

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM50352495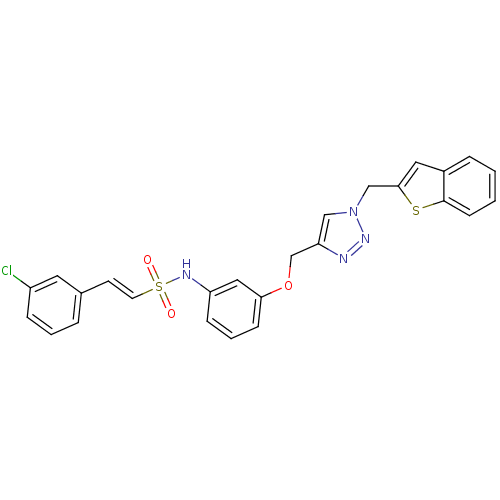 (CHEMBL1824555) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University Curated by ChEMBL | Assay Description Inhibition of human thrombin using chromogenic Sar-Pro-Arg p-nitroanilide dihydrochloride as substrate pre-incubated for 10 mins by spectrophotometry | Bioorg Med Chem Lett 21: 5305-9 (2011) Article DOI: 10.1016/j.bmcl.2011.07.023 BindingDB Entry DOI: 10.7270/Q23F4Q1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50352496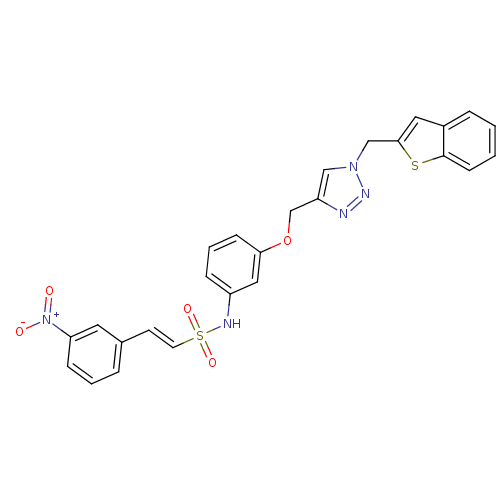 (CHEMBL1824556) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University Curated by ChEMBL | Assay Description Inhibition of human thrombin using chromogenic Sar-Pro-Arg p-nitroanilide dihydrochloride as substrate pre-incubated for 10 mins by spectrophotometry | Bioorg Med Chem Lett 21: 5305-9 (2011) Article DOI: 10.1016/j.bmcl.2011.07.023 BindingDB Entry DOI: 10.7270/Q23F4Q1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50352497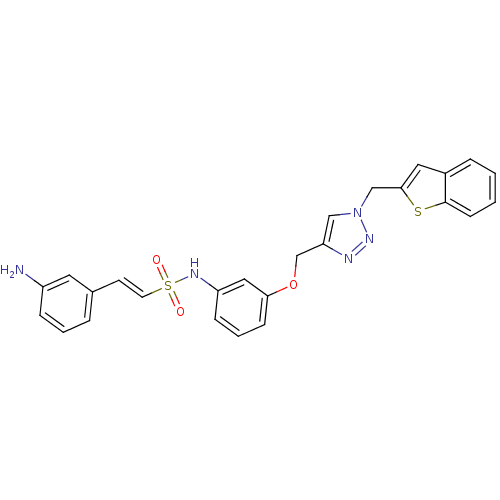 (CHEMBL1824553) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University Curated by ChEMBL | Assay Description Inhibition of human thrombin using chromogenic Sar-Pro-Arg p-nitroanilide dihydrochloride as substrate pre-incubated for 10 mins by spectrophotometry | Bioorg Med Chem Lett 21: 5305-9 (2011) Article DOI: 10.1016/j.bmcl.2011.07.023 BindingDB Entry DOI: 10.7270/Q23F4Q1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50352498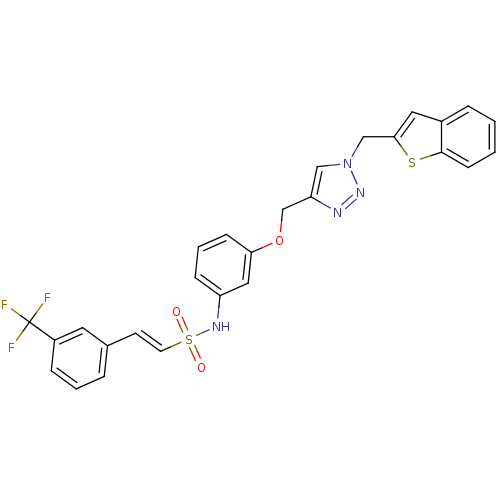 (CHEMBL1824554) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.97E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University Curated by ChEMBL | Assay Description Inhibition of human thrombin using chromogenic Sar-Pro-Arg p-nitroanilide dihydrochloride as substrate pre-incubated