 Found 427 hits with Last Name = 'wilcken' and Initial = 'r'
Found 427 hits with Last Name = 'wilcken' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM50222709
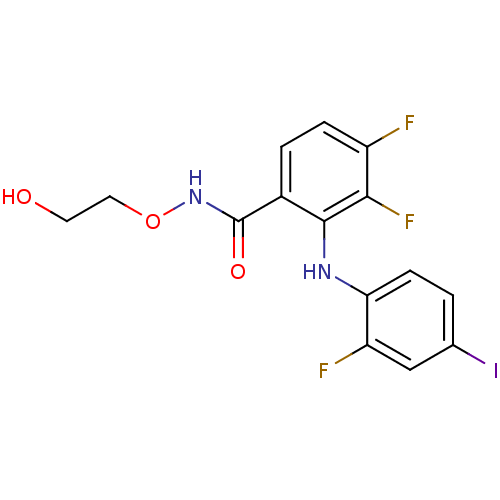
(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)-N-(2-h...)Show InChI InChI=1S/C15H12F3IN2O3/c16-10-3-2-9(15(23)21-24-6-5-22)14(13(10)18)20-12-4-1-8(19)7-11(12)17/h1-4,7,20,22H,5-6H2,(H,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| MMDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University
Curated by ChEMBL
| Assay Description
Inhibition of MEK1 (unknown origin) |
J Med Chem 56: 1363-88 (2013)
Article DOI: 10.1021/jm3012068
BindingDB Entry DOI: 10.7270/Q2GT5PH4 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50335827
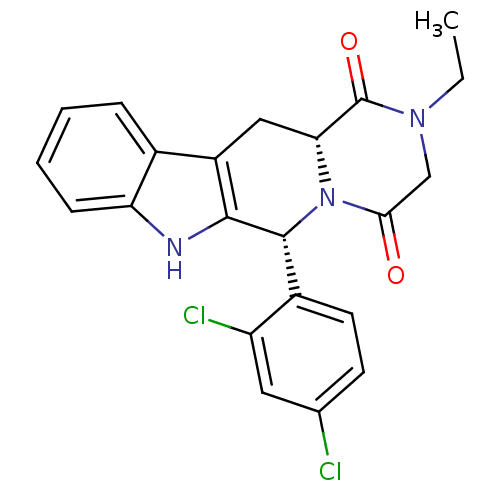
((6R,12aR)6-(2,4-Dichlorophenyl)-2-ethyl-2,3,6,7,12...)Show SMILES CCN1CC(=O)N2[C@H](Cc3c([nH]c4ccccc34)[C@H]2c2ccc(Cl)cc2Cl)C1=O |r| Show InChI InChI=1S/C22H19Cl2N3O2/c1-2-26-11-19(28)27-18(22(26)29)10-15-13-5-3-4-6-17(13)25-20(15)21(27)14-8-7-12(23)9-16(14)24/h3-9,18,21,25H,2,10-11H2,1H3/t18-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
German University in Cairo
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE5 after 30 mins by fluorescence polarization assay |
J Med Chem 54: 495-509 (2011)
Article DOI: 10.1021/jm100842v
BindingDB Entry DOI: 10.7270/Q2RR1ZHQ |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50335827

((6R,12aR)6-(2,4-Dichlorophenyl)-2-ethyl-2,3,6,7,12...)Show SMILES CCN1CC(=O)N2[C@H](Cc3c([nH]c4ccccc34)[C@H]2c2ccc(Cl)cc2Cl)C1=O |r| Show InChI InChI=1S/C22H19Cl2N3O2/c1-2-26-11-19(28)27-18(22(26)29)10-15-13-5-3-4-6-17(13)25-20(15)21(27)14-8-7-12(23)9-16(14)24/h3-9,18,21,25H,2,10-11H2,1H3/t18-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
German University in Cairo
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE5A-mediated hydrolysis of cGMP after 30 mins by fluorescence polarization assay |
J Med Chem 54: 495-509 (2011)
Article DOI: 10.1021/jm100842v
BindingDB Entry DOI: 10.7270/Q2RR1ZHQ |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM14777

((2R,8R)-2-(2H-1,3-benzodioxol-5-yl)-6-methyl-3,6,1...)Show SMILES [H][C@]12Cc3c([nH]c4ccccc34)[C@H](N1C(=O)CN(C)C2=O)c1ccc2OCOc2c1 |r| Show InChI InChI=1S/C22H19N3O4/c1-24-10-19(26)25-16(22(24)27)9-14-13-4-2-3-5-15(13)23-20(14)21(25)12-6-7-17-18(8-12)29-11-28-17/h2-8,16,21,23H,9-11H2,1H3/t16-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
German University in Cairo
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE5A-mediated hydrolysis of cGMP after 30 mins by fluorescence polarization assay |
J Med Chem 54: 495-509 (2011)
Article DOI: 10.1021/jm100842v
BindingDB Entry DOI: 10.7270/Q2RR1ZHQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
GTPase KRas
(Homo sapiens (Human)) | BDBM608937
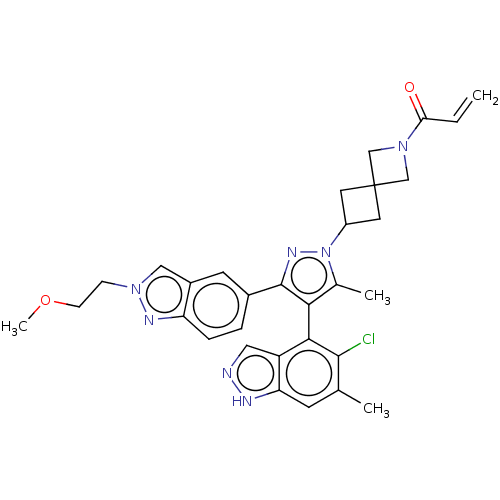
(1-(6-{(4M)-4-(5-Chloro-6- methyl-1H-indazol-4-yl)-...)Show SMILES COCCn1cc2cc(ccc2n1)-c1nn(C2CC3(C2)CN(C3)C(=O)C=C)c(C)c1-c1c(Cl)c(C)cc2[nH]ncc12 |(9.82,-.8,;9.42,-2.28,;7.94,-2.68,;6.85,-1.59,;5.36,-1.99,;4.21,-.96,;2.88,-1.73,;1.42,-1.26,;.27,-2.29,;.59,-3.79,;2.06,-4.27,;3.2,-3.24,;4.73,-3.4,;-1.19,-1.81,;-1.67,-.35,;-3.21,-.35,;-3.98,.99,;-5.47,1.39,;-5.07,2.87,;-3.58,2.48,;-6.55,3.27,;-6.16,4.76,;-4.67,4.36,;-7.25,5.85,;-6.85,7.34,;-8.73,5.45,;-9.82,6.54,;-3.68,-1.81,;-5.15,-2.29,;-2.44,-2.72,;-2.44,-4.26,;-3.77,-5.03,;-5.11,-4.26,;-3.77,-6.57,;-5.11,-7.34,;-2.44,-7.34,;-1.1,-6.57,;.36,-7.04,;1.27,-5.8,;.36,-4.55,;-1.1,-5.03,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50428483
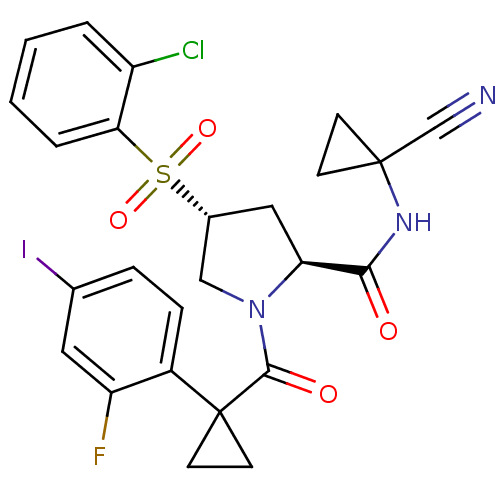
(CHEMBL2335184)Show SMILES Fc1cc(I)ccc1C1(CC1)C(=O)N1C[C@@H](C[C@H]1C(=O)NC1(CC1)C#N)S(=O)(=O)c1ccccc1Cl |r| Show InChI InChI=1S/C25H22ClFIN3O4S/c26-18-3-1-2-4-21(18)36(34,35)16-12-20(22(32)30-24(14-29)7-8-24)31(13-16)23(33)25(9-10-25)17-6-5-15(28)11-19(17)27/h1-6,11,16,20H,7-10,12-13H2,(H,30,32)/t16-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L |
J Med Chem 56: 1363-88 (2013)
Article DOI: 10.1021/jm3012068
BindingDB Entry DOI: 10.7270/Q2GT5PH4 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50203680
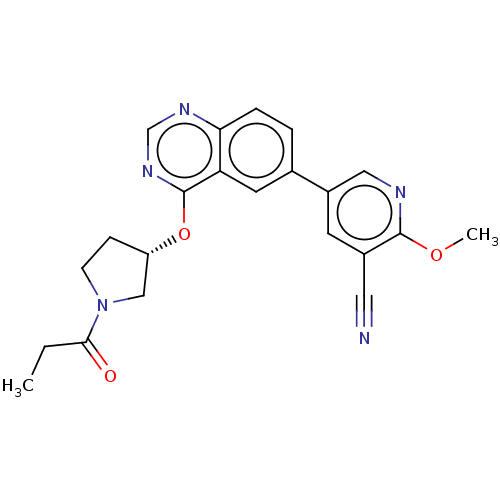
(CHEMBL3960012)Show SMILES CCC(=O)N1CC[C@@H](C1)Oc1ncnc2ccc(cc12)-c1cnc(OC)c(c1)C#N |r| Show InChI InChI=1S/C22H21N5O3/c1-3-20(28)27-7-6-17(12-27)30-22-18-9-14(4-5-19(18)25-13-26-22)16-8-15(10-23)21(29-2)24-11-16/h4-5,8-9,11,13,17H,3,6-7,12H2,1-2H3/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3K-delta (unknown origin) by KinaseGlo assay |
