 Found 460 hits with Last Name = 'smirk' and Initial = 'r'
Found 460 hits with Last Name = 'smirk' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
11-beta-hydroxysteroid dehydrogenase 1 [23-292,A23S,F278E]
(Homo sapiens (Human)) | BDBM32522
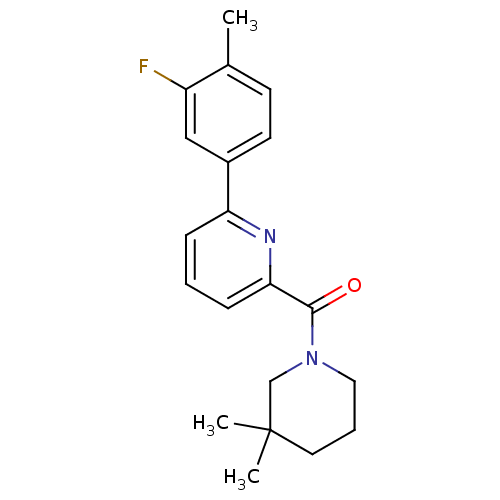
(pyridine amide, 30)Show InChI InChI=1S/C20H23FN2O/c1-14-8-9-15(12-16(14)21)17-6-4-7-18(22-17)19(24)23-11-5-10-20(2,3)13-23/h4,6-9,12H,5,10-11,13H2,1-3H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 6.5 | 23 |
Bristol-Myers Squibb Company
| Assay Description
11beta-HSD1 microsomes isolated from HEK 293 cells over-expressing human 11beta-HSD1 were incubated with the substrate cortisone and cofactor NADPH a... |
Bioorg Med Chem Lett 18: 3168-72 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.069
BindingDB Entry DOI: 10.7270/Q2P26WGT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
11-beta-hydroxysteroid dehydrogenase 1 [23-292,A23S,F278E]
(Homo sapiens (Human)) | BDBM32520
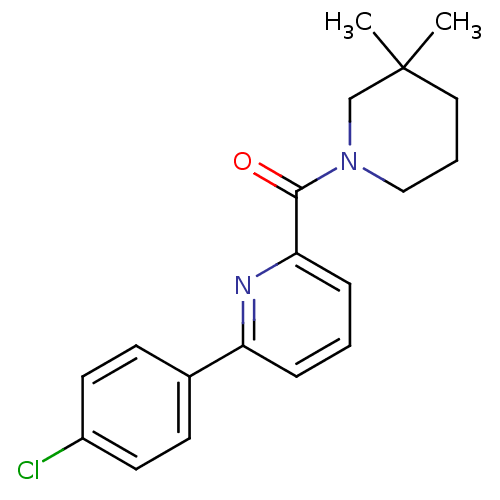
(pyridine amide, 28)Show InChI InChI=1S/C19H21ClN2O/c1-19(2)11-4-12-22(13-19)18(23)17-6-3-5-16(21-17)14-7-9-15(20)10-8-14/h3,5-10H,4,11-13H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 6.5 | 23 |
Bristol-Myers Squibb Company
| Assay Description
11beta-HSD1 microsomes isolated from HEK 293 cells over-expressing human 11beta-HSD1 were incubated with the substrate cortisone and cofactor NADPH a... |
Bioorg Med Chem Lett 18: 3168-72 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.069
BindingDB Entry DOI: 10.7270/Q2P26WGT |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
11-beta-hydroxysteroid dehydrogenase 1 [23-292,A23S,F278E]
(Homo sapiens (Human)) | BDBM32521

(pyridine amide, 29)Show SMILES CC1(C)CCCN(C1)C(=O)c1cccc(n1)-c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C20H21F3N2O2/c1-19(2)11-4-12-25(13-19)18(26)17-6-3-5-16(24-17)14-7-9-15(10-8-14)27-20(21,22)23/h3,5-10H,4,11-13H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 6.5 | 23 |
Bristol-Myers Squibb Company
| Assay Description
11beta-HSD1 microsomes isolated from HEK 293 cells over-expressing human 11beta-HSD1 were incubated with the substrate cortisone and cofactor NADPH a... |
Bioorg Med Chem Lett 18: 3168-72 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.069
BindingDB Entry DOI: 10.7270/Q2P26WGT |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
11-beta-hydroxysteroid dehydrogenase 1 [23-292,A23S,F278E]
(Homo sapiens (Human)) | BDBM32527

(pyridine amide, 35)Show SMILES [H][C@]12C[C@](C)(CN1C(=O)c1cncc(COc3c(Cl)cccc3Cl)c1)CC(C)(C)C2 |r| Show InChI InChI=1S/C23H26Cl2N2O2/c1-22(2)8-17-9-23(3,13-22)14-27(17)21(28)16-7-15(10-26-11-16)12-29-20-18(24)5-4-6-19(20)25/h4-7,10-11,17H,8-9,12-14H2,1-3H3/t17-,23-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company
| Assay Description
11beta-HSD1 microsomes isolated from HEK 293 cells over-expressing human 11beta-HSD1 were incubated with the substrate cortisone and cofactor NADPH a... |
Bioorg Med Chem Lett 18: 3168-72 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.069
BindingDB Entry DOI: 10.7270/Q2P26WGT |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1 [23-292,A23S,F278E]
(Homo sapiens (Human)) | BDBM32523

(pyridine amide, 31)Show SMILES [O-][N+](=O)c1ccccc1-c1cccc(n1)C(=O)N1CCCC2CCCCC12 Show InChI InChI=1S/C21H23N3O3/c25-21(23-14-6-8-15-7-1-3-12-19(15)23)18-11-5-10-17(22-18)16-9-2-4-13-20(16)24(26)27/h2,4-5,9-11,13,15,19H,1,3,6-8,12,14H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 6.5 | 23 |
Bristol-Myers Squibb Company
| Assay Description
11beta-HSD1 microsomes isolated from HEK 293 cells over-expressing human 11beta-HSD1 were incubated with the substrate cortisone and cofactor NADPH a... |
Bioorg Med Chem Lett 18: 3168-72 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.069
BindingDB Entry DOI: 10.7270/Q2P26WGT |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
11-beta-hydroxysteroid dehydrogenase 1 [23-292,A23S,F278E]
(Homo sapiens (Human)) | BDBM32526

(pyridine amide, 34)Show SMILES Clc1cccc(Cl)c1OCc1cncc(c1)C(=O)N1CCCCC1c1cccnc1 Show InChI InChI=1S/C23H21Cl2N3O2/c24-19-6-3-7-20(25)22(19)30-15-16-11-18(14-27-12-16)23(29)28-10-2-1-8-21(28)17-5-4-9-26-13-17/h3-7,9,11-14,21H,1-2,8,10,15H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company
| Assay Description
11beta-HSD1 microsomes isolated from HEK 293 cells over-expressing human 11beta-HSD1 were incubated with the substrate cortisone and cofactor NADPH a... |
Bioorg Med Chem Lett 18: 3168-72 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.069
BindingDB Entry DOI: 10.7270/Q2P26WGT |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1 [23-292,A23S,F278E]
(Homo sapiens (Human)) | BDBM32508

(pyridine amide, 23)Show SMILES Clc1cccc(Cl)c1S(=O)(=O)Cc1cncc(c1)C(=O)N1CCCCCCCC1 Show InChI InChI=1S/C21H24Cl2N2O3S/c22-18-8-7-9-19(23)20(18)29(27,28)15-16-12-17(14-24-13-16)21(26)25-10-5-3-1-2-4-6-11-25/h7-9,12-14H,1-6,10-11,15H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | 6.5 | 23 |
Bristol-Myers Squibb Company
| Assay Description
11beta-HSD1 microsomes isolated from HEK 293 cells over-expressing human 11beta-HSD1 were incubated with the substrate cortisone and cofactor NADPH a... |
Bioorg Med Chem Lett 18: 3168-72 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.069
BindingDB Entry DOI: 10.7270/Q2P26WGT |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1 [23-292,A23S,F278E]
(Homo sapiens (Human)) | BDBM32519