for 10 mins by spectrophotometry | Bioorg Med Chem Lett 21: 5305-9 (2011) Article DOI: 10.1016/j.bmcl.2011.07.023 BindingDB Entry DOI: 10.7270/Q23F4Q1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50352499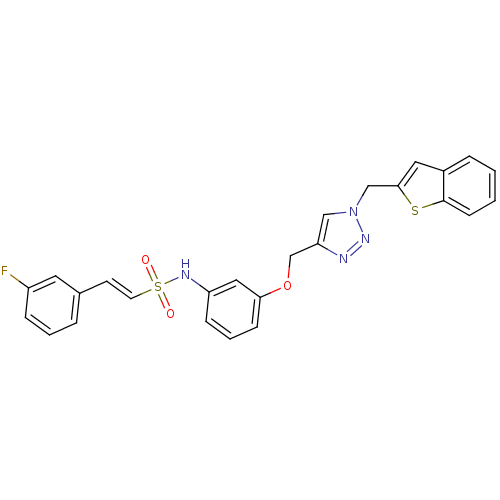 (CHEMBL1824551) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University Curated by ChEMBL | Assay Description Inhibition of human thrombin using chromogenic Sar-Pro-Arg p-nitroanilide dihydrochloride as substrate pre-incubated for 10 mins by spectrophotometry | Bioorg Med Chem Lett 21: 5305-9 (2011) Article DOI: 10.1016/j.bmcl.2011.07.023 BindingDB Entry DOI: 10.7270/Q23F4Q1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50352500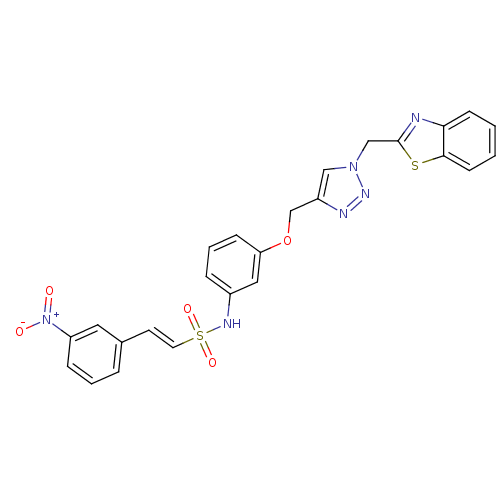 (CHEMBL1824550) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University Curated by ChEMBL | Assay Description Inhibition of human thrombin using chromogenic Sar-Pro-Arg p-nitroanilide dihydrochloride as substrate pre-incubated for 10 mins by spectrophotometry | Bioorg Med Chem Lett 21: 5305-9 (2011) Article DOI: 10.1016/j.bmcl.2011.07.023 BindingDB Entry DOI: 10.7270/Q23F4Q1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50352501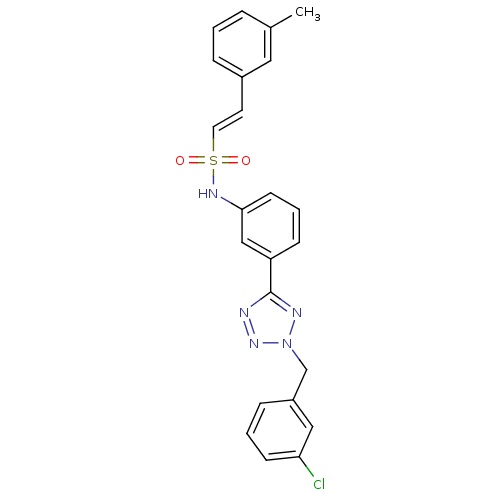 (CHEMBL1824559) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University Curated by ChEMBL | Assay Description Inhibition of human thrombin using chromogenic Sar-Pro-Arg p-nitroanilide dihydrochloride as substrate pre-incubated for 10 mins by spectrophotometry | Bioorg Med Chem Lett 21: 5305-9 (2011) Article DOI: 10.1016/j.bmcl.2011.07.023 BindingDB Entry DOI: 10.7270/Q23F4Q1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50352502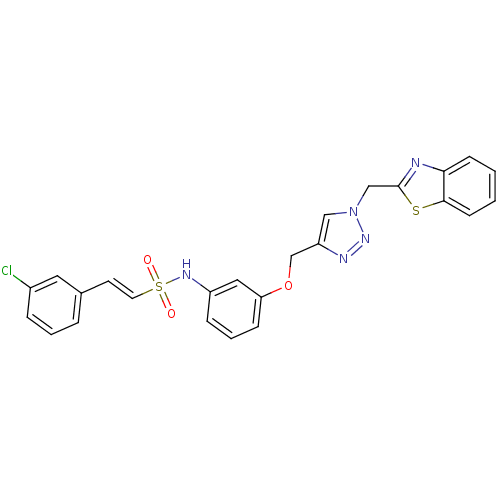 (CHEMBL1824549) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University Curated by ChEMBL | Assay Description Inhibition of