Bioorg Med Chem Lett 26: 5657-5662 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.069
BindingDB Entry DOI: 10.7270/Q20Z7574 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50428484
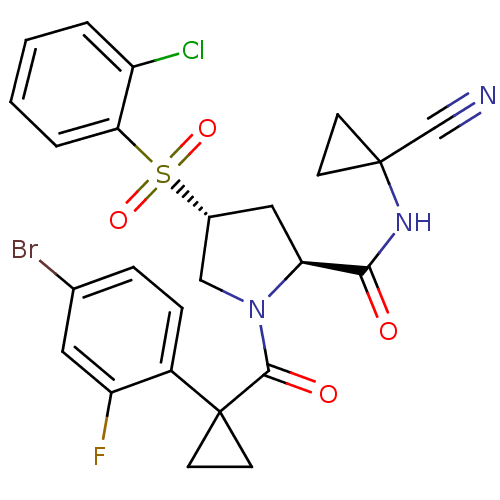
(CHEMBL2335183)Show SMILES Fc1cc(Br)ccc1C1(CC1)C(=O)N1C[C@@H](C[C@H]1C(=O)NC1(CC1)C#N)S(=O)(=O)c1ccccc1Cl |r| Show InChI InChI=1S/C25H22BrClFN3O4S/c26-15-5-6-17(19(28)11-15)25(9-10-25)23(33)31-13-16(36(34,35)21-4-2-1-3-18(21)27)12-20(31)22(32)30-24(14-29)7-8-24/h1-6,11,16,20H,7-10,12-13H2,(H,30,32)/t16-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L |
J Med Chem 56: 1363-88 (2013)
Article DOI: 10.1021/jm3012068
BindingDB Entry DOI: 10.7270/Q2GT5PH4 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50428486
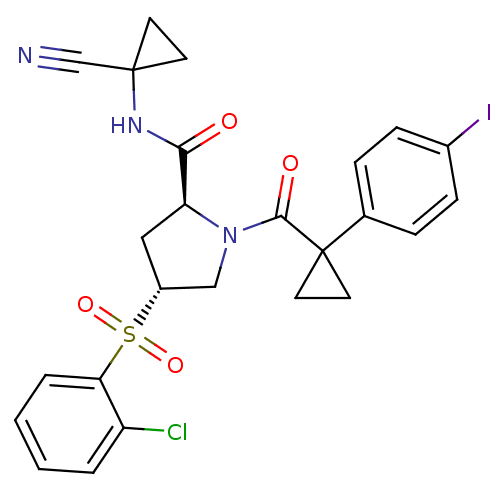
(CHEMBL2335181)Show SMILES Clc1ccccc1S(=O)(=O)[C@@H]1C[C@H](N(C1)C(=O)C1(CC1)c1ccc(I)cc1)C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C25H23ClIN3O4S/c26-19-3-1-2-4-21(19)35(33,34)18-13-20(22(31)29-24(15-28)9-10-24)30(14-18)23(32)25(11-12-25)16-5-7-17(27)8-6-16/h1-8,18,20H,9-14H2,(H,29,31)/t18-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L |
J Med Chem 56: 1363-88 (2013)
Article DOI: 10.1021/jm3012068
BindingDB Entry DOI: 10.7270/Q2GT5PH4 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50335843
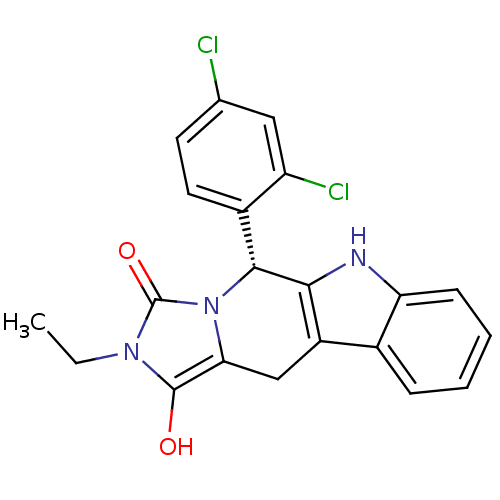
((5R,11aR)5-(2,4-Dichlorophenyl)-2-ethyl-5,6,11,11a...)Show SMILES CCn1c(O)c2Cc3c([nH]c4ccccc34)[C@@H](c3ccc(Cl)cc3Cl)n2c1=O |r| Show InChI InChI=1S/C21H17Cl2N3O2/c1-2-25-20(27)17-10-14-12-5-3-4-6-16(12)24-18(14)19(26(17)21(25)28)13-8-7-11(22)9-15(13)23/h3-9,19,24,27H,2,10H2,1H3/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
German University in Cairo
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE5A-mediated hydrolysis of cGMP after 30 mins by fluorescence polarization assay |
J Med Chem 54: 495-509 (2011)
Article DOI: 10.1021/jm100842v
BindingDB Entry DOI: 10.7270/Q2RR1ZHQ |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50335843

((5R,11aR)5-(2,4-Dichlorophenyl)-2-ethyl-5,6,11,11a...)Show SMILES CCn1c(O)c2Cc3c([nH]c4ccccc34)[C@@H](c3ccc(Cl)cc3Cl)n2c1=O |r| Show InChI InChI=1S/C21H17Cl2N3O2/c1-2-25-20(27)17-10-14-12-5-3-4-6-16(12)24-18(14)19(26(17)21(25)28)13-8-7-11(22)9-15(13)23/h3-9,19,24,27H,2,10H2,1H3/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
German University in Cairo
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE5 after 30 mins by fluorescence polarization assay |
J Med Chem 54: 495-509 (2011)
Article DOI: 10.1021/jm100842v
BindingDB Entry DOI: 10.7270/Q2RR1ZHQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50203699
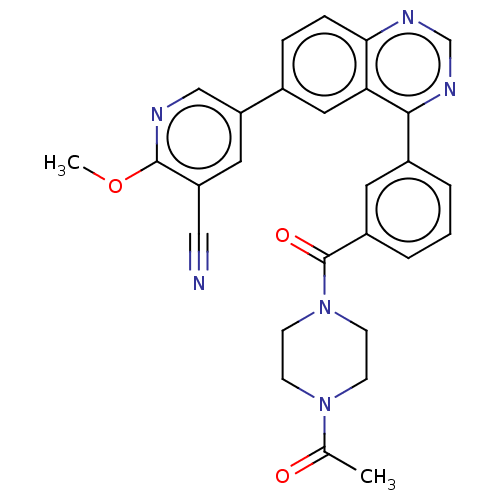
(CHEMBL3977066)Show SMILES COc1ncc(cc1C#N)-c1ccc2ncnc(-c3cccc(c3)C(=O)N3CCN(CC3)C(C)=O)c2c1 Show InChI InChI=1S/C28H24N6O3/c1-18(35)33-8-10-34(11-9-33)28(36)21-5-3-4-20(12-21)26-24-14-19(6-7-25(24)31-17-32-26)23-13-22(15-29)27(37-2)30-16-23/h3-7,12-14,16-17H,8-11H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3K-delta (unknown origin) by KinaseGlo assay |
Bioorg Med Chem Lett 26: 5657-5662 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.069
BindingDB Entry DOI: 10.7270/Q20Z7574 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM50428481
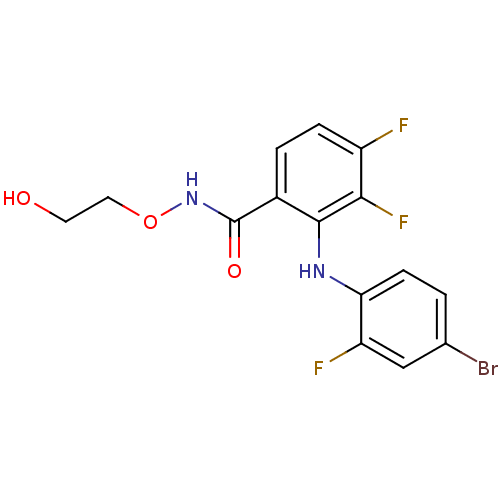
(CHEMBL2335186)Show InChI InChI=1S/C15H12BrF3N2O3/c16-8-1-4-12(11(18)7-8)20-14-9(2-3-10(17)13(14)19)15(23)21-24-6-5-22/h1-4,7,20,22H,5-6H2,(H,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University
Curated by ChEMBL
| Assay Description
Inhibition of MEK1 (unknown origin) |
J Med Chem 56: 1363-88 (2013)
Article DOI: 10.1021/jm3012068
BindingDB Entry DOI: 10.7270/Q2GT5PH4 |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50609524
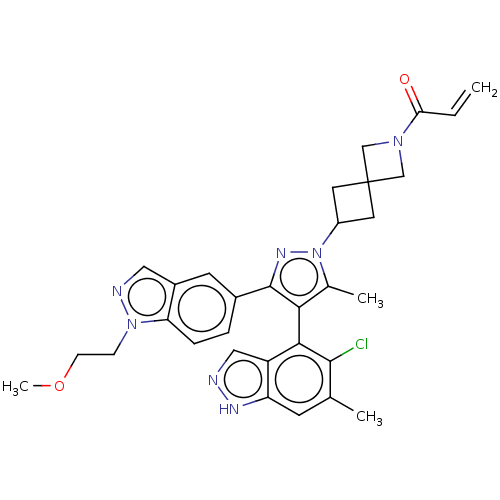
(CHEMBL5281254)Show SMILES COCCn1ncc2cc(ccc12)-c1nn(C2CC3(C2)CN(C3)C(=O)C=C)c(C)c1-c1c(Cl)c(C)cc2[nH]ncc12 |(6.51,-8.61,;5.19,-9.32,;3.92,-8.53,;2.6,-9.24,;1.33,-8.44,;-.1,-9.02,;-1.09,-7.85,;-.28,-6.54,;-.71,-5.06,;.35,-3.95,;1.85,-4.31,;2.28,-5.79,;1.22,-6.91,;.03,-2.44,;1.07,-1.29,;.29,.05,;.92,1.46,;2.28,2.07,;1.62,3.49,;.22,2.79,;3.03,4.13,;2.4,5.53,;.99,4.9,;2.94,6.97,;4.17,7.17,;1.97,8.17,;2.4,9.32,;-1.21,-.28,;-2.14,.54,;-1.35,-1.8,;-2.7,-2.58,;-2.7,-4.12,;-1.62,-4.74,;-4.03,-4.89,;-4.03,-6.12,;-5.37,-4.12,;-5.37,-2.58,;-6.51,-1.54,;-5.88,-.13,;-4.35,-.29,;-4.03,-1.8,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