(pyridine amide, 27)Show InChI InChI=1S/C19H21ClN2O/c1-19(2)11-6-12-22(13-19)18(23)17-10-5-9-16(21-17)14-7-3-4-8-15(14)20/h3-5,7-10H,6,11-13H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | 6.5 | 23 |
Bristol-Myers Squibb Company
| Assay Description
11beta-HSD1 microsomes isolated from HEK 293 cells over-expressing human 11beta-HSD1 were incubated with the substrate cortisone and cofactor NADPH a... |
Bioorg Med Chem Lett 18: 3168-72 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.069
BindingDB Entry DOI: 10.7270/Q2P26WGT |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
11-beta-hydroxysteroid dehydrogenase 1 [23-292,A23S,F278E]
(Homo sapiens (Human)) | BDBM32524

(pyridine amide, 32)Show SMILES FC(F)(F)c1ccc(cc1)-c1cccc(n1)C(=O)N1CCCC2CCCCC12 Show InChI InChI=1S/C22H23F3N2O/c23-22(24,25)17-12-10-15(11-13-17)18-7-3-8-19(26-18)21(28)27-14-4-6-16-5-1-2-9-20(16)27/h3,7-8,10-13,16,20H,1-2,4-6,9,14H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | 6.5 | 23 |
Bristol-Myers Squibb Company
| Assay Description
11beta-HSD1 microsomes isolated from HEK 293 cells over-expressing human 11beta-HSD1 were incubated with the substrate cortisone and cofactor NADPH a... |
Bioorg Med Chem Lett 18: 3168-72 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.069
BindingDB Entry DOI: 10.7270/Q2P26WGT |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
11-beta-hydroxysteroid dehydrogenase 1 [23-292,A23S,F278E]
(Homo sapiens (Human)) | BDBM32529
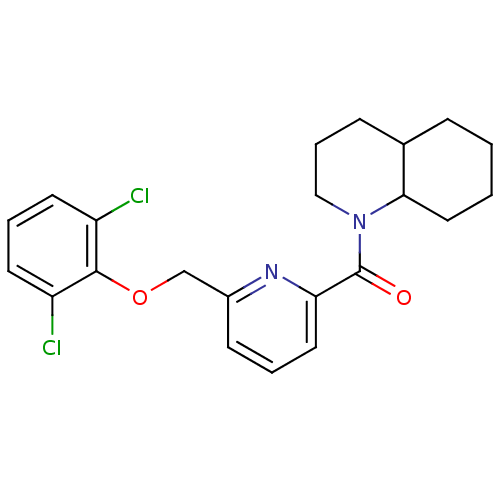
(pyridine amide, 37)Show SMILES Clc1cccc(Cl)c1OCc1cccc(n1)C(=O)N1CCCC2CCCCC12 Show InChI InChI=1S/C22H24Cl2N2O2/c23-17-9-4-10-18(24)21(17)28-14-16-8-3-11-19(25-16)22(27)26-13-5-7-15-6-1-2-12-20(15)26/h3-4,8-11,15,20H,1-2,5-7,12-14H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company
| Assay Description
11beta-HSD1 microsomes isolated from HEK 293 cells over-expressing human 11beta-HSD1 were incubated with the substrate cortisone and cofactor NADPH a... |
Bioorg Med Chem Lett 18: 3168-72 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.069
BindingDB Entry DOI: 10.7270/Q2P26WGT |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
11-beta-hydroxysteroid dehydrogenase 1 [23-292,A23S,F278E]
(Homo sapiens (Human)) | BDBM32525
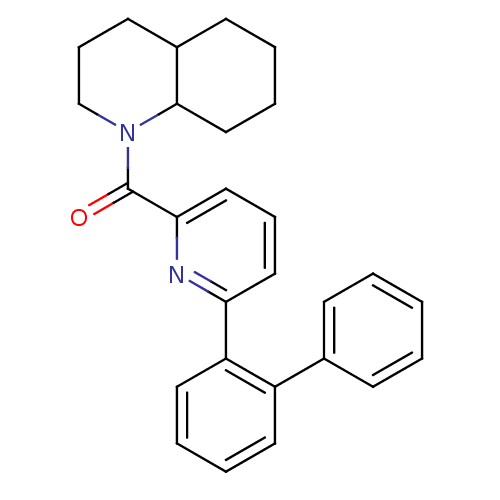
(pyridine amide, 33)Show SMILES O=C(N1CCCC2CCCCC12)c1cccc(n1)-c1ccccc1-c1ccccc1 Show InChI InChI=1S/C27H28N2O/c30-27(29-19-9-13-21-12-4-7-18-26(21)29)25-17-8-16-24(28-25)23-15-6-5-14-22(23)20-10-2-1-3-11-20/h1-3,5-6,8,10-11,14-17,21,26H,4,7,9,12-13,18-19H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | 6.5 | 23 |
Bristol-Myers Squibb Company
| Assay Description
11beta-HSD1 microsomes isolated from HEK 293 cells over-expressing human 11beta-HSD1 were incubated with the substrate cortisone and cofactor NADPH a... |
Bioorg Med Chem Lett 18: 3168-72 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.069
BindingDB Entry DOI: 10.7270/Q2P26WGT |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM13592
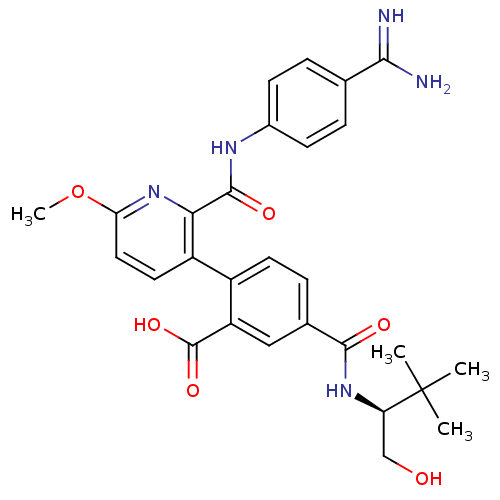
(2-{2-[(4-carbamimidoylphenyl)carbamoyl]-6-methoxyp...)Show SMILES COc1ccc(-c2ccc(cc2C(O)=O)C(=O)N[C@H](CO)C(C)(C)C)c(n1)C(=O)Nc1ccc(cc1)C(N)=N |r| Show InChI InChI=1S/C28H31N5O6/c1-28(2,3)21(14-34)32-25(35)16-7-10-18(20(13-16)27(37)38)19-11-12-22(39-4)33-23(19)26(36)31-17-8-5-15(6-9-17)24(29)30/h5-13,21,34H,14H2,1-4H3,(H3,29,30)(H,31,36)(H,32,35)(H,37,38)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human F7a using D-Ile-Pro-Arg-AFC as substrate after 3 mins |
Bioorg Med Chem Lett 23: 5239-43 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.028
BindingDB Entry DOI: 10.7270/Q2CC123C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
11-beta-hydroxysteroid dehydrogenase 1 [23-292,A23S,F278E]
(Homo sapiens (Human)) | BDBM32528
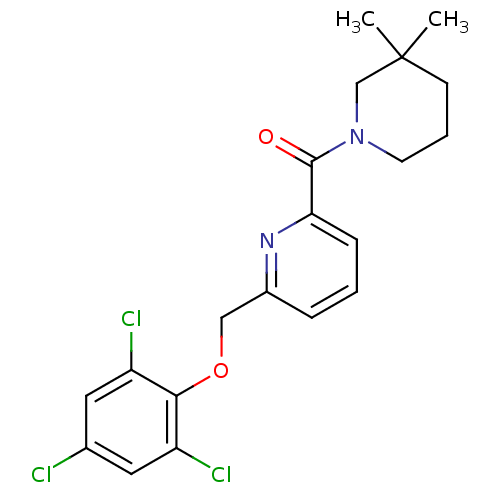
(pyridine amide, 36)Show SMILES CC1(C)CCCN(C1)C(=O)c1cccc(COc2c(Cl)cc(Cl)cc2Cl)n1 Show InChI InChI=1S/C20H21Cl3N2O2/c1-20(2)7-4-8-25(12-20)19(26)17-6-3-5-14(24-17)11-27-18-15(22)9-13(21)10-16(18)23/h3,5-6,9-10H,4,7-8,11-12H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company
| Assay Description
11beta-HSD1 microsomes isolated from HEK 293 cells over-expressing human 11beta-HSD1 were incubated with the substrate cortisone and cofactor NADPH a... |
Bioorg Med Chem Lett 18: 3168-72 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.069
BindingDB Entry DOI: 10.7270/Q2P26WGT |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM50439474
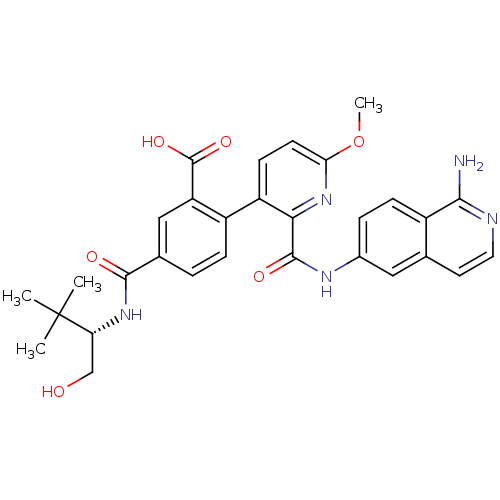
(CHEMBL2417906)Show SMILES COc1ccc(-c2ccc(cc2C(O)=O)C(=O)N[C@H](CO)C(C)(C)C)c(n1)C(=O)Nc1ccc2c(N)nccc2c1 |r| Show InChI InChI=1S/C30H31N5O6/c1-30(2,3)23(15-36)34-27(37)17-5-7-20(22(14-17)29(39)40)21-9-10-24(41-4)35-25(21)28(38)33-18-6-8-19-16(13-18)11-12-32-26(19)31/h5-14,23,36H,15H2,1-4H3,(H2,31,32)(H,33,38)(H,34,37)(H,39,40)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human F7a using D-Ile-Pro-Arg-AFC as substrate after 3 mins |
Bioorg Med Chem Lett 23: 5239-43 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.028
BindingDB Entry DOI: 10.7270/Q2CC123C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
11-beta-hydroxysteroid dehydrogenase 1 [23-292,A23S,F278E]
(Homo sapiens (Human)) | BDBM32512

