.2004.03.100 BindingDB Entry DOI: 10.7270/Q23T9GNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM50111211 (2-((R)-3-Methyl-2,6-dioxo-piperidin-3-yl)-5-nitro-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Inhibitory activity (RA2) of the compound against Prostaglandin G/H synthase 2 was calculated relative to aspirin | Bioorg Med Chem Lett 12: 1043-6 (2002) BindingDB Entry DOI: 10.7270/Q28K78D9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50261103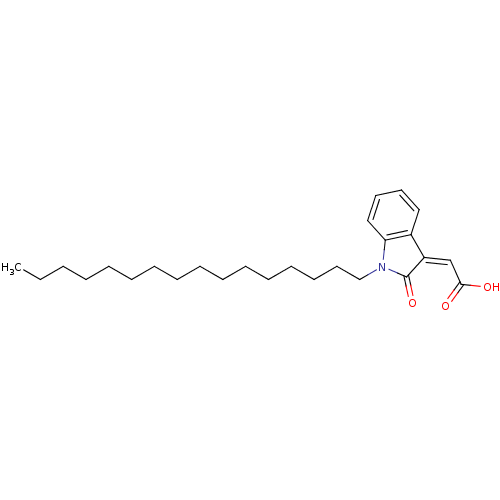 ((Z)-2-(1-hexadecyl-2-oxoindolin-3-ylidene)acetic a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Doshisha Women's College of Liberal Arts Curated by ChEMBL | Assay Description Inhibition of human Cdc25A phosphatase activity | Bioorg Med Chem Lett 18: 3350-3 (2008) Article DOI: 10.1016/j.bmcl.2008.04.027 BindingDB Entry DOI: 10.7270/Q2P55N9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50147587 ((S)-4-{2-[(3R,7R)-1-((2R,3R)-1,5-Dimethyl-hexyl)-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Concentration required to inhibit human Cell division cycle 25B activity | Bioorg Med Chem Lett 14: 3291-4 (2004) Article DOI: 10.1016/j.bmcl.2004.03.100 BindingDB Entry DOI: 10.7270/Q23T9GNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50261059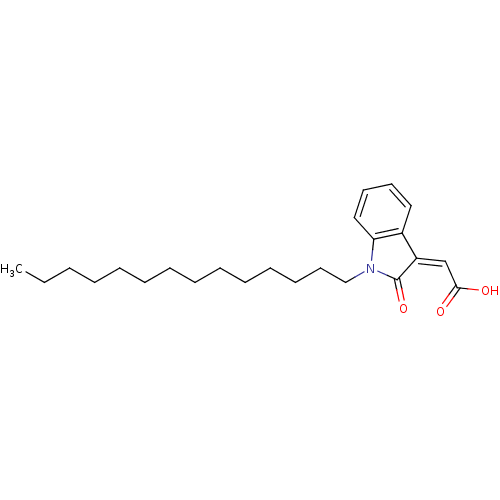 ((Z)-2-(2-oxo-1-tetradecylindolin-3-ylidene)acetic ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Doshisha Women's College of Liberal Arts Curated by ChEMBL | Assay Description Inhibition of human Cdc25A phosphatase activity | Bioorg Med Chem Lett 18: 3350-3 (2008) Article DOI: 10.1016/j.bmcl.2008.04.027 BindingDB Entry DOI: 10.7270/Q2P55N9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50150608 (2-[1-(1,5-dimethylhexyl)-4,7a-dimethylperhydro-4-i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Inhibitory concentration agaunst dual-specificity phosphatase Cell division cycle (Cdc) 25B | Bioorg Med Chem Lett 14: 4339-42 (2004) Article DOI: 10.1016/j.bmcl.2004.05.083 BindingDB Entry DOI: 10.7270/Q2Q52P37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM50111222 (4-Amino-2-((R)-3-methyl-2,6-dioxo-piperidin-3-yl)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Inhibitory activity (RA2) of the compound against Prostaglandin G/H synthase 2 was calculated relative to aspirin | Bioorg Med Chem Lett 12: 1043-6 (2002) BindingDB Entry DOI: 10.7270/Q28K78D9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50147582 (4-{(R)-2-[(3aR,4S)-1-((1R,2R)-1,5-Dimethyl-hexyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Concentration required to inhibit human Cell division cycle 25B activity | Bioorg Med Chem Lett 14: 3291-4 (2004) Article DOI: 10.1016/j.bmcl.2004.03.100 BindingDB Entry DOI: 10.7270/Q23T9GNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 183 total ) | Next | Last >> |