GTPase KRas [G12C]
(Homo sapiens (Human)) | BDBM609011
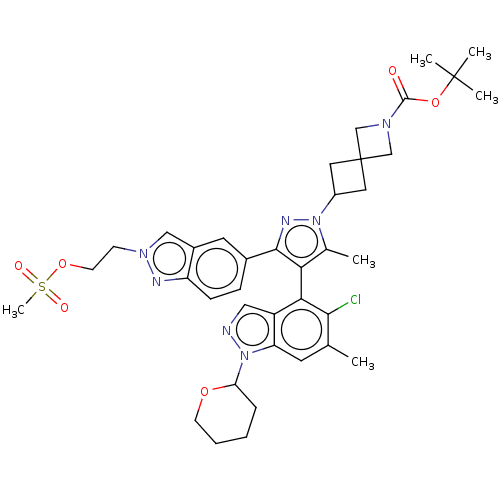
(US11702409, Example 80a | US11702409, Example 80b)Show SMILES Cc1c(c(nn1C1CC2(C1)CN(C2)C(=O)OC(C)(C)C)-c1ccc2nn(CCOS(C)(=O)=O)cc2c1)-c1c(Cl)c(C)cc2n(ncc12)C1CCCCO1 |(-8.56,21.58,;-7.07,21.98,;-5.82,21.07,;-4.58,21.98,;-5.05,23.44,;-6.59,23.44,;-7.36,24.77,;-6.96,26.26,;-8.45,26.66,;-8.85,25.17,;-8.05,28.15,;-9.54,28.55,;-9.94,27.06,;-10.63,29.64,;-10.23,31.12,;-12.12,29.24,;-13.21,30.33,;-14.69,29.93,;-12.81,31.81,;-11.72,30.72,;-3.09,21.58,;-2,22.67,;-.51,22.27,;-.11,20.78,;1.26,20.08,;1.02,18.56,;2.11,17.47,;1.71,15.98,;2.8,14.89,;2.4,13.41,;3.89,13.01,;2,11.92,;.91,13.81,;-.5,18.32,;-1.2,19.69,;-2.69,20.09,;-5.82,19.53,;-7.16,18.76,;-8.49,19.53,;-7.16,17.22,;-8.49,16.45,;-5.82,16.45,;-4.49,17.22,;-3.02,16.74,;-2.12,17.99,;-3.02,19.24,;-4.49,18.76,;-2.55,15.28,;-3.58,14.14,;-3.1,12.67,;-1.6,12.35,;-.57,13.5,;-1.04,14.96,)| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20V8HXK |
More data for this
Ligand-Target Pair | |
Complement factor D
(Homo sapiens (Human)) | BDBM50524338
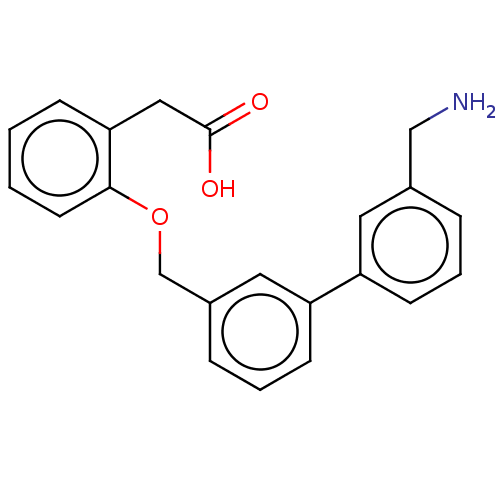
(CHEMBL4468000)Show InChI InChI=1S/C22H21NO3/c23-14-16-5-3-8-18(11-16)19-9-4-6-17(12-19)15-26-21-10-2-1-7-20(21)13-22(24)25/h1-12H,13-15,23H2,(H,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human CFD expressed in Escherichia coli incubated up to 2 hrs by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01020
BindingDB Entry DOI: 10.7270/Q20K2D8S |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50203701
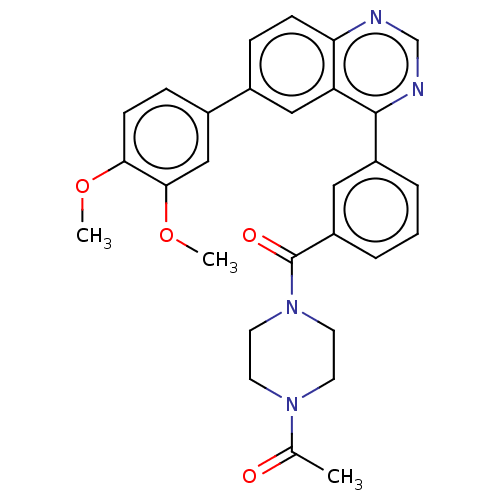
(CHEMBL3944013)Show SMILES COc1ccc(cc1OC)-c1ccc2ncnc(-c3cccc(c3)C(=O)N3CCN(CC3)C(C)=O)c2c1 Show InChI InChI=1S/C29H28N4O4/c1-19(34)32-11-13-33(14-12-32)29(35)23-6-4-5-22(15-23)28-24-16-20(7-9-25(24)30-18-31-28)21-8-10-26(36-2)27(17-21)37-3/h4-10,15-18H,11-14H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3K-delta (unknown origin) by KinaseGlo assay |
Bioorg Med Chem Lett 26: 5657-5662 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.069
BindingDB Entry DOI: 10.7270/Q20Z7574 |
More data for this
Ligand-Target Pair | |
GTPase KRas [G12C]
(Homo sapiens (Human)) | BDBM50579992
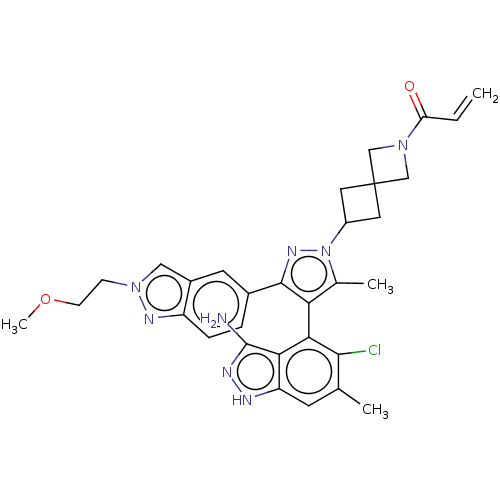
(CHEMBL5080264 | US11702409, Example 69b)Show SMILES COCCn1cc2cc(ccc2n1)-c1nn(C2CC3(C2)CN(C3)C(=O)C=C)c(C)c1-c1c(Cl)c(C)cc2[nH]nc(N)c12 |(88.07,-36.3,;87.02,-35.17,;87.48,-33.7,;86.43,-32.57,;84.93,-32.91,;83.77,-31.89,;82.45,-32.68,;80.99,-32.22,;79.85,-33.25,;80.17,-34.76,;81.64,-35.23,;82.79,-34.19,;84.32,-34.33,;78.39,-32.78,;77.91,-31.32,;76.37,-31.32,;75.47,-30.08,;75.7,-28.56,;74.18,-28.32,;73.95,-29.84,;74.58,-26.83,;73.08,-26.43,;72.69,-27.92,;72.31,-25.1,;73.08,-23.76,;70.77,-25.1,;70,-23.77,;75.9,-32.79,;74.43,-33.27,;77.14,-33.69,;77.16,-35.23,;75.83,-36,;74.5,-35.23,;75.83,-37.54,;74.5,-38.31,;77.16,-38.32,;78.5,-37.54,;79.96,-38.01,;80.87,-36.76,;79.96,-35.52,;79.56,-34.03,;78.5,-35.99,)| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20V8HXK |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50203696
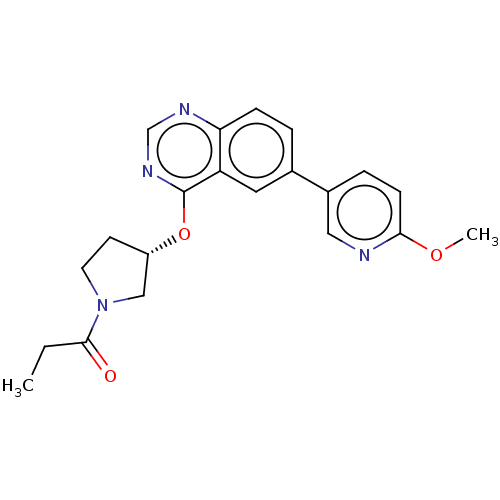
(CHEMBL3896413)Show SMILES CCC(=O)N1CC[C@@H](C1)Oc1ncnc2ccc(cc12)-c1ccc(OC)nc1 |r| Show InChI InChI=1S/C21H22N4O3/c1-3-20(26)25-9-8-16(12-25)28-21-17-10-14(4-6-18(17)23-13-24-21)15-5-7-19(27-2)22-11-15/h4-7,10-11,13,16H,3,8-9,12H2,1-2H3/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3K-delta (unknown origin) by KinaseGlo assay |
Bioorg Med Chem Lett 26: 5657-5662 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.069
BindingDB Entry DOI: 10.7270/Q20Z7574 |
More data for this
Ligand-Target Pair | |
GTPase KRas [G12C]
(Homo sapiens (Human)) | BDBM608941
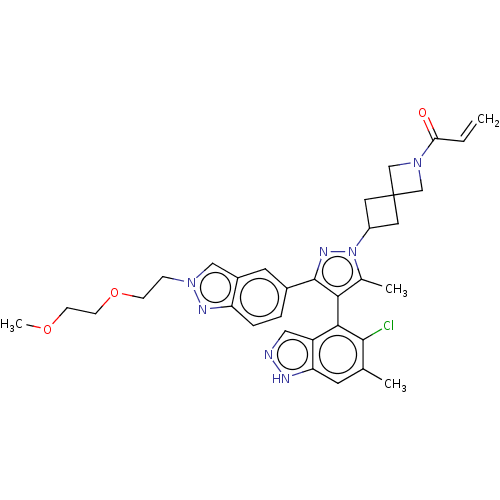
(US11702409, Example 43a | US11702409, Example 43b)Show SMILES COCCOCCn1cc2cc(ccc2n1)-c1nn(C2CC3(C2)CN(C3)C(=O)C=C)c(C)c1-c1c(Cl)c(C)cc2[nH]ncc12 |(8.04,2.87,;9.13,1.78,;8.73,.29,;9.82,-.8,;9.42,-2.28,;7.94,-2.68,;6.85,-1.59,;5.36,-1.99,;4.21,-.96,;2.88,-1.73,;1.42,-1.26,;.27,-2.29,;.59,-3.79,;2.06,-4.27,;3.2,-3.24,;4.73,-3.4,;-1.19,-1.81,;-1.67,-.35,;-3.21,-.35,;-3.98,.99,;-5.47,1.39,;-5.07,2.87,;-3.58,2.48,;-6.55,3.27,;-6.16,4.76,;-4.67,4.36,;-7.25,5.85,;-6.85,7.34,;-8.73,5.45,;-9.82,6.54,;-3.68,-1.81,;-5.15,-2.29,;-2.44,-2.72,;-2.44,-4.26,;-3.77,-5.03,;-5.11,-4.26,;-3.77,-6.57,;-5.11,-7.34,;-2.44,-7.34,;-1.1,-6.57,;.36,-7.04,;1.27,-5.8,;.36,-4.55,;-1.1,-5.03,)| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20V8HXK |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50203698