(pyridine amide, 6)Show InChI InChI=1S/C19H20Cl2N2OS/c1-13-8-10-23(11-9-13)19(24)17-7-2-4-14(22-17)12-25-18-15(20)5-3-6-16(18)21/h2-7,13H,8-12H2,1H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | 6.5 | 23 |
Bristol-Myers Squibb Company
| Assay Description
11beta-HSD1 microsomes isolated from HEK 293 cells over-expressing human 11beta-HSD1 were incubated with the substrate cortisone and cofactor NADPH a... |
Bioorg Med Chem Lett 18: 3168-72 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.069
BindingDB Entry DOI: 10.7270/Q2P26WGT |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM50439477
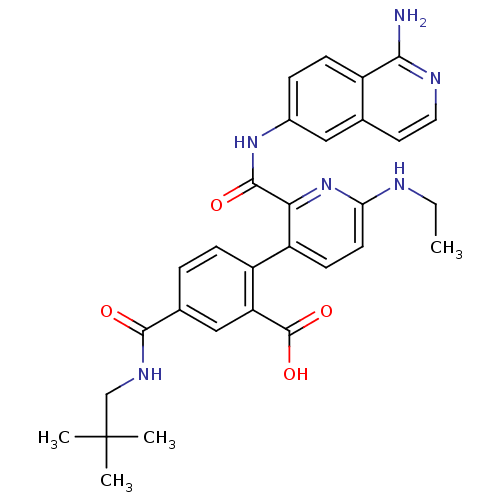
(CHEMBL2417903)Show SMILES CCNc1ccc(c(n1)C(=O)Nc1ccc2c(N)nccc2c1)-c1ccc(cc1C(O)=O)C(=O)NCC(C)(C)C Show InChI InChI=1S/C30H32N6O4/c1-5-32-24-11-10-22(21-8-6-18(15-23(21)29(39)40)27(37)34-16-30(2,3)4)25(36-24)28(38)35-19-7-9-20-17(14-19)12-13-33-26(20)31/h6-15H,5,16H2,1-4H3,(H2,31,33)(H,32,36)(H,34,37)(H,35,38)(H,39,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human F7a using D-Ile-Pro-Arg-AFC as substrate after 3 mins |
Bioorg Med Chem Lett 23: 5239-43 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.028
BindingDB Entry DOI: 10.7270/Q2CC123C |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM50439475
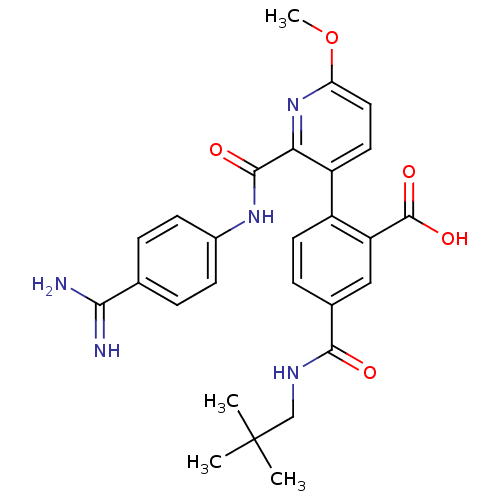
(CHEMBL2417905)Show SMILES COc1ccc(-c2ccc(cc2C(O)=O)C(=O)NCC(C)(C)C)c(n1)C(=O)Nc1ccc(cc1)C(N)=N Show InChI InChI=1S/C27H29N5O5/c1-27(2,3)14-30-24(33)16-7-10-18(20(13-16)26(35)36)19-11-12-21(37-4)32-22(19)25(34)31-17-8-5-15(6-9-17)23(28)29/h5-13H,14H2,1-4H3,(H3,28,29)(H,30,33)(H,31,34)(H,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human F7a using D-Ile-Pro-Arg-AFC as substrate after 3 mins |
Bioorg Med Chem Lett 23: 5239-43 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.028
BindingDB Entry DOI: 10.7270/Q2CC123C |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM50439487

(CHEMBL2417893)Show SMILES CCOc1ccc(-c2ccc(cc2C(O)=O)C(=O)NCC(C)(C)C)c(n1)C(=O)Nc1ccc2c(N)nccc2c1 Show InChI InChI=1S/C30H31N5O5/c1-5-40-24-11-10-22(21-8-6-18(15-23(21)29(38)39)27(36)33-16-30(2,3)4)25(35-24)28(37)34-19-7-9-20-17(14-19)12-13-32-26(20)31/h6-15H,5,16H2,1-4H3,(H2,31,32)(H,33,36)(H,34,37)(H,38,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human F7a using D-Ile-Pro-Arg-AFC as substrate after 3 mins |
Bioorg Med Chem Lett 23: 5239-43 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.028
BindingDB Entry DOI: 10.7270/Q2CC123C |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1 [23-292,A23S,F278E]
(Homo sapiens (Human)) | BDBM32507
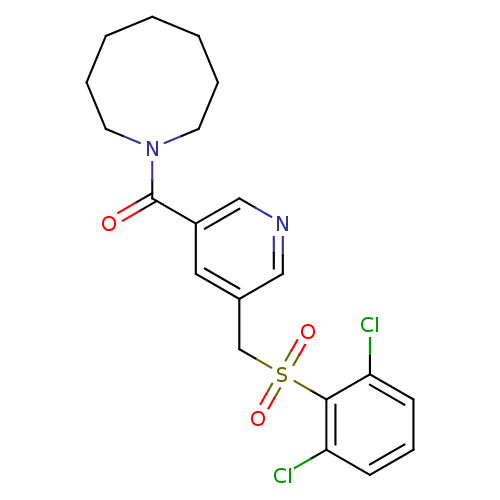
(pyridine amide, 22)Show SMILES Clc1cccc(Cl)c1S(=O)(=O)Cc1cncc(c1)C(=O)N1CCCCCCC1 Show InChI InChI=1S/C20H22Cl2N2O3S/c21-17-7-6-8-18(22)19(17)28(26,27)14-15-11-16(13-23-12-15)20(25)24-9-4-2-1-3-5-10-24/h6-8,11-13H,1-5,9-10,14H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | 6.5 | 23 |
Bristol-Myers Squibb Company
| Assay Description
11beta-HSD1 microsomes isolated from HEK 293 cells over-expressing human 11beta-HSD1 were incubated with the substrate cortisone and cofactor NADPH a... |
Bioorg Med Chem Lett 18: 3168-72 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.069
BindingDB Entry DOI: 10.7270/Q2P26WGT |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM50439486