human thrombin using chromogenic Sar-Pro-Arg p-nitroanilide dihydrochloride as substrate pre-incubated for 10 mins by spectrophotometry | Bioorg Med Chem Lett 21: 5305-9 (2011) Article DOI: 10.1016/j.bmcl.2011.07.023 BindingDB Entry DOI: 10.7270/Q23F4Q1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50352503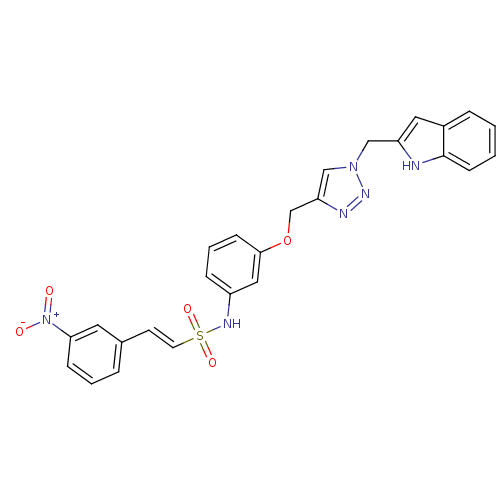 (CHEMBL1824552) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 6.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University Curated by ChEMBL | Assay Description Inhibition of human thrombin using chromogenic Sar-Pro-Arg p-nitroanilide dihydrochloride as substrate pre-incubated for 10 mins by spectrophotometry | Bioorg Med Chem Lett 21: 5305-9 (2011) Article DOI: 10.1016/j.bmcl.2011.07.023 BindingDB Entry DOI: 10.7270/Q23F4Q1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50352504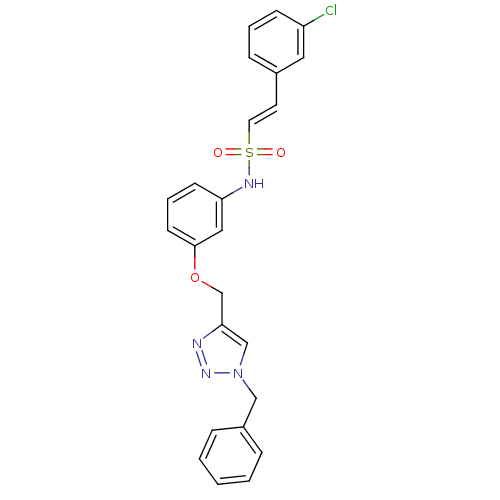 (CHEMBL1824546) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University Curated by ChEMBL | Assay Description Inhibition of human thrombin using chromogenic Sar-Pro-Arg p-nitroanilide dihydrochloride as substrate pre-incubated for 10 mins by spectrophotometry | Bioorg Med Chem Lett 21: 5305-9 (2011) Article DOI: 10.1016/j.bmcl.2011.07.023 BindingDB Entry DOI: 10.7270/Q23F4Q1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50352505 (CHEMBL1824544) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University Curated by ChEMBL | Assay Description Inhibition of human thrombin using chromogenic Sar-Pro-Arg p-nitroanilide dihydrochloride as substrate pre-incubated for 10 mins by spectrophotometry | Bioorg Med Chem Lett 21: 5305-9 (2011) Article DOI: 10.1016/j.bmcl.2011.07.023 BindingDB Entry DOI: 10.7270/Q23F4Q1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50352506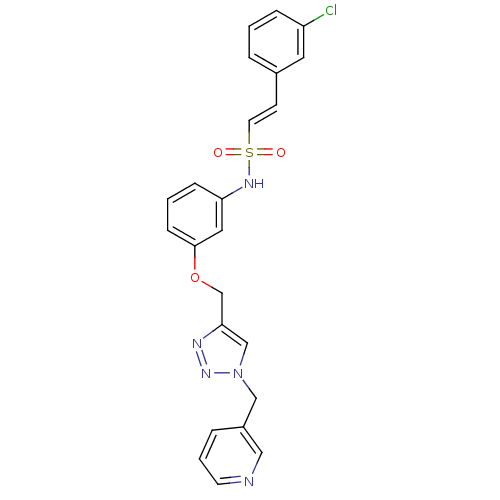 (CHEMBL1824558) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University Curated by ChEMBL | Assay Description Inhibition of human thrombin using chromogenic Sar-Pro-Arg p-nitroanilide dihydrochloride as substrate pre-incubated for 10 mins by spectrophotometry | Bioorg Med Chem Lett 21: 5305-9 (2011) Article DOI: 10.1016/j.bmcl.2011.07.023 BindingDB Entry DOI: 10.7270/Q23F4Q1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50352507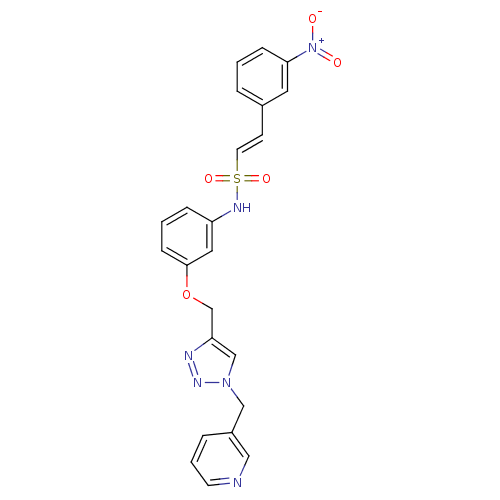 (CHEMBL1824542) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University Curated by ChEMBL | Assay Description Inhibition of human thrombin using chromogenic Sar-Pro-Arg p-nitroanilide dihydrochloride as substrate pre-incubated for 10 mins by spectrophotometry | Bioorg Med Chem Lett 21: 5305-9 (2011) Article DOI: 10.1016/j.bmcl.2011.07.023 BindingDB Entry DOI: 10.7270/Q23F4Q1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50189273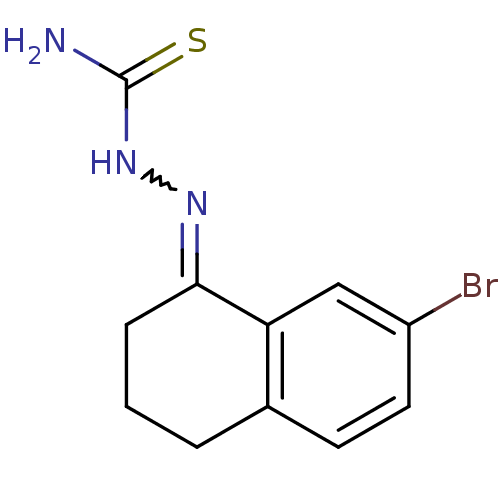 (1-(7-bromo-3,4-dihydronaphthalen-1(2H)-ylidene)thi...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor University Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi recombinant cruzain | Bioorg Med Chem Lett 16: 4405-9 (2006) Article DOI: 10.1016/j.bmcl.2006.05.041 BindingDB Entry DOI: 10.7270/Q23R0SHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50189279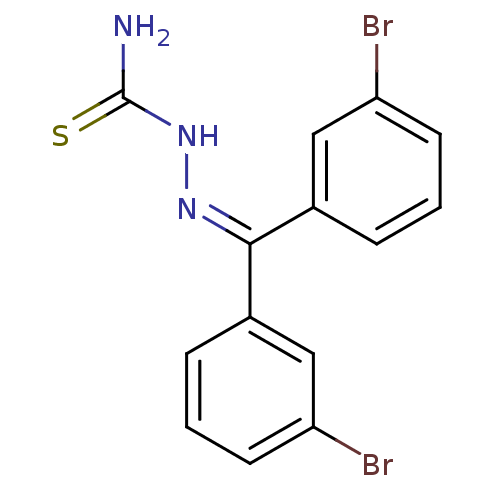 (1-(bis(3-bromophenyl)methylene)thiosemicarbazide |...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor University Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi recombinant cruzain | Bioorg Med Chem Lett 16: 4405-9 (2006) Article DOI: 10.1016/j.bmcl.2006.05.041 BindingDB Entry DOI: 10.7270/Q23R0SHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50189280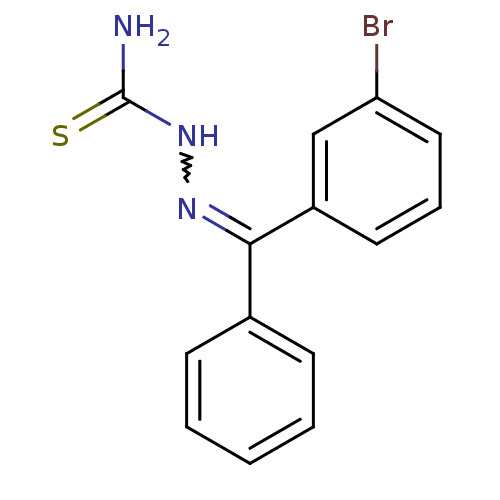 (1-((3-bromophenyl)(phenyl)methylene)thiosemicarbaz...