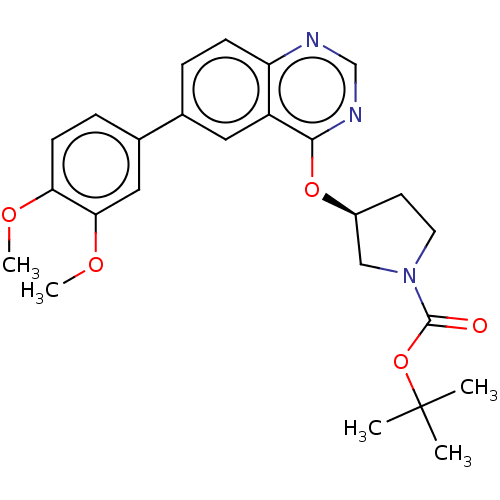
(CHEMBL3930000)Show SMILES COc1ccc(cc1OC)-c1ccc2ncnc(O[C@H]3CCN(C3)C(=O)OC(C)(C)C)c2c1 |r| Show InChI InChI=1S/C25H29N3O5/c1-25(2,3)33-24(29)28-11-10-18(14-28)32-23-19-12-16(6-8-20(19)26-15-27-23)17-7-9-21(30-4)22(13-17)31-5/h6-9,12-13,15,18H,10-11,14H2,1-5H3/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3K-delta (unknown origin) by KinaseGlo assay |
Bioorg Med Chem Lett 26: 5657-5662 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.069
BindingDB Entry DOI: 10.7270/Q20Z7574 |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50609523
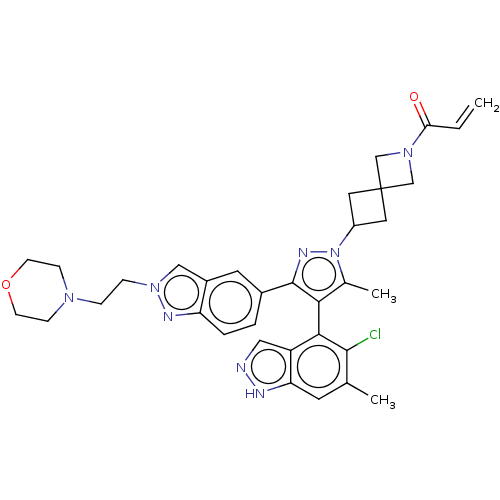
(CHEMBL5271997)Show SMILES Cc1c(c(nn1C1CC2(C1)CN(C2)C(=O)C=C)-c1ccc2nn(CCN3CCOCC3)cc2c1)-c1c(Cl)c(C)cc2[nH]ncc12 |(-1.82,5.46,;-1.41,4.3,;-2.27,3.04,;-1.37,1.81,;.09,2.31,;.06,3.85,;1.29,4.77,;2.79,4.65,;2.9,6.21,;1.34,6.28,;4.43,6.08,;4.56,7.61,;3.03,7.74,;5.73,8.61,;6.89,8.19,;5.46,10.12,;6.4,10.92,;-1.83,.34,;-3.29,-.12,;-3.64,-1.62,;-2.51,-2.66,;-2.53,-4.2,;-1.07,-4.7,;-.61,-6.17,;.89,-6.51,;1.35,-7.98,;2.85,-8.32,;3.31,-9.79,;2.26,-10.92,;.76,-10.58,;.3,-9.11,;-.15,-3.47,;-1.04,-2.21,;-.69,-.71,;-3.81,3.01,;-4.56,1.67,;-3.93,.61,;-6.1,1.65,;-6.7,.57,;-6.89,2.97,;-6.14,4.31,;-6.64,5.77,;-5.41,6.69,;-4.15,5.81,;-4.6,4.34,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50335830

((6R,12aS)6-(2,4-Dichlorophenyl)-2-ethyl-2,3,6,7,12...)Show SMILES CCN1CC(=O)N2[C@@H](Cc3c([nH]c4ccccc34)[C@H]2c2ccc(Cl)cc2Cl)C1=O |r| Show InChI InChI=1S/C22H19Cl2N3O2/c1-2-26-11-19(28)27-18(22(26)29)10-15-13-5-3-4-6-17(13)25-20(15)21(27)14-8-7-12(23)9-16(14)24/h3-9,18,21,25H,2,10-11H2,1H3/t18-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
German University in Cairo
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE5 after 30 mins by fluorescence polarization assay |
J Med Chem 54: 495-509 (2011)
Article DOI: 10.1021/jm100842v
BindingDB Entry DOI: 10.7270/Q2RR1ZHQ |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50335843

((5R,11aR)5-(2,4-Dichlorophenyl)-2-ethyl-5,6,11,11a...)Show SMILES CCn1c(O)c2Cc3c([nH]c4ccccc34)[C@@H](c3ccc(Cl)cc3Cl)n2c1=O |r| Show InChI InChI=1S/C21H17Cl2N3O2/c1-2-25-20(27)17-10-14-12-5-3-4-6-16(12)24-18(14)19(26(17)21(25)28)13-8-7-11(22)9-15(13)23/h3-9,19,24,27H,2,10H2,1H3/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
German University in Cairo
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE5 after 30 mins by fluorescence polarization assay |
J Med Chem 54: 495-509 (2011)
Article DOI: 10.1021/jm100842v
BindingDB Entry DOI: 10.7270/Q2RR1ZHQ |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50335843

((5R,11aR)5-(2,4-Dichlorophenyl)-2-ethyl-5,6,11,11a...)Show SMILES CCn1c(O)c2Cc3c([nH]c4ccccc34)[C@@H](c3ccc(Cl)cc3Cl)n2c1=O |r| Show InChI InChI=1S/C21H17Cl2N3O2/c1-2-25-20(27)17-10-14-12-5-3-4-6-16(12)24-18(14)19(26(17)21(25)28)13-8-7-11(22)9-15(13)23/h3-9,19,24,27H,2,10H2,1H3/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
German University in Cairo
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE5A-mediated hydrolysis of cGMP after 30 mins by fluorescence polarization assay |
J Med Chem 54: 495-509 (2011)
Article DOI: 10.1021/jm100842v
BindingDB Entry DOI: 10.7270/Q2RR1ZHQ |
More data for this
Ligand-Target Pair | |
GTPase KRas [G12C]
(Homo sapiens (Human)) | BDBM608937

(1-(6-{(4M)-4-(5-Chloro-6- methyl-1H-indazol-4-yl)-...)Show SMILES COCCn1cc2cc(ccc2n1)-c1nn(C2CC3(C2)CN(C3)C(=O)C=C)c(C)c1-c1c(Cl)c(C)cc2[nH]ncc12 |(9.82,-.8,;9.42,-2.28,;7.94,-2.68,;6.85,-1.59,;5.36,-1.99,;4.21,-.96,;2.88,-1.73,;1.42,-1.26,;.27,-2.29,;.59,-3.79,;2.06,-4.27,;3.2,-3.24,;4.73,-3.4,;-1.19,-1.81,;-1.67,-.35,;-3.21,-.35,;-3.98,.99,;-5.47,1.39,;-5.07,2.87,;-3.58,2.48,;-6.55,3.27,;-6.16,4.76,;-4.67,4.36,;-7.25,5.85,;-6.85,7.34,;-8.73,5.45,;-9.82,6.54,;-3.68,-1.81,;-5.15,-2.29,;-2.44,-2.72,;-2.44,-4.26,;-3.77,-5.03,;-5.11,-4.26,;-3.77,-6.57,;-5.11,-7.34,;-2.44,-7.34,;-1.1,-6.57,;.36,-7.04,;1.27,-5.8,;.36,-4.55,;-1.1,-5.03,)| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20V8HXK |
More data for this
Ligand-Target Pair | |
GTPase KRas [G12C]
(Homo sapiens (Human)) | BDBM608859
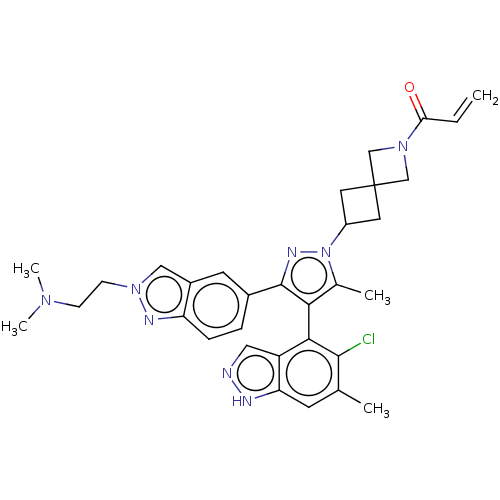