(CHEMBL2417894)Show SMILES CCCOc1ccc(-c2ccc(cc2C(O)=O)C(=O)NCC(C)(C)C)c(n1)C(=O)Nc1ccc2c(N)nccc2c1 Show InChI InChI=1S/C31H33N5O5/c1-5-14-41-25-11-10-23(22-8-6-19(16-24(22)30(39)40)28(37)34-17-31(2,3)4)26(36-25)29(38)35-20-7-9-21-18(15-20)12-13-33-27(21)32/h6-13,15-16H,5,14,17H2,1-4H3,(H2,32,33)(H,34,37)(H,35,38)(H,39,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human F7a using D-Ile-Pro-Arg-AFC as substrate after 3 mins |
Bioorg Med Chem Lett 23: 5239-43 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.028
BindingDB Entry DOI: 10.7270/Q2CC123C |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM50439473

(CHEMBL2417907)Show SMILES COc1ccc(-c2ccc(cc2C(O)=O)C(=O)NCC(C)(C)C)c(n1)C(=O)Nc1ccc2c(N)nccc2c1 Show InChI InChI=1S/C29H29N5O5/c1-29(2,3)15-32-26(35)17-5-7-20(22(14-17)28(37)38)21-9-10-23(39-4)34-24(21)27(36)33-18-6-8-19-16(13-18)11-12-31-25(19)30/h5-14H,15H2,1-4H3,(H2,30,31)(H,32,35)(H,33,36)(H,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human F7a using D-Ile-Pro-Arg-AFC as substrate after 3 mins |
Bioorg Med Chem Lett 23: 5239-43 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.028
BindingDB Entry DOI: 10.7270/Q2CC123C |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1 [23-292,A23S,F278E]
(Homo sapiens (Human)) | BDBM32518
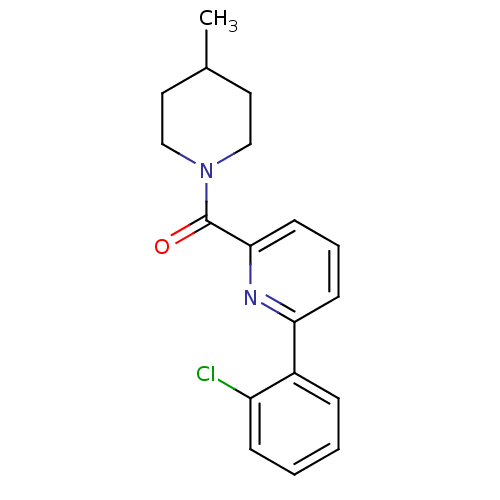
(pyridine amide, 9)Show InChI InChI=1S/C18H19ClN2O/c1-13-9-11-21(12-10-13)18(22)17-8-4-7-16(20-17)14-5-2-3-6-15(14)19/h2-8,13H,9-12H2,1H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | 6.5 | 23 |
Bristol-Myers Squibb Company
| Assay Description
11beta-HSD1 microsomes isolated from HEK 293 cells over-expressing human 11beta-HSD1 were incubated with the substrate cortisone and cofactor NADPH a... |
Bioorg Med Chem Lett 18: 3168-72 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.069
BindingDB Entry DOI: 10.7270/Q2P26WGT |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50377302
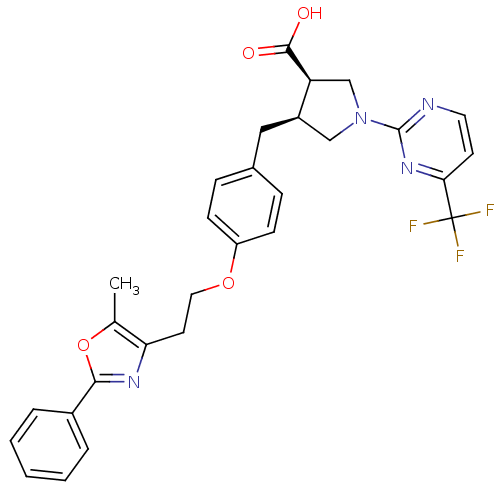
(CHEMBL255930)Show SMILES Cc1oc(nc1CCOc1ccc(C[C@@H]2CN(C[C@@H]2C(O)=O)c2nccc(n2)C(F)(F)F)cc1)-c1ccccc1 Show InChI InChI=1S/C29H27F3N4O4/c1-18-24(34-26(40-18)20-5-3-2-4-6-20)12-14-39-22-9-7-19(8-10-22)15-21-16-36(17-23(21)27(37)38)28-33-13-11-25(35-28)29(30,31)32/h2-11,13,21,23H,12,14-17H2,1H3,(H,37,38)/t21-,23+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development (R&D)
Curated by ChEMBL
| Assay Description
Binding affinity to PPARgamma (unknown origin) using fluorescein-tagged dual PPARalpha/gamma activator by homogeneous fluorescence polarization bindi... |
Bioorg Med Chem Lett 25: 1196-205 (2015)
Article DOI: 10.1016/j.bmcl.2015.01.066
BindingDB Entry DOI: 10.7270/Q20866ZP |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50377302