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor University Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi recombinant cruzain | Bioorg Med Chem Lett 16: 4405-9 (2006) Article DOI: 10.1016/j.bmcl.2006.05.041 BindingDB Entry DOI: 10.7270/Q23R0SHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50189271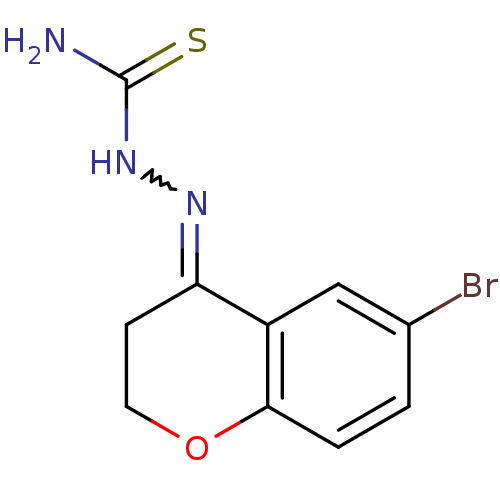 (1-(6-bromo-2,3-dihydrochromen-4-ylidene)thiosemica...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor University Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi recombinant cruzain | Bioorg Med Chem Lett 16: 4405-9 (2006) Article DOI: 10.1016/j.bmcl.2006.05.041 BindingDB Entry DOI: 10.7270/Q23R0SHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50189278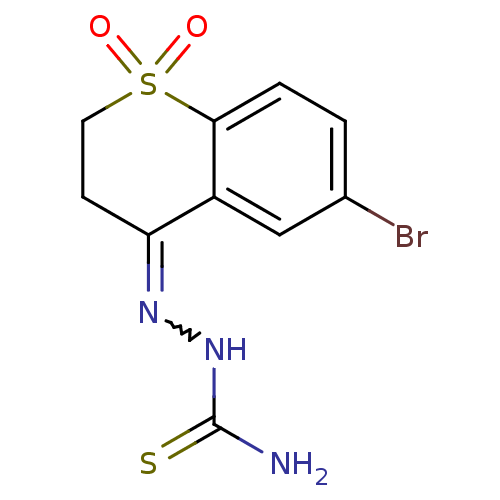 (6-bromo-2,3-dihydro-4H-thiochromen-4-one thiosemic...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor University Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi recombinant cruzain | Bioorg Med Chem Lett 16: 4405-9 (2006) Article DOI: 10.1016/j.bmcl.2006.05.041 BindingDB Entry DOI: 10.7270/Q23R0SHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50189263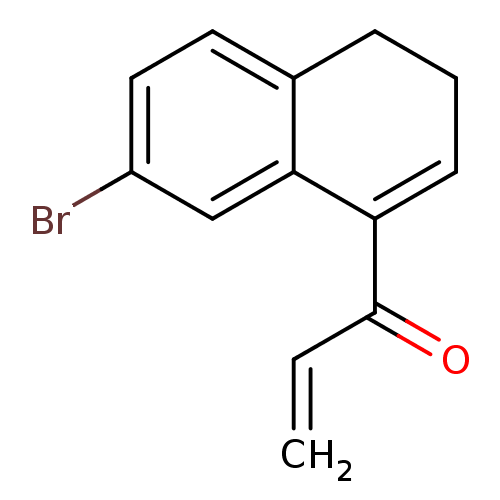 (1-(7-bromo-3,4-dihydronaphthalen-1-yl)prop-2-en-1-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor University Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi recombinant cruzain | Bioorg Med Chem Lett 16: 4405-9 (2006) Article DOI: 10.1016/j.bmcl.2006.05.041 BindingDB Entry DOI: 10.7270/Q23R0SHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50189274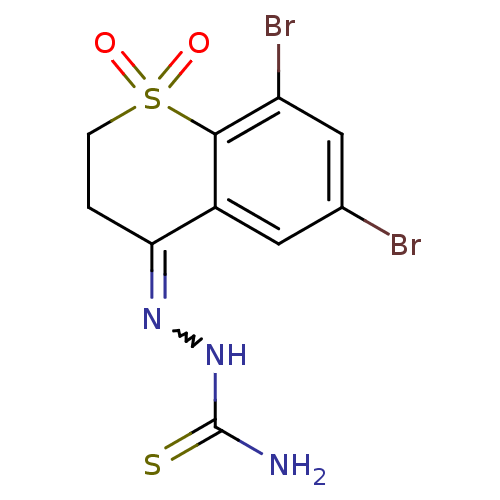 (6,8-dibromo-2,3-dihydro-4H-thiochromen-4-one thios...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor University Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi recombinant cruzain | Bioorg Med Chem Lett 16: 4405-9 (2006) Article DOI: 10.1016/j.bmcl.2006.05.041 BindingDB Entry DOI: 10.7270/Q23R0SHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50189265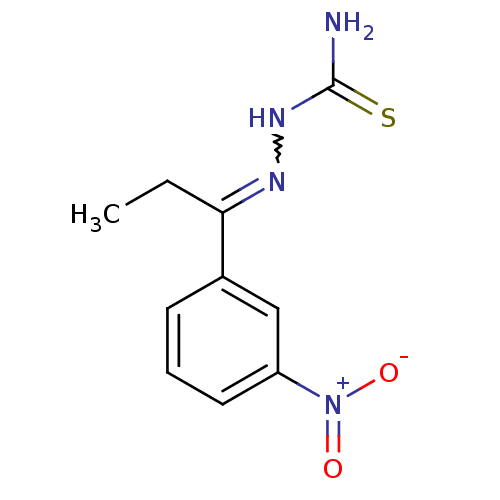 (1-(1-(3-nitrophenyl)propylidene)thiosemicarbazide ...