(US11702409, Example 16a | US11702409, Example 16b)Show SMILES CN(C)CCn1cc2cc(ccc2n1)-c1nn(C2CC3(C2)CN(C3)C(=O)C=C)c(C)c1-c1c(Cl)c(C)cc2[nH]ncc12 |(5.96,.58,;7.44,.98,;7.84,2.47,;8.53,-.11,;8.13,-1.59,;6.65,-1.99,;5.5,-.96,;4.17,-1.73,;2.7,-1.26,;1.56,-2.29,;1.88,-3.79,;3.35,-4.27,;4.49,-3.24,;6.02,-3.4,;.1,-1.81,;-.38,-.35,;-1.92,-.35,;-2.69,.99,;-4.18,1.39,;-3.78,2.87,;-2.29,2.48,;-5.27,3.27,;-4.87,4.76,;-3.38,4.36,;-5.96,5.85,;-5.56,7.34,;-7.44,5.45,;-8.53,6.54,;-2.4,-1.81,;-3.86,-2.29,;-1.15,-2.72,;-1.15,-4.26,;-2.48,-5.03,;-3.82,-4.26,;-2.48,-6.57,;-3.82,-7.34,;-1.15,-7.34,;.18,-6.57,;1.65,-7.04,;2.55,-5.8,;1.65,-4.55,;.18,-5.03,)| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20V8HXK |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50203678

(CHEMBL3905342)Show SMILES CCC(=O)N1CC[C@@H](C1)Oc1ccnc2ccc(cc12)-c1ccc(OC)nc1 |r| Show InChI InChI=1S/C22H23N3O3/c1-3-22(26)25-11-9-17(14-25)28-20-8-10-23-19-6-4-15(12-18(19)20)16-5-7-21(27-2)24-13-16/h4-8,10,12-13,17H,3,9,11,14H2,1-2H3/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3K-delta (unknown origin) by KinaseGlo assay |
Bioorg Med Chem Lett 26: 5657-5662 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.069
BindingDB Entry DOI: 10.7270/Q20Z7574 |
More data for this
Ligand-Target Pair | |
Dual 3',5'-cyclic-AMP and -GMP phosphodiesterase 11A
(Homo sapiens (Human)) | BDBM50335827

((6R,12aR)6-(2,4-Dichlorophenyl)-2-ethyl-2,3,6,7,12...)Show SMILES CCN1CC(=O)N2[C@H](Cc3c([nH]c4ccccc34)[C@H]2c2ccc(Cl)cc2Cl)C1=O |r| Show InChI InChI=1S/C22H19Cl2N3O2/c1-2-26-11-19(28)27-18(22(26)29)10-15-13-5-3-4-6-17(13)25-20(15)21(27)14-8-7-12(23)9-16(14)24/h3-9,18,21,25H,2,10-11H2,1H3/t18-,21-/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
German University in Cairo
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE11A-mediated hydrolysis of cGMP after 30 mins by fluorescence polarization assay |
J Med Chem 54: 495-509 (2011)
Article DOI: 10.1021/jm100842v
BindingDB Entry DOI: 10.7270/Q2RR1ZHQ |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50428487
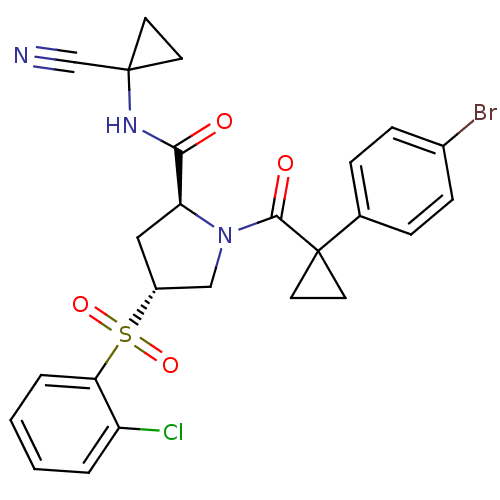
(CHEMBL2335180)Show SMILES Clc1ccccc1S(=O)(=O)[C@@H]1C[C@H](N(C1)C(=O)C1(CC1)c1ccc(Br)cc1)C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C25H23BrClN3O4S/c26-17-7-5-16(6-8-17)25(11-12-25)23(32)30-14-18(35(33,34)21-4-2-1-3-19(21)27)13-20(30)22(31)29-24(15-28)9-10-24/h1-8,18,20H,9-14H2,(H,29,31)/t18-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L |
J Med Chem 56: 1363-88 (2013)
Article DOI: 10.1021/jm3012068
BindingDB Entry DOI: 10.7270/Q2GT5PH4 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50335846
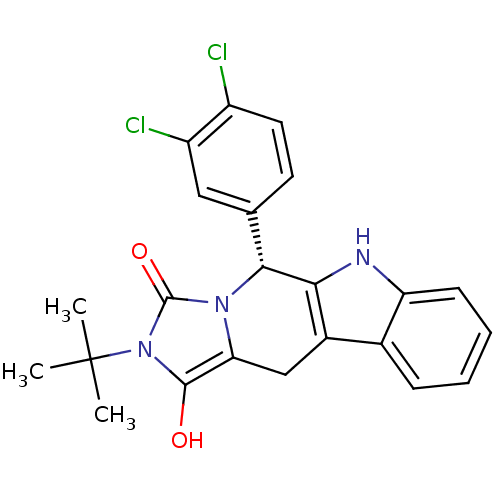
((5R,11aR)5-(3,4-Dichlorophenyl)-2-tert-butyl-5,6,1...)Show SMILES CC(C)(C)n1c(O)c2Cc3c([nH]c4ccccc34)[C@@H](c3ccc(Cl)c(Cl)c3)n2c1=O |r| Show InChI InChI=1S/C23H21Cl2N3O2/c1-23(2,3)28-21(29)18-11-14-13-6-4-5-7-17(13)26-19(14)20(27(18)22(28)30)12-8-9-15(24)16(25)10-12/h4-10,20,26,29H,11H2,1-3H3/t20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
German University in Cairo
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE5 after 30 mins by fluorescence polarization assay |
J Med Chem 54: 495-509 (2011)
Article DOI: 10.1021/jm100842v
BindingDB Entry DOI: 10.7270/Q2RR1ZHQ |
More data for this
Ligand-Target Pair | |
GTPase KRas [G12C]
(Homo sapiens (Human)) | BDBM609015
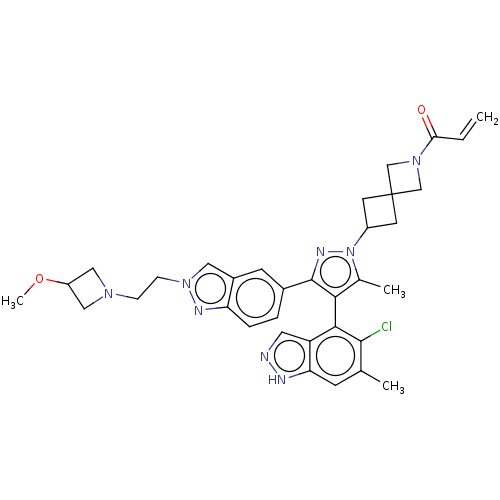
(US11702409, Example 82a | US11702409, Example 82b)Show SMILES COC1CN(CCn2cc3cc(ccc3n2)-c2nn(C3CC4(C3)CN(C4)C(=O)C=C)c(C)c2-c2c(Cl)c(C)cc3[nH]ncc23)C1 |(11.96,-2.41,;10.87,-1.32,;9.39,-1.72,;8.62,-3.05,;7.28,-2.28,;5.79,-2.68,;4.71,-1.59,;3.22,-1.99,;2.07,-.96,;.74,-1.73,;-.72,-1.26,;-1.87,-2.29,;-1.55,-3.79,;-.08,-4.27,;1.06,-3.24,;2.59,-3.4,;-3.33,-1.81,;-3.81,-.35,;-5.35,-.35,;-6.12,.99,;-7.61,1.39,;-7.21,2.87,;-5.72,2.48,;-8.7,3.27,;-8.3,4.76,;-6.81,4.36,;-9.39,5.85,;-8.99,7.34,;-10.87,5.45,;-11.96,6.54,;-5.83,-1.81,;-7.29,-2.29,;-4.58,-2.72,;-4.58,-4.26,;-3.25,-5.03,;-1.76,-4.63,;-3.25,-6.57,;-1.91,-7.34,;-4.58,-7.34,;-5.91,-6.57,;-7.38,-7.04,;-8.28,-5.8,;-7.38,-4.55,;-5.91,-5.03,;8.05,-.95,)| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20V8HXK |
More data for this
Ligand-Target Pair | |
GTPase KRas [G12C]
(Homo sapiens (Human)) | BDBM608939
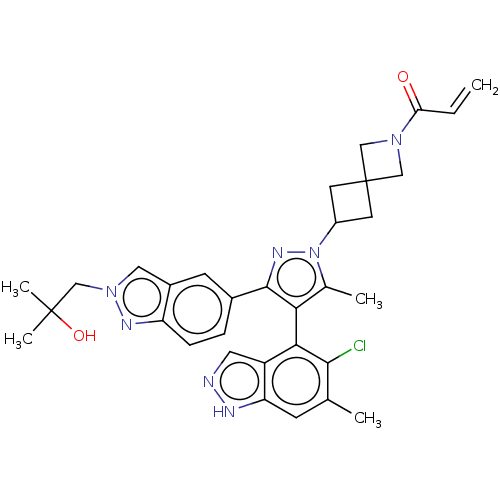