(CHEMBL255930)Show SMILES Cc1oc(nc1CCOc1ccc(C[C@@H]2CN(C[C@@H]2C(O)=O)c2nccc(n2)C(F)(F)F)cc1)-c1ccccc1 Show InChI InChI=1S/C29H27F3N4O4/c1-18-24(34-26(40-18)20-5-3-2-4-6-20)12-14-39-22-9-7-19(8-10-22)15-21-16-36(17-23(21)27(37)38)28-33-13-11-25(35-28)29(30,31)32/h2-11,13,21,23H,12,14-17H2,1H3,(H,37,38)/t21-,23+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development (R&D)
Curated by ChEMBL
| Assay Description
Binding affinity to PPARgamma (unknown origin) using fluorescein-tagged dual PPARalpha/gamma activator by homogeneous fluorescence polarization bindi... |
Bioorg Med Chem Lett 25: 1196-205 (2015)
Article DOI: 10.1016/j.bmcl.2015.01.066
BindingDB Entry DOI: 10.7270/Q20866ZP |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50063315
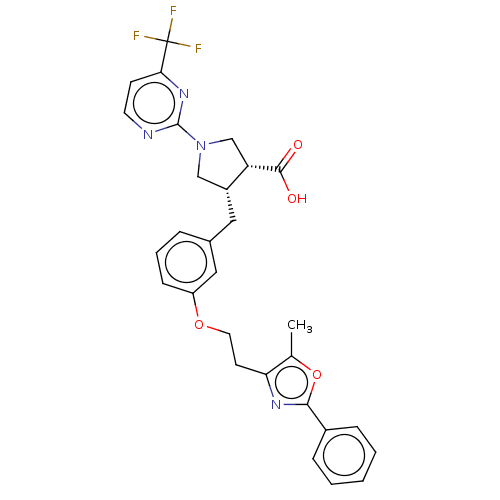
(CHEMBL3398446)Show SMILES Cc1oc(nc1CCOc1cccc(C[C@@H]2CN(C[C@@H]2C(O)=O)c2nccc(n2)C(F)(F)F)c1)-c1ccccc1 |r| Show InChI InChI=1S/C29H27F3N4O4/c1-18-24(34-26(40-18)20-7-3-2-4-8-20)11-13-39-22-9-5-6-19(15-22)14-21-16-36(17-23(21)27(37)38)28-33-12-10-25(35-28)29(30,31)32/h2-10,12,15,21,23H,11,13-14,16-17H2,1H3,(H,37,38)/t21-,23+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development (R&D)
Curated by ChEMBL
| Assay Description
Binding affinity to PPARalpha (unknown origin) using fluorescein-tagged dual PPARalpha/gamma activator by homogeneous fluorescence polarization bindi... |
Bioorg Med Chem Lett 25: 1196-205 (2015)
Article DOI: 10.1016/j.bmcl.2015.01.066
BindingDB Entry DOI: 10.7270/Q20866ZP |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1 [23-292,A23S,F278E]
(Homo sapiens (Human)) | BDBM32506
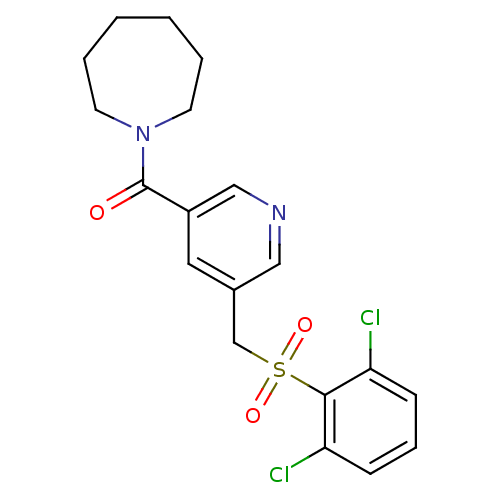
(pyridine amide, 21)Show SMILES Clc1cccc(Cl)c1S(=O)(=O)Cc1cncc(c1)C(=O)N1CCCCCC1 Show InChI InChI=1S/C19H20Cl2N2O3S/c20-16-6-5-7-17(21)18(16)27(25,26)13-14-10-15(12-22-11-14)19(24)23-8-3-1-2-4-9-23/h5-7,10-12H,1-4,8-9,13H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | 6.5 | 23 |
Bristol-Myers Squibb Company
| Assay Description
11beta-HSD1 microsomes isolated from HEK 293 cells over-expressing human 11beta-HSD1 were incubated with the substrate cortisone and cofactor NADPH a... |
Bioorg Med Chem Lett 18: 3168-72 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.069
BindingDB Entry DOI: 10.7270/Q2P26WGT |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1 [23-292,A23S,F278E]
(Homo sapiens (Human)) | BDBM32500
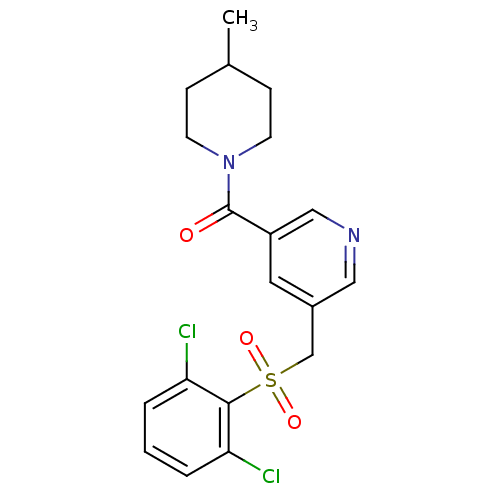
(pyridine amide, 15)Show SMILES CC1CCN(CC1)C(=O)c1cncc(CS(=O)(=O)c2c(Cl)cccc2Cl)c1 Show InChI InChI=1S/C19H20Cl2N2O3S/c1-13-5-7-23(8-6-13)19(24)15-9-14(10-22-11-15)12-27(25,26)18-16(20)3-2-4-17(18)21/h2-4,9-11,13H,5-8,12H2,1H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | 6.5 | 23 |
Bristol-Myers Squibb Company
| Assay Description
11beta-HSD1 microsomes isolated from HEK 293 cells over-expressing human 11beta-HSD1 were incubated with the substrate cortisone and cofactor NADPH a... |
Bioorg Med Chem Lett 18: 3168-72 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.069
BindingDB Entry DOI: 10.7270/Q2P26WGT |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM50439488
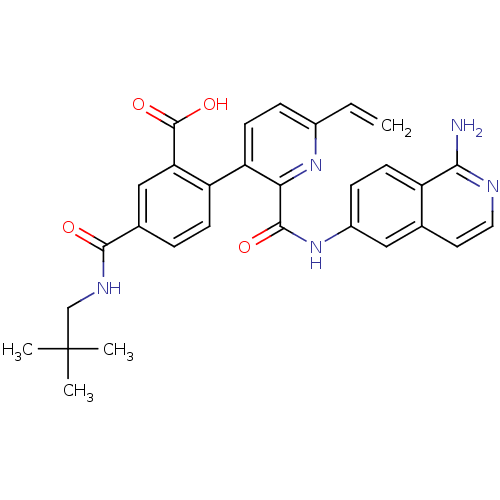
(CHEMBL2417911)Show SMILES CC(C)(C)CNC(=O)c1ccc(c(c1)C(O)=O)-c1ccc(C=C)nc1C(=O)Nc1ccc2c(N)nccc2c1 Show InChI InChI=1S/C30H29N5O4/c1-5-19-7-11-23(22-9-6-18(15-24(22)29(38)39)27(36)33-16-30(2,3)4)25(34-19)28(37)35-20-8-10-21-17(14-20)12-13-32-26(21)31/h5-15H,1,16H2,2-4H3,(H2,31,32)(H,33,36)(H,35,37)(H,38,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human F7a using D-Ile-Pro-Arg-AFC as substrate after 3 mins |
Bioorg Med Chem Lett 23: 5239-43 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.028
BindingDB Entry DOI: 10.7270/Q2CC123C |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM50439484
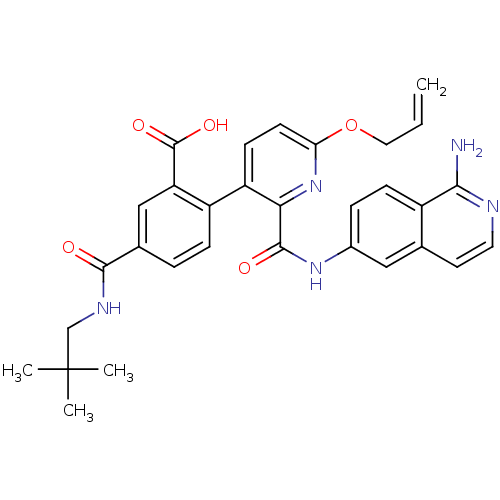
(CHEMBL2417896)Show SMILES CC(C)(C)CNC(=O)c1ccc(c(c1)C(O)=O)-c1ccc(OCC=C)nc1C(=O)Nc1ccc2c(N)nccc2c1 Show InChI InChI=1S/C31H31N5O5/c1-5-14-41-25-11-10-23(22-8-6-19(16-24(22)30(39)40)28(37)34-17-31(2,3)4)26(36-25)29(38)35-20-7-9-21-18(15-20)12-13-33-27(21)32/h5-13,15-16H,1,14,17H2,2-4H3,(H2,32,33)(H,34,37)(H,35,38)(H,39,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human F7a using D-Ile-Pro-Arg-AFC as substrate after 3 mins |
Bioorg Med Chem Lett 23: 5239-43 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.028
BindingDB Entry DOI: 10.7270/Q2CC123C |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM13592

(2-{2-[(4-carbamimidoylphenyl)carbamoyl]-6-methoxyp...)Show SMILES COc1ccc(-c2ccc(cc2C(O)=O)C(=O)N[C@H](CO)C(C)(C)C)c(n1)C(=O)Nc1ccc(cc1)C(N)=N |r| Show InChI InChI=1S/C28H31N5O6/c1-28(2,3)21(14-34)32-25(35)16-7-10-18(20(13-16)27(37)38)19-11-12-22(39-4)33-23(19)26(36)31-17-8-5-15(6-9-17)24(29)30/h5-13,21,34H,14H2,1-4H3,(H3,29,30)(H,31,36)(H,32,35)(H,37,38)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human F10a using S-2222 as substrate after 3 mins |
Bioorg Med Chem Lett 23: 5239-43 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.028
BindingDB Entry DOI: 10.7270/Q2CC123C |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1 [23-292,A23S,F278E]
(Homo sapiens (Human)) | BDBM32499