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor University Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi recombinant cruzain | Bioorg Med Chem Lett 16: 4405-9 (2006) Article DOI: 10.1016/j.bmcl.2006.05.041 BindingDB Entry DOI: 10.7270/Q23R0SHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50189261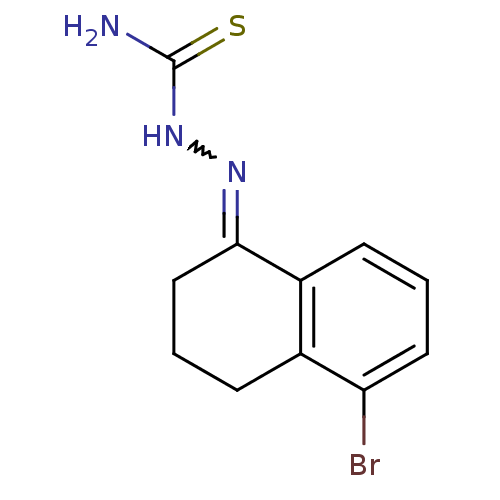 (1-(5-bromo-3,4-dihydronaphthalen-1(2H)-ylidene)thi...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor University Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi recombinant cruzain | Bioorg Med Chem Lett 16: 4405-9 (2006) Article DOI: 10.1016/j.bmcl.2006.05.041 BindingDB Entry DOI: 10.7270/Q23R0SHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50189272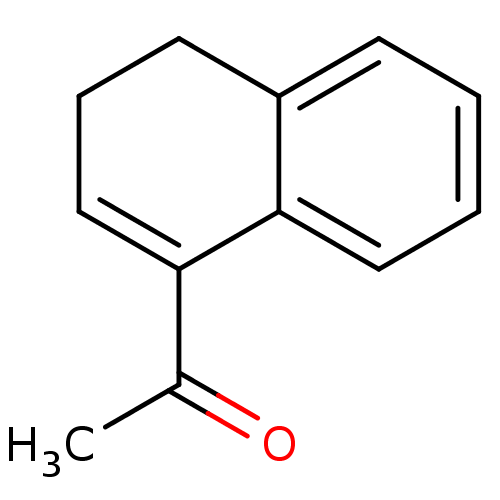 (1-(3,4-dihydronaphthalen-1-yl)ethanone | CHEMBL378...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor University Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi recombinant cruzain | Bioorg Med Chem Lett 16: 4405-9 (2006) Article DOI: 10.1016/j.bmcl.2006.05.041 BindingDB Entry DOI: 10.7270/Q23R0SHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50189270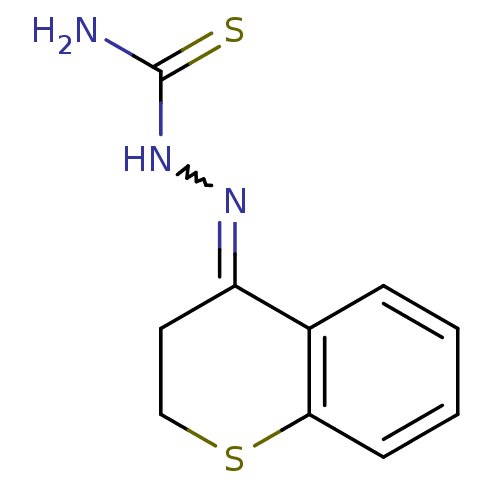 (1-(2,3-dihydrothiochromen-4-ylidene)thiosemicarbaz...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor University Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi recombinant cruzain | Bioorg Med Chem Lett 16: 4405-9 (2006) Article DOI: 10.1016/j.bmcl.2006.05.041 BindingDB Entry DOI: 10.7270/Q23R0SHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50189267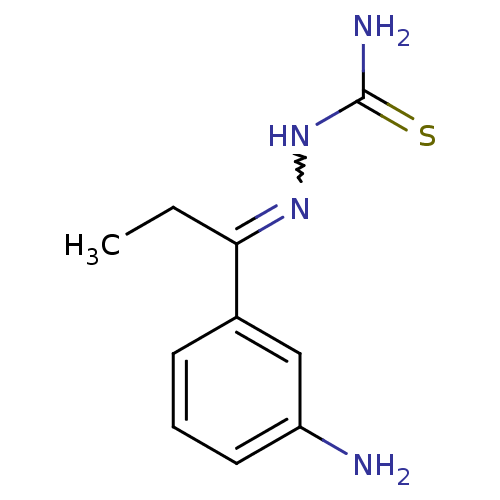 (1-(1-(3-aminophenyl)propylidene)thiosemicarbazide ...