(US11702409, Example 42a | US11702409, Example 42b)Show SMILES Cc1c(c(nn1C1CC2(C1)CN(C2)C(=O)C=C)-c1ccc2nn(CC(C)(C)O)cc2c1)-c1c(Cl)c(C)cc2[nH]ncc12 |(-4.95,-2.29,;-3.49,-1.81,;-2.24,-2.72,;-.99,-1.81,;-1.47,-.35,;-3.01,-.35,;-3.78,.99,;-5.27,1.39,;-4.87,2.87,;-3.38,2.48,;-6.36,3.27,;-5.96,4.76,;-4.47,4.36,;-7.05,5.85,;-6.65,7.34,;-8.53,5.45,;-9.62,6.54,;.47,-2.29,;.79,-3.79,;2.26,-4.27,;3.4,-3.24,;4.93,-3.4,;5.56,-1.99,;7.05,-1.59,;8.13,-2.68,;9.62,-2.28,;8.53,-4.17,;7.05,-3.77,;4.41,-.96,;3.08,-1.73,;1.62,-1.26,;-2.24,-4.26,;-3.57,-5.03,;-4.91,-4.26,;-3.57,-6.57,;-4.91,-7.34,;-2.24,-7.34,;-.91,-6.57,;.56,-7.04,;1.46,-5.8,;.56,-4.55,;-.91,-5.03,)| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20V8HXK |
More data for this
Ligand-Target Pair | |
GTPase KRas [G12C]
(Homo sapiens (Human)) | BDBM608864
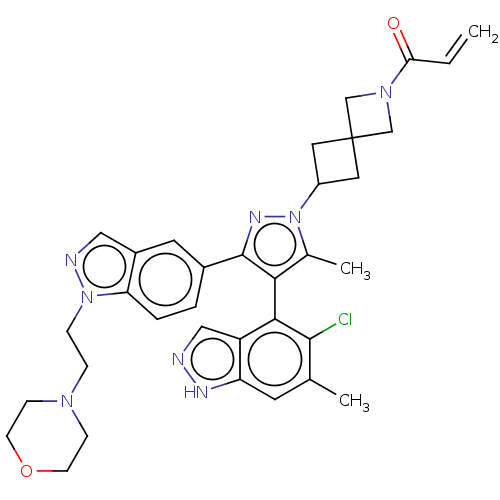
(US11702409, Example 17a | US11702409, Example 17b)Show SMILES Cc1c(c(nn1C1CC2(C1)CN(C2)C(=O)C=C)-c1ccc2n(CCN3CCOCC3)ncc2c1)-c1c(Cl)c(C)cc2[nH]ncc12 |(-4.14,-.92,;-2.68,-.44,;-1.43,-1.35,;-.19,-.44,;-.66,1.02,;-2.2,1.02,;-2.97,2.35,;-4.46,2.75,;-4.06,4.24,;-2.58,3.84,;-5.55,4.64,;-5.15,6.13,;-3.66,5.73,;-6.24,7.21,;-5.84,8.7,;-7.73,6.82,;-8.82,7.9,;1.28,-.92,;1.6,-2.43,;3.06,-2.9,;4.21,-1.87,;5.74,-2.03,;6.51,-3.37,;5.74,-4.7,;6.51,-6.03,;5.74,-7.37,;6.51,-8.7,;8.05,-8.7,;8.82,-7.37,;8.05,-6.03,;6.36,-.63,;5.22,.4,;3.89,-.37,;2.42,.11,;-1.43,-2.89,;-2.77,-3.66,;-4.1,-2.89,;-2.77,-5.2,;-4.1,-5.97,;-1.43,-5.97,;-.1,-5.2,;1.36,-5.68,;2.27,-4.43,;1.36,-3.18,;-.1,-3.66,)| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20V8HXK |
More data for this
Ligand-Target Pair | |
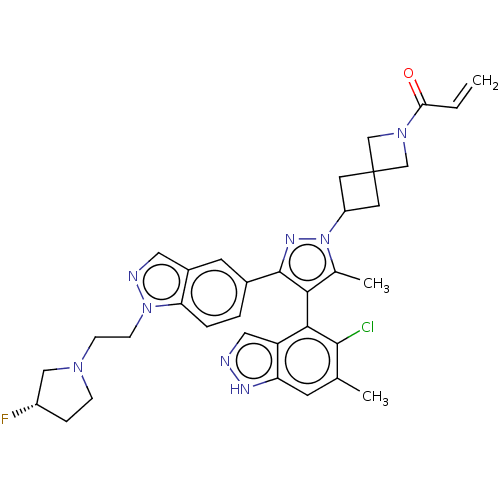
GTPase KRas [G12C]
(Homo sapiens (Human)) | BDBM50579987
(CHEMBL5083497 | US11702409, Example 18b)Show SMILES Cc1c(c(nn1C1CC2(C1)CN(C2)C(=O)C=C)-c1ccc2n(CCN3CC[C@H](F)C3)ncc2c1)-c1c(Cl)c(C)cc2[nH]ncc12 |r,wD:27.30,(54.94,-11.31,;56.4,-10.83,;57.65,-11.73,;58.9,-10.82,;58.42,-9.35,;56.88,-9.36,;55.97,-8.11,;56.21,-6.59,;54.69,-6.35,;54.45,-7.88,;55.08,-4.86,;53.59,-4.46,;53.19,-5.96,;52.82,-3.13,;53.58,-1.79,;51.28,-3.13,;50.5,-1.8,;60.36,-11.29,;60.68,-12.79,;62.14,-13.26,;63.28,-12.22,;64.82,-12.36,;65.6,-13.68,;64.85,-15.02,;65.63,-16.35,;67.17,-16.48,;67.51,-17.99,;66.19,-18.77,;66.04,-20.31,;65.03,-17.76,;65.42,-10.95,;64.27,-9.93,;62.95,-10.72,;61.48,-10.26,;57.67,-13.27,;56.34,-14.04,;55.01,-13.27,;56.34,-15.58,;55,-16.35,;57.67,-16.35,;59,-15.57,;60.47,-16.05,;61.38,-14.8,;60.47,-13.55,;59,-14.03,)| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20V8HXK |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50203685
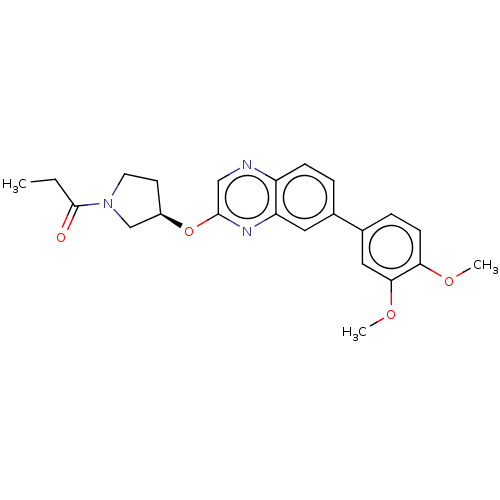
(CHEMBL3966263)Show SMILES CCC(=O)N1CC[C@H](C1)Oc1cnc2ccc(cc2n1)-c1ccc(OC)c(OC)c1 |r| Show InChI InChI=1S/C23H25N3O4/c1-4-23(27)26-10-9-17(14-26)30-22-13-24-18-7-5-15(11-19(18)25-22)16-6-8-20(28-2)21(12-16)29-3/h5-8,11-13,17H,4,9-10,14H2,1-3H3/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3K-delta (unknown origin) by KinaseGlo assay |
Bioorg Med Chem Lett 26: 5657-5662 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.069
BindingDB Entry DOI: 10.7270/Q20Z7574 |
More data for this
Ligand-Target Pair | |
GTPase KRas [G12C]
(Homo sapiens (Human)) | BDBM609006
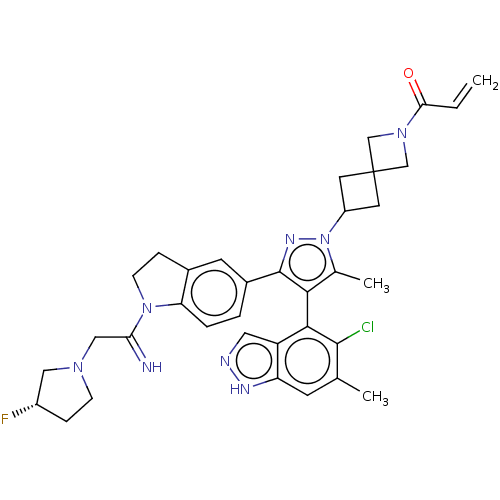
(US11702409, Example 76a | US11702409, Example 76b)Show SMILES Cc1c(c(nn1C1CC2(C1)CN(C2)C(=O)C=C)-c1ccc2N(CCc2c1)C(=N)CN1CC[C@H](F)C1)-c1c(Cl)c(C)cc2[nH]ncc12 |r,wU:32.37,(-2.78,3.57,;-1.53,2.66,;-1.53,1.12,;-.07,.65,;.84,1.89,;-.07,3.14,;.33,4.63,;1.67,5.4,;.9,6.73,;-.44,5.96,;2.23,7.5,;1.46,8.83,;.13,8.06,;1.86,10.32,;.77,11.41,;3.35,10.72,;4.43,9.63,;.33,-.84,;-.07,-2.33,;1.02,-3.42,;2.51,-3.02,;3.8,-3.86,;5,-2.89,;4.45,-1.45,;2.91,-1.53,;1.82,-.44,;3.8,-5.4,;2.47,-6.17,;5.14,-6.17,;5.14,-7.71,;3.89,-8.61,;4.37,-10.08,;5.91,-10.08,;6.68,-11.41,;6.38,-8.61,;-2.78,.22,;-2.62,-1.31,;-1.53,-2.4,;-3.86,-2.22,;-3.7,-3.75,;-5.27,-1.59,;-5.43,-.06,;-6.68,.84,;-6.2,2.31,;-4.66,2.31,;-4.18,.84,)| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20V8HXK |
More data for this
Ligand-Target Pair | |
GTPase KRas [G12C]
(Homo sapiens (Human)) | BDBM608857
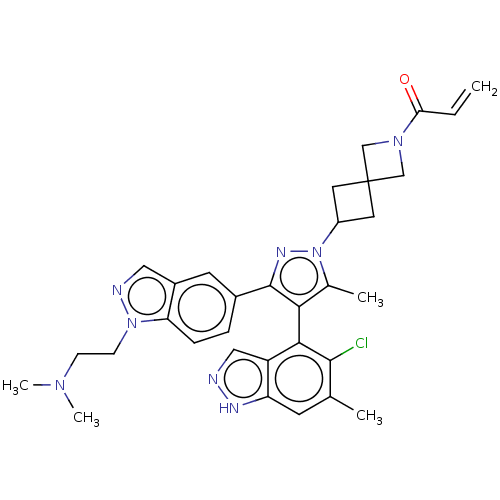