(pyridine amide, 8)Show SMILES CC1CCN(CC1)C(=O)c1cccc(CS(=O)(=O)c2c(Cl)cccc2Cl)n1 Show InChI InChI=1S/C19H20Cl2N2O3S/c1-13-8-10-23(11-9-13)19(24)17-7-2-4-14(22-17)12-27(25,26)18-15(20)5-3-6-16(18)21/h2-7,13H,8-12H2,1H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | 6.5 | 23 |
Bristol-Myers Squibb Company
| Assay Description
11beta-HSD1 microsomes isolated from HEK 293 cells over-expressing human 11beta-HSD1 were incubated with the substrate cortisone and cofactor NADPH a... |
Bioorg Med Chem Lett 18: 3168-72 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.069
BindingDB Entry DOI: 10.7270/Q2P26WGT |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50063311

(CHEMBL3398444)Show SMILES Cc1oc(nc1CCOc1cccc(C[C@@H]2CN(C[C@@H]2C(O)=O)c2ccccc2)c1)-c1ccccc1 |r| Show InChI InChI=1S/C30H30N2O4/c1-21-28(31-29(36-21)23-10-4-2-5-11-23)15-16-35-26-14-8-9-22(18-26)17-24-19-32(20-27(24)30(33)34)25-12-6-3-7-13-25/h2-14,18,24,27H,15-17,19-20H2,1H3,(H,33,34)/t24-,27+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development (R&D)
Curated by ChEMBL
| Assay Description
Binding affinity to PPARalpha (unknown origin) using fluorescein-tagged dual PPARalpha/gamma activator by homogeneous fluorescence polarization bindi... |
Bioorg Med Chem Lett 25: 1196-205 (2015)
Article DOI: 10.1016/j.bmcl.2015.01.066
BindingDB Entry DOI: 10.7270/Q20866ZP |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50063311

(CHEMBL3398444)Show SMILES Cc1oc(nc1CCOc1cccc(C[C@@H]2CN(C[C@@H]2C(O)=O)c2ccccc2)c1)-c1ccccc1 |r| Show InChI InChI=1S/C30H30N2O4/c1-21-28(31-29(36-21)23-10-4-2-5-11-23)15-16-35-26-14-8-9-22(18-26)17-24-19-32(20-27(24)30(33)34)25-12-6-3-7-13-25/h2-14,18,24,27H,15-17,19-20H2,1H3,(H,33,34)/t24-,27+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development (R&D)
Curated by ChEMBL
| Assay Description
Binding affinity to PPARalpha (unknown origin) using fluorescein-tagged dual PPARalpha/gamma activator by homogeneous fluorescence polarization bindi... |
Bioorg Med Chem Lett 25: 1196-205 (2015)
Article DOI: 10.1016/j.bmcl.2015.01.066
BindingDB Entry DOI: 10.7270/Q20866ZP |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50063315

(CHEMBL3398446)Show SMILES Cc1oc(nc1CCOc1cccc(C[C@@H]2CN(C[C@@H]2C(O)=O)c2nccc(n2)C(F)(F)F)c1)-c1ccccc1 |r| Show InChI InChI=1S/C29H27F3N4O4/c1-18-24(34-26(40-18)20-7-3-2-4-8-20)11-13-39-22-9-5-6-19(15-22)14-21-16-36(17-23(21)27(37)38)28-33-12-10-25(35-28)29(30,31)32/h2-10,12,15,21,23H,11,13-14,16-17H2,1H3,(H,37,38)/t21-,23+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development (R&D)
Curated by ChEMBL
| Assay Description
Binding affinity to PPARgamma (unknown origin) using fluorescein-tagged dual PPARalpha/gamma activator by homogeneous fluorescence polarization bindi... |
Bioorg Med Chem Lett 25: 1196-205 (2015)
Article DOI: 10.1016/j.bmcl.2015.01.066
BindingDB Entry DOI: 10.7270/Q20866ZP |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50063313

(CHEMBL3398454)Show SMILES Cc1oc(nc1CCOc1ccc(C[C@@H]2CN(C[C@@H]2C(O)=O)c2nc(co2)C(F)(F)F)cc1)-c1ccccc1 |r| Show InChI InChI=1S/C28H26F3N3O5/c1-17-23(32-25(39-17)19-5-3-2-4-6-19)11-12-37-21-9-7-18(8-10-21)13-20-14-34(15-22(20)26(35)36)27-33-24(16-38-27)28(29,30)31/h2-10,16,20,22H,11-15H2,1H3,(H,35,36)/t20-,22+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development (R&D)
Curated by ChEMBL
| Assay Description
Binding affinity to PPARgamma (unknown origin) using fluorescein-tagged dual PPARalpha/gamma activator by homogeneous fluorescence polarization bindi... |
Bioorg Med Chem Lett 25: 1196-205 (2015)
Article DOI: 10.1016/j.bmcl.2015.01.066
BindingDB Entry DOI: 10.7270/Q20866ZP |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM50439478
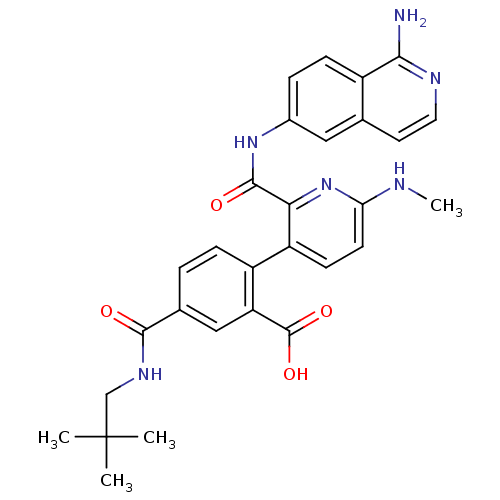
(CHEMBL2417902)Show SMILES CNc1ccc(c(n1)C(=O)Nc1ccc2c(N)nccc2c1)-c1ccc(cc1C(O)=O)C(=O)NCC(C)(C)C Show InChI InChI=1S/C29H30N6O4/c1-29(2,3)15-33-26(36)17-5-7-20(22(14-17)28(38)39)21-9-10-23(31-4)35-24(21)27(37)34-18-6-8-19-16(13-18)11-12-32-25(19)30/h5-14H,15H2,1-4H3,(H2,30,32)(H,31,35)(H,33,36)(H,34,37)(H,38,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human F7a using D-Ile-Pro-Arg-AFC as substrate after 3 mins |
Bioorg Med Chem Lett 23: 5239-43 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.028
BindingDB Entry DOI: 10.7270/Q2CC123C |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM50439472
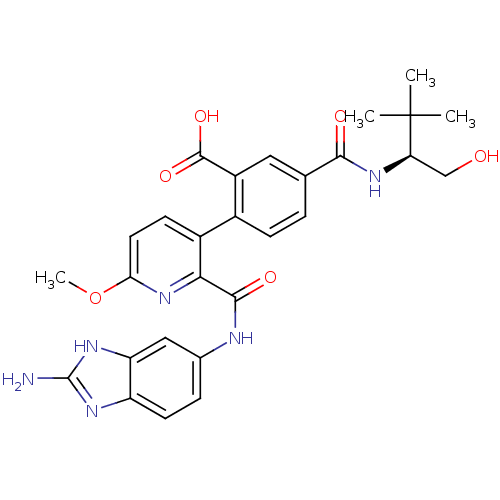
(CHEMBL2417908)Show SMILES COc1ccc(-c2ccc(cc2C(O)=O)C(=O)N[C@H](CO)C(C)(C)C)c(n1)C(=O)Nc1ccc2nc(N)[nH]c2c1 |r| Show InChI InChI=1S/C28H30N6O6/c1-28(2,3)21(13-35)33-24(36)14-5-7-16(18(11-14)26(38)39)17-8-10-22(40-4)34-23(17)25(37)30-15-6-9-19-20(12-15)32-27(29)31-19/h5-12,21,35H,13H2,1-4H3,(H,30,37)(H,33,36)(H,38,39)(H3,29,31,32)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human F7a using D-Ile-Pro-Arg-AFC as substrate after 3 mins |
Bioorg Med Chem Lett 23: 5239-43 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.028
BindingDB Entry DOI: 10.7270/Q2CC123C |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1 [23-292,A23S,F278E]
(Homo sapiens (Human)) | BDBM32513