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >3.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor University Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi recombinant cruzain | Bioorg Med Chem Lett 16: 4405-9 (2006) Article DOI: 10.1016/j.bmcl.2006.05.041 BindingDB Entry DOI: 10.7270/Q23R0SHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50189266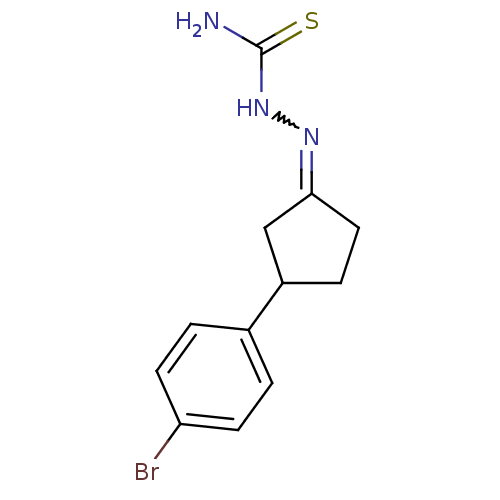 (1-(3-(4-bromophenyl)cyclopentylidene)thiosemicarba...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor University Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi recombinant cruzain | Bioorg Med Chem Lett 16: 4405-9 (2006) Article DOI: 10.1016/j.bmcl.2006.05.041 BindingDB Entry DOI: 10.7270/Q23R0SHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50189269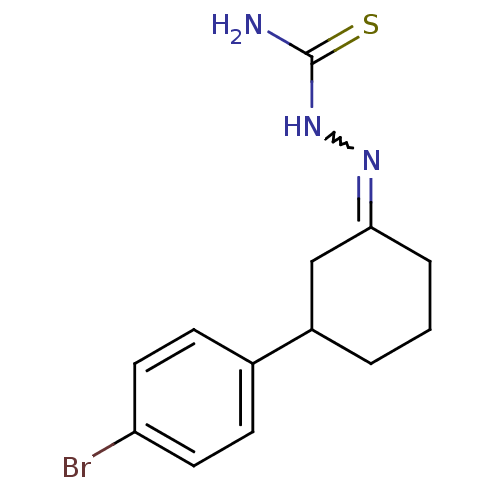 (1-(3-(4-bromophenyl)cyclohexylidene)thiosemicarbaz...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor University Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi recombinant cruzain | Bioorg Med Chem Lett 16: 4405-9 (2006) Article DOI: 10.1016/j.bmcl.2006.05.041 BindingDB Entry DOI: 10.7270/Q23R0SHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50189262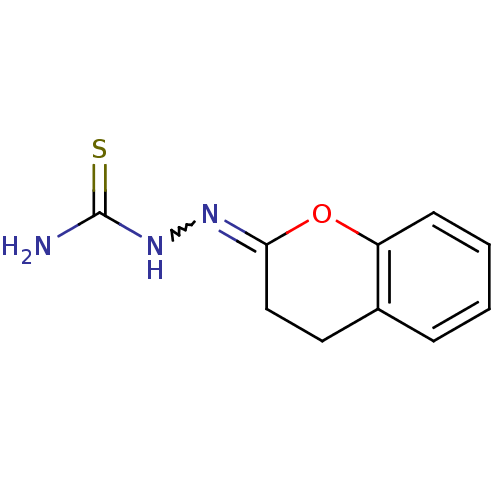 (1-(3,4-dihydrochromen-2-ylidene)thiosemicarbazide ...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor University Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi recombinant cruzain | Bioorg Med Chem Lett 16: 4405-9 (2006) Article DOI: 10.1016/j.bmcl.2006.05.041 BindingDB Entry DOI: 10.7270/Q23R0SHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50189264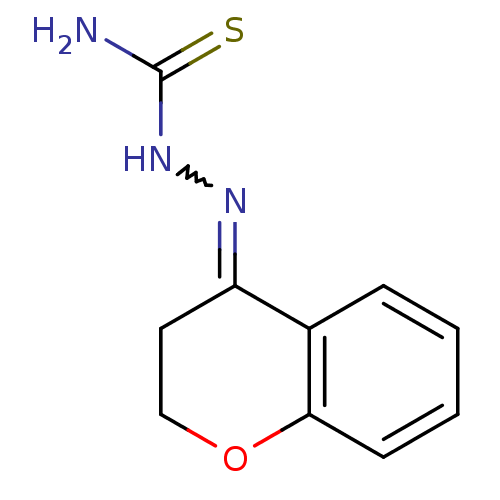 (1-(2,3-dihydrochromen-4-ylidene)thiosemicarbazide ...