(US11702409, Example 15a | US11702409, Example 15b)Show SMILES CN(C)CCn1ncc2cc(ccc12)-c1nn(C2CC3(C2)CN(C3)C(=O)C=C)c(C)c1-c1c(Cl)c(C)cc2[nH]ncc12 |(6.12,-8.04,;6.89,-6.7,;8.43,-6.7,;6.12,-5.37,;6.89,-4.03,;6.12,-2.7,;6.75,-1.29,;5.6,-.26,;4.27,-1.03,;2.81,-.56,;1.66,-1.59,;1.98,-3.09,;3.45,-3.57,;4.59,-2.54,;.2,-1.11,;-.28,.35,;-1.82,.35,;-2.59,1.69,;-4.08,2.09,;-3.68,3.57,;-2.19,3.17,;-5.17,3.97,;-4.77,5.46,;-3.28,5.06,;-5.86,6.55,;-5.46,8.04,;-7.34,6.15,;-8.43,7.24,;-2.29,-1.11,;-3.76,-1.59,;-1.05,-2.02,;-1.05,-3.56,;-2.38,-4.33,;-3.72,-3.56,;-2.38,-5.87,;-3.72,-6.64,;-1.05,-6.64,;.28,-5.87,;1.75,-6.34,;2.65,-5.1,;1.75,-3.85,;.28,-4.33,)| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20V8HXK |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50203697
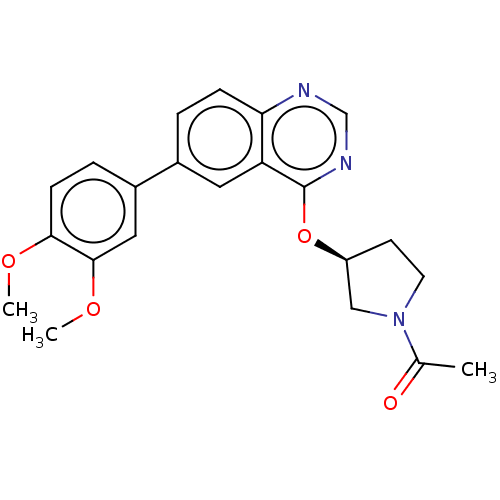
(CHEMBL3969716)Show SMILES COc1ccc(cc1OC)-c1ccc2ncnc(O[C@H]3CCN(C3)C(C)=O)c2c1 |r| Show InChI InChI=1S/C22H23N3O4/c1-14(26)25-9-8-17(12-25)29-22-18-10-15(4-6-19(18)23-13-24-22)16-5-7-20(27-2)21(11-16)28-3/h4-7,10-11,13,17H,8-9,12H2,1-3H3/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3K-delta (unknown origin) by KinaseGlo assay |
Bioorg Med Chem Lett 26: 5657-5662 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.069
BindingDB Entry DOI: 10.7270/Q20Z7574 |
More data for this
Ligand-Target Pair | |
GTPase KRas [G12C]
(Homo sapiens (Human)) | BDBM50579985

(JDQ-443 | JDQ443 | Jdq 443 | Jdq-443 | Nvp-jdq-443...)Show SMILES Cc1c(c(nn1C1CC2(C1)CN(C2)C(=O)C=C)-c1ccc2n(C)ncc2c1)-c1c(Cl)c(C)cc2[nH]ncc12 |(8.62,-12.01,;10.09,-11.52,;11.34,-12.43,;12.58,-11.52,;12.1,-10.05,;10.56,-10.06,;9.66,-8.81,;9.89,-7.29,;8.37,-7.05,;8.13,-8.58,;8.77,-5.56,;7.27,-5.16,;6.88,-6.65,;6.5,-3.83,;7.27,-2.49,;4.96,-3.83,;4.19,-2.5,;14.04,-11.99,;15.18,-10.95,;16.64,-11.42,;16.97,-12.93,;18.31,-13.69,;19.71,-13.06,;18,-15.2,;16.47,-15.37,;15.84,-13.97,;14.37,-13.49,;11.35,-13.97,;10.02,-14.74,;8.69,-13.97,;10.02,-16.28,;8.69,-17.05,;11.35,-17.05,;12.69,-16.27,;14.15,-16.75,;15.06,-15.5,;14.15,-14.25,;12.69,-14.73,)| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20V8HXK |
More data for this
Ligand-Target Pair | |
GTPase KRas [G12C]
(Homo sapiens (Human)) | BDBM609017
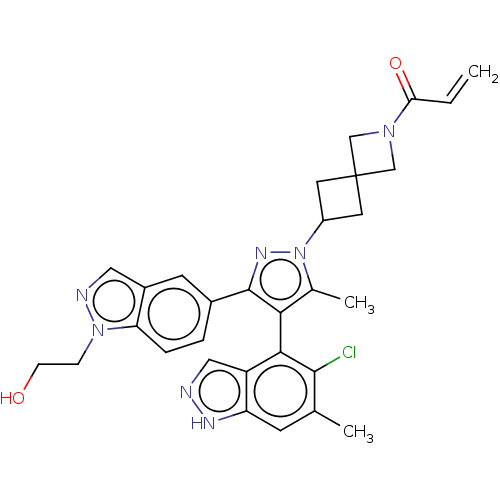
(US11702409, Example 83a | US11702409, Example 83b)Show SMILES Cc1c(c(nn1C1CC2(C1)CN(C2)C(=O)C=C)-c1ccc2n(CCO)ncc2c1)-c1c(Cl)c(C)cc2[nH]ncc12 |(-2.96,-2.11,;-1.49,-1.63,;-.25,-2.54,;1,-1.63,;.52,-.17,;-1.02,-.17,;-1.92,1.08,;-1.68,2.6,;-3.2,2.84,;-3.44,1.32,;-2.96,4.36,;-4.48,4.6,;-4.72,3.08,;-5.39,5.85,;-4.69,7.22,;-6.93,5.85,;-7.7,7.18,;2.47,-2.11,;2.79,-3.61,;4.25,-4.09,;5.39,-3.06,;6.93,-3.22,;7.7,-4.55,;6.93,-5.89,;7.7,-7.22,;7.55,-1.81,;6.41,-.78,;5.07,-1.55,;3.61,-1.08,;-.25,-4.08,;-1.58,-4.85,;-2.91,-4.08,;-1.58,-6.39,;-2.91,-7.16,;-.25,-7.16,;1.09,-6.39,;2.55,-6.86,;3.46,-5.62,;2.55,-4.37,;1.09,-4.85,)| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20V8HXK |
More data for this
Ligand-Target Pair | |
GTPase KRas [G12C]
(Homo sapiens (Human)) | BDBM608852
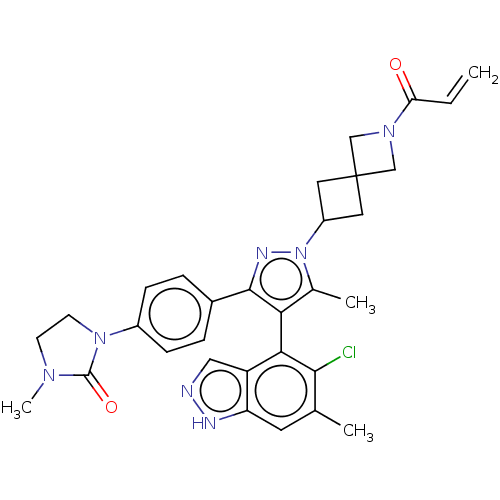
(US11702409, Example 13a | US11702409, Example 13b)Show SMILES CN1CCN(C1=O)c1ccc(cc1)-c1nn(C2CC3(C2)CN(C3)C(=O)C=C)c(C)c1-c1c(Cl)c(C)cc2[nH]ncc12 |(9.19,-2.83,;7.78,-3.45,;7.46,-4.96,;5.93,-5.12,;5.3,-3.71,;6.45,-2.68,;6.05,-1.2,;3.84,-3.24,;3.52,-1.73,;2.05,-1.26,;.91,-2.29,;1.23,-3.79,;2.69,-4.27,;-.56,-1.81,;-1.03,-.35,;-2.57,-.35,;-3.34,.99,;-4.83,1.39,;-4.43,2.87,;-2.94,2.48,;-5.92,3.27,;-5.52,4.76,;-4.03,4.36,;-6.61,5.85,;-6.21,7.34,;-8.1,5.45,;-9.19,6.54,;-3.05,-1.81,;-4.51,-2.29,;-1.8,-2.72,;-1.8,-4.26,;-3.14,-5.03,;-4.47,-4.26,;-3.14,-6.57,;-4.47,-7.34,;-1.8,-7.34,;-.47,-6.57,;1,-7.04,;1.9,-5.8,;1,-4.55,;-.47,-5.03,)| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20V8HXK |
More data for this
Ligand-Target Pair | |
GTPase KRas [G12C]
(Homo sapiens (Human)) | BDBM608943
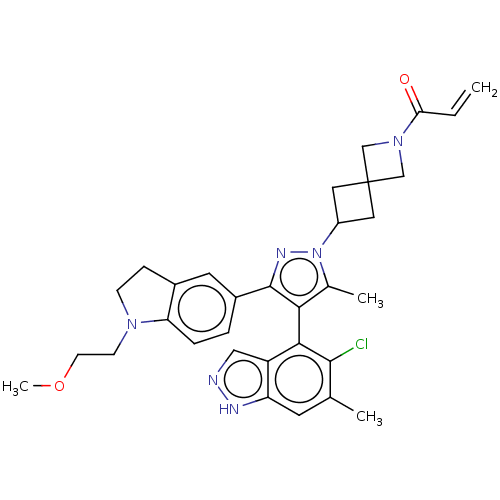
(US11702409, Example 44a | US11702409, Example 44b)Show SMILES COCCN1CCc2cc(ccc12)-c1nn(C2CC3(C2)CN(C3)C(=O)C=C)c(C)c1-c1c(Cl)c(C)cc2[nH]ncc12 |(9.59,-6.07,;8.05,-6.07,;7.28,-4.73,;5.74,-4.73,;4.97,-3.4,;5.59,-1.99,;4.45,-.96,;3.12,-1.73,;1.65,-1.26,;.51,-2.29,;.83,-3.79,;2.29,-4.27,;3.44,-3.24,;-.96,-1.81,;-1.43,-.35,;-2.97,-.35,;-3.74,.99,;-5.23,1.39,;-4.83,2.87,;-3.35,2.48,;-6.32,3.27,;-5.92,4.76,;-4.43,4.36,;-7.01,5.85,;-6.61,7.34,;-8.5,5.45,;-9.59,6.54,;-3.45,-1.81,;-4.91,-2.29,;-2.2,-2.72,;-2.2,-4.26,;-3.54,-5.03,;-4.87,-4.26,;-3.54,-6.57,;-4.87,-7.34,;-2.2,-7.34,;-.87,-6.57,;.59,-7.04,;1.5,-5.8,;.59,-4.55,;-.87,-5.03,)| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20V8HXK |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50203682
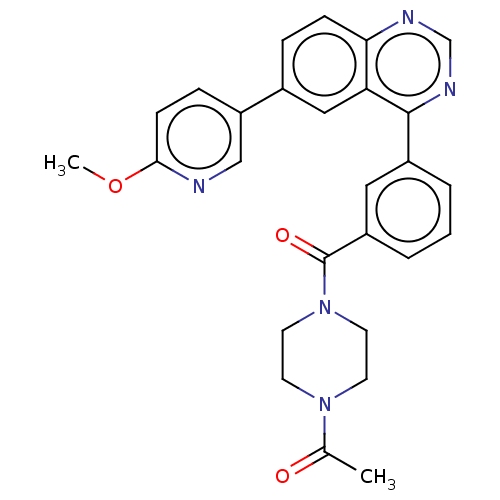