(pyridine amide, 7)Show InChI InChI=1S/C19H20Cl2N2O2/c1-13-8-10-23(11-9-13)19(24)17-7-2-4-14(22-17)12-25-18-15(20)5-3-6-16(18)21/h2-7,13H,8-12H2,1H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | 6.5 | 23 |
Bristol-Myers Squibb Company
| Assay Description
11beta-HSD1 microsomes isolated from HEK 293 cells over-expressing human 11beta-HSD1 were incubated with the substrate cortisone and cofactor NADPH a... |
Bioorg Med Chem Lett 18: 3168-72 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.069
BindingDB Entry DOI: 10.7270/Q2P26WGT |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50063089

(CHEMBL3398443)Show SMILES Cc1oc(nc1CCOc1ccc(C[C@@H]2CN(C[C@@H]2C(O)=O)C(=O)Oc2ccccc2)cc1)-c1ccccc1 |r| Show InChI InChI=1S/C31H30N2O6/c1-21-28(32-29(38-21)23-8-4-2-5-9-23)16-17-37-25-14-12-22(13-15-25)18-24-19-33(20-27(24)30(34)35)31(36)39-26-10-6-3-7-11-26/h2-15,24,27H,16-20H2,1H3,(H,34,35)/t24-,27+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development (R&D)
Curated by ChEMBL
| Assay Description
Binding affinity to PPARgamma (unknown origin) using fluorescein-tagged dual PPARalpha/gamma activator by homogeneous fluorescence polarization bindi... |
Bioorg Med Chem Lett 25: 1196-205 (2015)
Article DOI: 10.1016/j.bmcl.2015.01.066
BindingDB Entry DOI: 10.7270/Q20866ZP |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50063311

(CHEMBL3398444)Show SMILES Cc1oc(nc1CCOc1cccc(C[C@@H]2CN(C[C@@H]2C(O)=O)c2ccccc2)c1)-c1ccccc1 |r| Show InChI InChI=1S/C30H30N2O4/c1-21-28(31-29(36-21)23-10-4-2-5-11-23)15-16-35-26-14-8-9-22(18-26)17-24-19-32(20-27(24)30(33)34)25-12-6-3-7-13-25/h2-14,18,24,27H,15-17,19-20H2,1H3,(H,33,34)/t24-,27+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development (R&D)
Curated by ChEMBL
| Assay Description
Binding affinity to PPARgamma (unknown origin) using fluorescein-tagged dual PPARalpha/gamma activator by homogeneous fluorescence polarization bindi... |
Bioorg Med Chem Lett 25: 1196-205 (2015)
Article DOI: 10.1016/j.bmcl.2015.01.066
BindingDB Entry DOI: 10.7270/Q20866ZP |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50063311

(CHEMBL3398444)Show SMILES Cc1oc(nc1CCOc1cccc(C[C@@H]2CN(C[C@@H]2C(O)=O)c2ccccc2)c1)-c1ccccc1 |r| Show InChI InChI=1S/C30H30N2O4/c1-21-28(31-29(36-21)23-10-4-2-5-11-23)15-16-35-26-14-8-9-22(18-26)17-24-19-32(20-27(24)30(33)34)25-12-6-3-7-13-25/h2-14,18,24,27H,15-17,19-20H2,1H3,(H,33,34)/t24-,27+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development (R&D)
Curated by ChEMBL
| Assay Description
Binding affinity to PPARgamma (unknown origin) using fluorescein-tagged dual PPARalpha/gamma activator by homogeneous fluorescence polarization bindi... |
Bioorg Med Chem Lett 25: 1196-205 (2015)
Article DOI: 10.1016/j.bmcl.2015.01.066
BindingDB Entry DOI: 10.7270/Q20866ZP |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50063089

(CHEMBL3398443)Show SMILES Cc1oc(nc1CCOc1ccc(C[C@@H]2CN(C[C@@H]2C(O)=O)C(=O)Oc2ccccc2)cc1)-c1ccccc1 |r| Show InChI InChI=1S/C31H30N2O6/c1-21-28(32-29(38-21)23-8-4-2-5-9-23)16-17-37-25-14-12-22(13-15-25)18-24-19-33(20-27(24)30(34)35)31(36)39-26-10-6-3-7-11-26/h2-15,24,27H,16-20H2,1H3,(H,34,35)/t24-,27+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development (R&D)
Curated by ChEMBL
| Assay Description
Binding affinity to PPARgamma (unknown origin) using fluorescein-tagged dual PPARalpha/gamma activator by homogeneous fluorescence polarization bindi... |
Bioorg Med Chem Lett 25: 1196-205 (2015)
Article DOI: 10.1016/j.bmcl.2015.01.066
BindingDB Entry DOI: 10.7270/Q20866ZP |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50063099
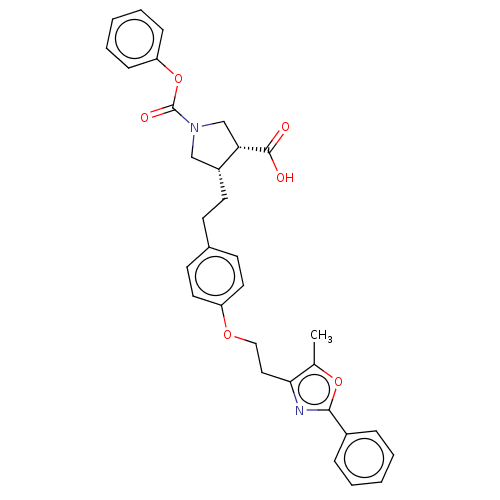
(CHEMBL3398459)Show SMILES Cc1oc(nc1CCOc1ccc(CC[C@@H]2CN(C[C@@H]2C(O)=O)C(=O)Oc2ccccc2)cc1)-c1ccccc1 |r| Show InChI InChI=1S/C32H32N2O6/c1-22-29(33-30(39-22)24-8-4-2-5-9-24)18-19-38-26-16-13-23(14-17-26)12-15-25-20-34(21-28(25)31(35)36)32(37)40-27-10-6-3-7-11-27/h2-11,13-14,16-17,25,28H,12,15,18-21H2,1H3,(H,35,36)/t25-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development (R&D)
Curated by ChEMBL
| Assay Description
Binding affinity to PPARgamma (unknown origin) using fluorescein-tagged dual PPARalpha/gamma activator by homogeneous fluorescence polarization bindi... |
Bioorg Med Chem Lett 25: 1196-205 (2015)
Article DOI: 10.1016/j.bmcl.2015.01.066
BindingDB Entry DOI: 10.7270/Q20866ZP |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50439475

(CHEMBL2417905)Show SMILES COc1ccc(-c2ccc(cc2C(O)=O)C(=O)NCC(C)(C)C)c(n1)C(=O)Nc1ccc(cc1)C(N)=N Show InChI InChI=1S/C27H29N5O5/c1-27(2,3)14-30-24(33)16-7-10-18(20(13-16)26(35)36)19-11-12-21(37-4)32-22(19)25(34)31-17-8-5-15(6-9-17)23(28)29/h5-13H,14H2,1-4H3,(H3,28,29)(H,30,33)(H,31,34)(H,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human F10a using S-2222 as substrate after 3 mins |
Bioorg Med Chem Lett 23: 5239-43 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.028
BindingDB Entry DOI: 10.7270/Q2CC123C |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM50439479