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor University Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi recombinant cruzain | Bioorg Med Chem Lett 16: 4405-9 (2006) Article DOI: 10.1016/j.bmcl.2006.05.041 BindingDB Entry DOI: 10.7270/Q23R0SHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50189268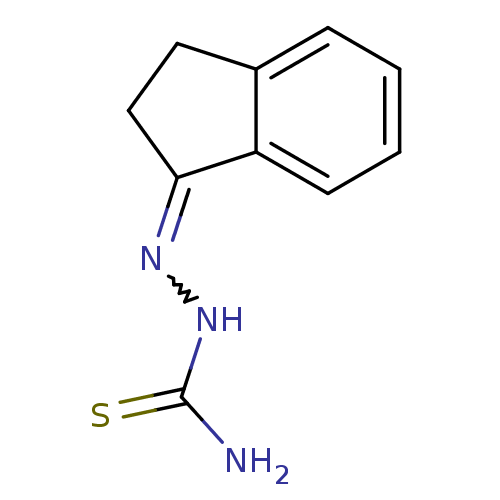 (1-(2,3-dihydroinden-1-ylidene)thiosemicarbazide | ...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor University Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi recombinant cruzain | Bioorg Med Chem Lett 16: 4405-9 (2006) Article DOI: 10.1016/j.bmcl.2006.05.041 BindingDB Entry DOI: 10.7270/Q23R0SHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50189276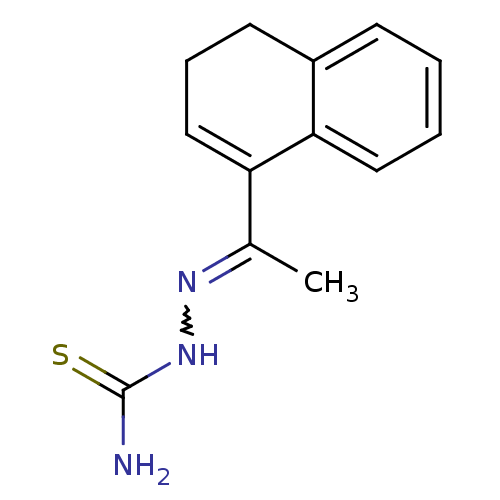 (1-(1-(3,4-dihydronaphthalen-1-yl)ethylidene)thiose...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor University Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi recombinant cruzain | Bioorg Med Chem Lett 16: 4405-9 (2006) Article DOI: 10.1016/j.bmcl.2006.05.041 BindingDB Entry DOI: 10.7270/Q23R0SHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50189277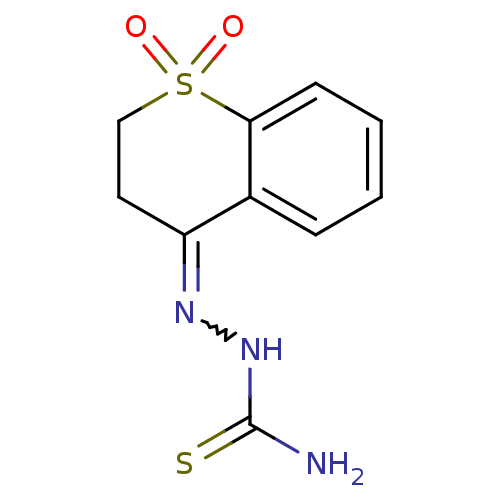 (2,3-dihydro-4H-thiochromen-4-one thiosemicarbazone...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor University Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi recombinant cruzain | Bioorg Med Chem Lett 16: 4405-9 (2006) Article DOI: 10.1016/j.bmcl.2006.05.041 BindingDB Entry DOI: 10.7270/Q23R0SHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50189275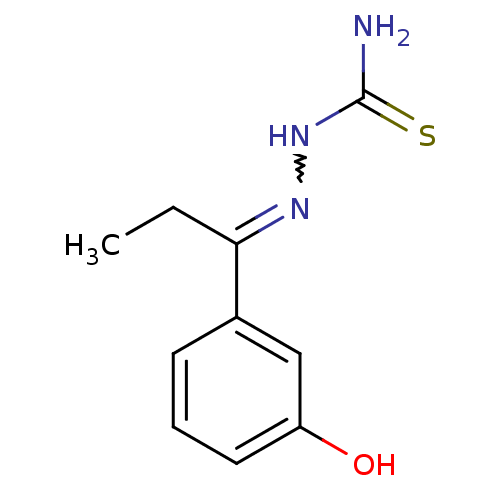 (1-(1-(3-hydroxyphenyl)propylidene)thiosemicarbazid...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor University Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi recombinant cruzain | Bioorg Med Chem Lett 16: 4405-9 (2006) Article DOI: 10.1016/j.bmcl.2006.05.041 BindingDB Entry DOI: 10.7270/Q23R0SHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||