(CHEMBL3947814)Show SMILES COc1ccc(cn1)-c1ccc2ncnc(-c3cccc(c3)C(=O)N3CCN(CC3)C(C)=O)c2c1 Show InChI InChI=1S/C27H25N5O3/c1-18(33)31-10-12-32(13-11-31)27(34)21-5-3-4-20(14-21)26-23-15-19(6-8-24(23)29-17-30-26)22-7-9-25(35-2)28-16-22/h3-9,14-17H,10-13H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3K-delta (unknown origin) by KinaseGlo assay |
Bioorg Med Chem Lett 26: 5657-5662 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.069
BindingDB Entry DOI: 10.7270/Q20Z7574 |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50579985

(JDQ-443 | JDQ443 | Jdq 443 | Jdq-443 | Nvp-jdq-443...)Show SMILES Cc1c(c(nn1C1CC2(C1)CN(C2)C(=O)C=C)-c1ccc2n(C)ncc2c1)-c1c(Cl)c(C)cc2[nH]ncc12 |(8.62,-12.01,;10.09,-11.52,;11.34,-12.43,;12.58,-11.52,;12.1,-10.05,;10.56,-10.06,;9.66,-8.81,;9.89,-7.29,;8.37,-7.05,;8.13,-8.58,;8.77,-5.56,;7.27,-5.16,;6.88,-6.65,;6.5,-3.83,;7.27,-2.49,;4.96,-3.83,;4.19,-2.5,;14.04,-11.99,;15.18,-10.95,;16.64,-11.42,;16.97,-12.93,;18.31,-13.69,;19.71,-13.06,;18,-15.2,;16.47,-15.37,;15.84,-13.97,;14.37,-13.49,;11.35,-13.97,;10.02,-14.74,;8.69,-13.97,;10.02,-16.28,;8.69,-17.05,;11.35,-17.05,;12.69,-16.27,;14.15,-16.75,;15.06,-15.5,;14.15,-14.25,;12.69,-14.73,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM608886
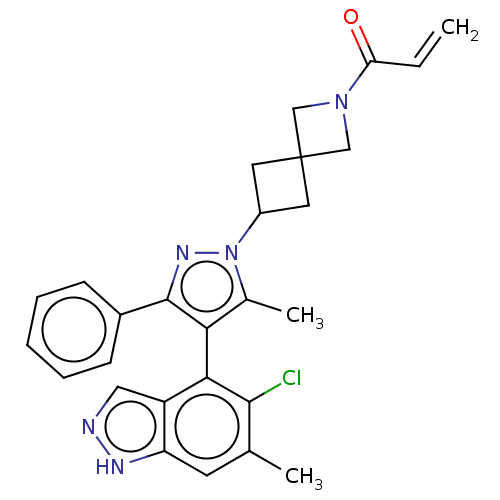
(1-{6-[(4M)-4-(5-Chloro-6- methyl-1H-indazol-4-yl)-...)Show SMILES Cc1c(c(nn1C1CC2(C1)CN(C2)C(=O)C=C)-c1ccccc1)-c1c(Cl)c(C)cc2[nH]ncc12 |(-2.38,-2.83,;-.92,-2.36,;.33,-3.26,;1.57,-2.36,;1.1,-.89,;-.44,-.89,;-1.21,.44,;-2.7,.84,;-2.3,2.33,;-.81,1.93,;-3.79,2.73,;-3.39,4.22,;-1.9,3.82,;-4.48,5.3,;-5.97,4.91,;-4.08,6.79,;-5.17,7.88,;3.04,-2.83,;3.36,-4.34,;4.82,-4.81,;5.97,-3.78,;5.65,-2.28,;4.18,-1.8,;.33,-4.8,;-1.01,-5.57,;-2.34,-4.8,;-1.01,-7.11,;-2.34,-7.88,;.33,-7.88,;1.66,-7.11,;3.13,-7.59,;4.03,-6.34,;3.13,-5.09,;1.66,-5.57,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM608850
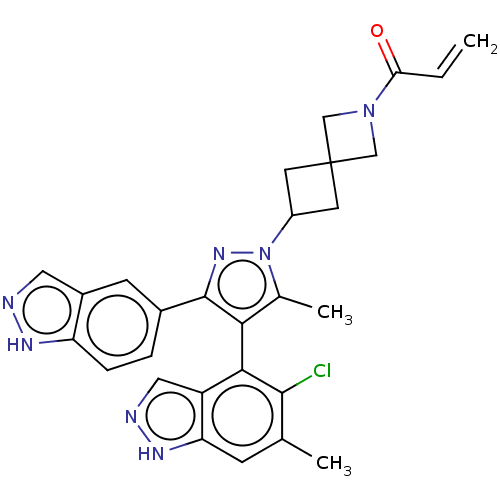
(US11702409, Example 12a | US11702409, Example 12b)Show SMILES Cc1c(c(nn1C1CC2(C1)CN(C2)C(=O)C=C)-c1ccc2[nH]ncc2c1)-c1c(Cl)c(C)cc2[nH]ncc12 |(-2.64,-2.29,;-1.17,-1.81,;.07,-2.72,;1.32,-1.81,;.84,-.35,;-.7,-.35,;-1.47,.99,;-2.95,1.39,;-2.56,2.87,;-1.07,2.48,;-4.04,3.27,;-3.65,4.76,;-2.16,4.36,;-4.73,5.85,;-4.34,7.34,;-6.22,5.45,;-7.31,6.54,;2.78,-2.29,;3.69,-1.04,;5.22,-1.2,;5.85,-2.61,;7.31,-3.08,;7.31,-4.62,;5.85,-5.1,;4.94,-3.85,;3.41,-3.69,;.07,-4.26,;-1.26,-5.03,;-2.59,-4.26,;-1.26,-6.57,;-2.59,-7.34,;.07,-7.34,;1.41,-6.57,;2.87,-7.04,;3.78,-5.8,;2.87,-4.55,;1.41,-5.03,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50203677

(CHEMBL3977135)Show SMILES CCC(=O)N1CC[C@@H](C1)Oc1ccnc2ccc(nc12)-c1ccc(OC)nc1 |r| Show InChI InChI=1S/C21H22N4O3/c1-3-20(26)25-11-9-15(13-25)28-18-8-10-22-17-6-5-16(24-21(17)18)14-4-7-19(27-2)23-12-14/h4-8,10,12,15H,3,9,11,13H2,1-2H3/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3K-delta (unknown origin) by KinaseGlo assay |
Bioorg Med Chem Lett 26: 5657-5662 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.069
BindingDB Entry DOI: 10.7270/Q20Z7574 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50428488
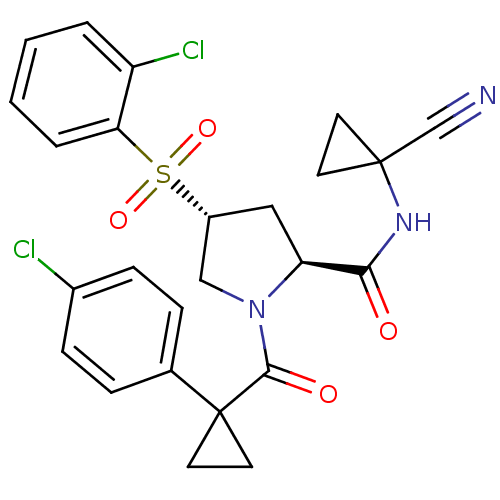
(CHEMBL2335179)Show SMILES Clc1ccc(cc1)C1(CC1)C(=O)N1C[C@@H](C[C@H]1C(=O)NC1(CC1)C#N)S(=O)(=O)c1ccccc1Cl |r| Show InChI InChI=1S/C25H23Cl2N3O4S/c26-17-7-5-16(6-8-17)25(11-12-25)23(32)30-14-18(35(33,34)21-4-2-1-3-19(21)27)13-20(30)22(31)29-24(15-28)9-10-24/h1-8,18,20H,9-14H2,(H,29,31)/t18-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L |
J Med Chem 56: 1363-88 (2013)
Article DOI: 10.1021/jm3012068
BindingDB Entry DOI: 10.7270/Q2GT5PH4 |
More data for this
Ligand-Target Pair | |
GTPase KRas [G12C]
(Homo sapiens (Human)) | BDBM608876
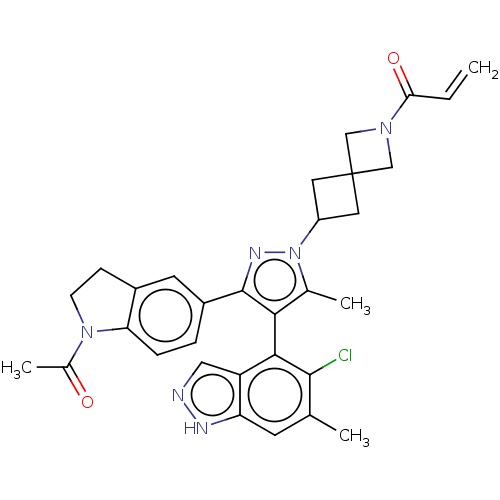
(US11702409, Example 22a | US11702409, Example 22b)Show SMILES CC(=O)N1CCc2cc(ccc12)-c1nn(C2CC3(C2)CN(C3)C(=O)C=C)c(C)c1-c1c(Cl)c(C)cc2[nH]ncc12 |(7.89,-5.28,;6.35,-5.28,;5.58,-6.61,;5.58,-3.94,;6.2,-2.54,;5.06,-1.51,;3.73,-2.28,;2.26,-1.8,;1.12,-2.83,;1.44,-4.34,;2.9,-4.81,;4.05,-3.78,;-.35,-2.36,;-.82,-.89,;-2.36,-.89,;-3.13,.44,;-4.62,.84,;-4.22,2.33,;-2.73,1.93,;-5.71,2.73,;-5.31,4.22,;-3.82,3.82,;-6.4,5.3,;-7.89,4.91,;-6,6.79,;-7.09,7.88,;-2.84,-2.36,;-4.3,-2.83,;-1.59,-3.26,;-1.59,-4.8,;-2.93,-5.57,;-4.26,-4.8,;-2.93,-7.11,;-4.26,-7.88,;-1.59,-7.88,;-.26,-7.11,;1.2,-7.59,;2.11,-6.34,;1.2,-5.09,;-.26,-5.57,)| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20V8HXK |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data