(CHEMBL2417901)Show SMILES CN(C)c1ccc(-c2ccc(cc2C(O)=O)C(=O)NCC(C)(C)C)c(n1)C(=O)Nc1ccc2c(N)nccc2c1 Show InChI InChI=1S/C30H32N6O4/c1-30(2,3)16-33-27(37)18-6-8-21(23(15-18)29(39)40)22-10-11-24(36(4)5)35-25(22)28(38)34-19-7-9-20-17(14-19)12-13-32-26(20)31/h6-15H,16H2,1-5H3,(H2,31,32)(H,33,37)(H,34,38)(H,39,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 101 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human F7a using D-Ile-Pro-Arg-AFC as substrate after 3 mins |
Bioorg Med Chem Lett 23: 5239-43 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.028
BindingDB Entry DOI: 10.7270/Q2CC123C |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1 [23-292,A23S,F278E]
(Homo sapiens (Human)) | BDBM32501
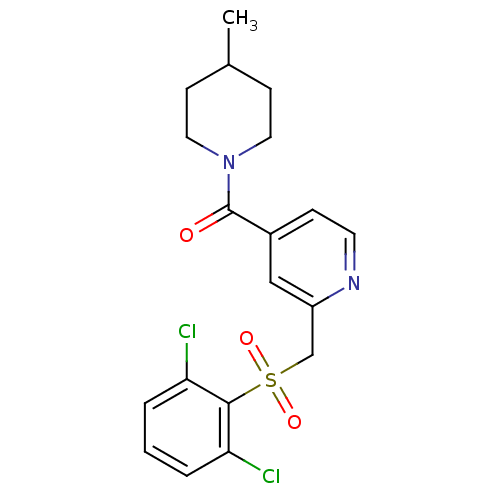
(pyridine amide, 16)Show SMILES CC1CCN(CC1)C(=O)c1ccnc(CS(=O)(=O)c2c(Cl)cccc2Cl)c1 Show InChI InChI=1S/C19H20Cl2N2O3S/c1-13-6-9-23(10-7-13)19(24)14-5-8-22-15(11-14)12-27(25,26)18-16(20)3-2-4-17(18)21/h2-5,8,11,13H,6-7,9-10,12H2,1H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 106 | n/a | n/a | n/a | n/a | 6.5 | 23 |
Bristol-Myers Squibb Company
| Assay Description
11beta-HSD1 microsomes isolated from HEK 293 cells over-expressing human 11beta-HSD1 were incubated with the substrate cortisone and cofactor NADPH a... |
Bioorg Med Chem Lett 18: 3168-72 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.069
BindingDB Entry DOI: 10.7270/Q2P26WGT |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50063316
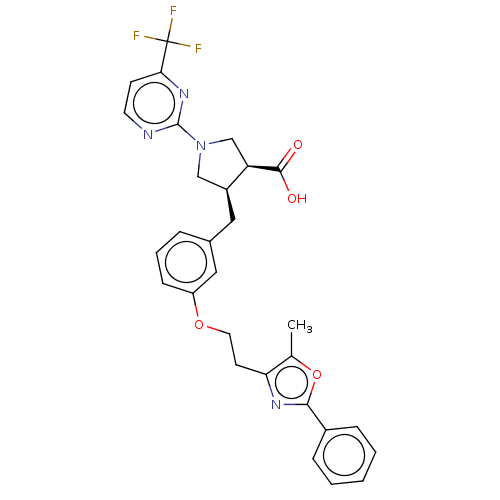
(CHEMBL3398447)Show SMILES Cc1oc(nc1CCOc1cccc(C[C@H]2CN(C[C@H]2C(O)=O)c2nccc(n2)C(F)(F)F)c1)-c1ccccc1 |r| Show InChI InChI=1S/C29H27F3N4O4/c1-18-24(34-26(40-18)20-7-3-2-4-8-20)11-13-39-22-9-5-6-19(15-22)14-21-16-36(17-23(21)27(37)38)28-33-12-10-25(35-28)29(30,31)32/h2-10,12,15,21,23H,11,13-14,16-17H2,1H3,(H,37,38)/t21-,23+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development (R&D)
Curated by ChEMBL
| Assay Description
Binding affinity to PPARgamma (unknown origin) using fluorescein-tagged dual PPARalpha/gamma activator by homogeneous fluorescence polarization bindi... |
Bioorg Med Chem Lett 25: 1196-205 (2015)
Article DOI: 10.1016/j.bmcl.2015.01.066
BindingDB Entry DOI: 10.7270/Q20866ZP |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM50439485
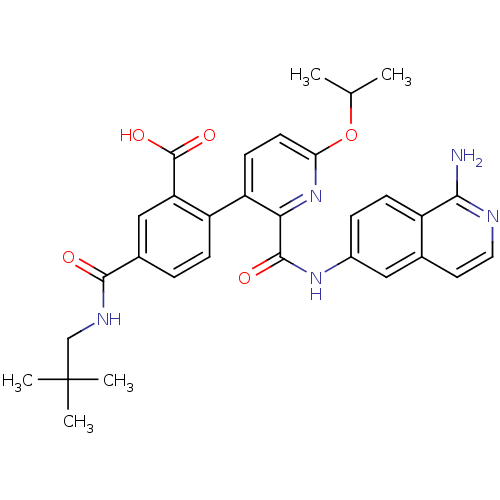
(CHEMBL2417895)Show SMILES CC(C)Oc1ccc(-c2ccc(cc2C(O)=O)C(=O)NCC(C)(C)C)c(n1)C(=O)Nc1ccc2c(N)nccc2c1 Show InChI InChI=1S/C31H33N5O5/c1-17(2)41-25-11-10-23(22-8-6-19(15-24(22)30(39)40)28(37)34-16-31(3,4)5)26(36-25)29(38)35-20-7-9-21-18(14-20)12-13-33-27(21)32/h6-15,17H,16H2,1-5H3,(H2,32,33)(H,34,37)(H,35,38)(H,39,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 111 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human F7a using D-Ile-Pro-Arg-AFC as substrate after 3 mins |
Bioorg Med Chem Lett 23: 5239-43 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.028
BindingDB Entry DOI: 10.7270/Q2CC123C |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM50439483
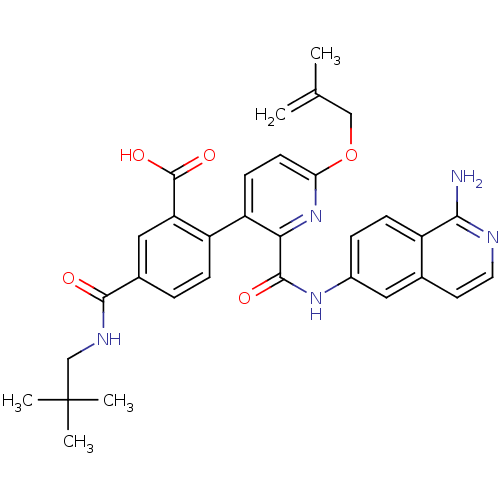
(CHEMBL2417897)Show SMILES CC(=C)COc1ccc(-c2ccc(cc2C(O)=O)C(=O)NCC(C)(C)C)c(n1)C(=O)Nc1ccc2c(N)nccc2c1 Show InChI InChI=1S/C32H33N5O5/c1-18(2)16-42-26-11-10-24(23-8-6-20(15-25(23)31(40)41)29(38)35-17-32(3,4)5)27(37-26)30(39)36-21-7-9-22-19(14-21)12-13-34-28(22)33/h6-15H,1,16-17H2,2-5H3,(H2,33,34)(H,35,38)(H,36,39)(H,40,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 113 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human F7a using D-Ile-Pro-Arg-AFC as substrate after 3 mins |
Bioorg Med Chem Lett 23: 5239-43 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.028
BindingDB Entry DOI: 10.7270/Q2CC123C |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50585812
(CHEMBL5091943)Show SMILES CCOP(=O)(Cc1csc(NC(=O)c2cc(O[C@@H](C)COC)cc(Oc3ccc(cc3)S(C)(=O)=O)c2)n1)OCC |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 122 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of fluorescent labeled derivative from recombinant human hepatic glucokinase incubated for 30 mins in presence of 12 mM glucose by fluor... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02110
BindingDB Entry DOI: 10.7270/Q2057KV7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data