

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hexokinase-4 (Homo sapiens (Human)) | BDBM50346026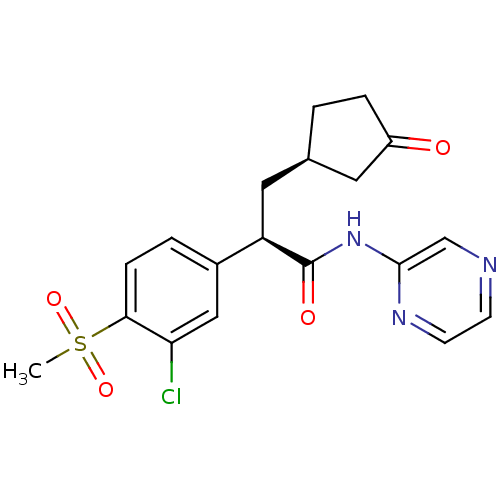 ((R)-2-(3-chloro-4-(methylsulfonyl)phenyl)-3-((R)-3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | 830 | n/a | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Displacement of TAFMT from human pancreatic N-terminal His6-tagged glucokinase isoform 1 expressed in Escherichia coli BL21(DE3) by stopped-flow fluo... | J Med Chem 59: 7167-76 (2016) Article DOI: 10.1021/acs.jmedchem.6b00632 BindingDB Entry DOI: 10.7270/Q2Z60SHM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Hexokinase-4 (Homo sapiens (Human)) | BDBM50533461 (CHEMBL4483049) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Displacement of TAFMT from human pancreatic N-terminal His6-tagged glucokinase isoform 1 expressed in Escherichia coli BL21(DE3) by stopped-flow fluo... | J Med Chem 59: 7167-76 (2016) Article DOI: 10.1021/acs.jmedchem.6b00632 BindingDB Entry DOI: 10.7270/Q2Z60SHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Homo sapiens (Human)) | BDBM50533462 (CHEMBL4534484) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Displacement of TAFMT from human pancreatic N-terminal His6-tagged glucokinase isoform 1 expressed in Escherichia coli BL21(DE3) by stopped-flow fluo... | J Med Chem 59: 7167-76 (2016) Article DOI: 10.1021/acs.jmedchem.6b00632 BindingDB Entry DOI: 10.7270/Q2Z60SHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Homo sapiens (Human)) | BDBM50533463 (CHEMBL4522750) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Displacement of TAFMT from human pancreatic N-terminal His6-tagged glucokinase isoform 1 expressed in Escherichia coli BL21(DE3) by stopped-flow fluo... | J Med Chem 59: 7167-76 (2016) Article DOI: 10.1021/acs.jmedchem.6b00632 BindingDB Entry DOI: 10.7270/Q2Z60SHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Homo sapiens (Human)) | BDBM34071 (benzamide derivative, 6) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Binding affinity to human pancreatic N-terminal His6-tagged glucokinase isoform 1 expressed in Escherichia coli BL21(DE3) by stopped-flow fluorometri... | J Med Chem 59: 7167-76 (2016) Article DOI: 10.1021/acs.jmedchem.6b00632 BindingDB Entry DOI: 10.7270/Q2Z60SHM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236342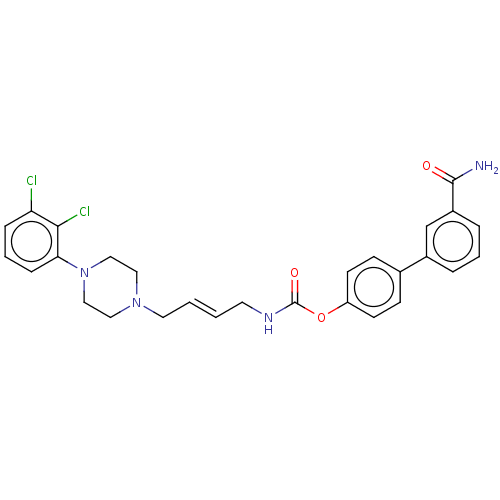 (CHEMBL4065510) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 38 | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human dopamine D3 receptor expressed in CHO cells assessed as inhibition of cAMP accumulation by flourescence assay | Citation and Details Article DOI: 10.1016/j.ejmech.2019.111975 BindingDB Entry DOI: 10.7270/Q261142R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Homo sapiens (Human)) | BDBM50533464 (CHEMBL4457828) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Displacement of TAFMT from human pancreatic N-terminal His6-tagged glucokinase isoform 1 expressed in Escherichia coli BL21(DE3) by stopped-flow fluo... | J Med Chem 59: 7167-76 (2016) Article DOI: 10.1021/acs.jmedchem.6b00632 BindingDB Entry DOI: 10.7270/Q2Z60SHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50564049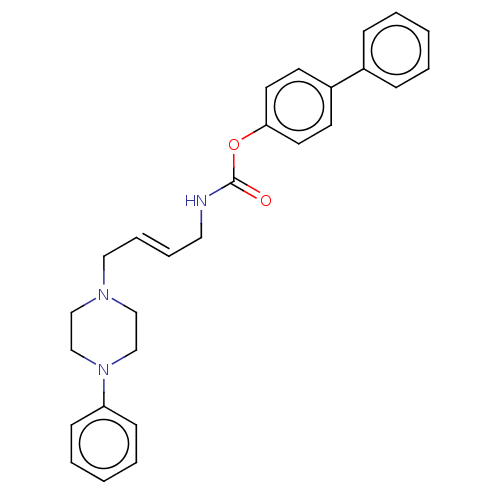 (CHEMBL4785109) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 23 | n/a | n/a | n/a | n/a |
TBA | Assay Description Partial agonist activity at human dopamine D3 receptor expressed in CHO cells assessed as inhibition of cAMP accumulation by flourescence assay | Citation and Details Article DOI: 10.1016/j.ejmech.2019.111975 BindingDB Entry DOI: 10.7270/Q261142R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236333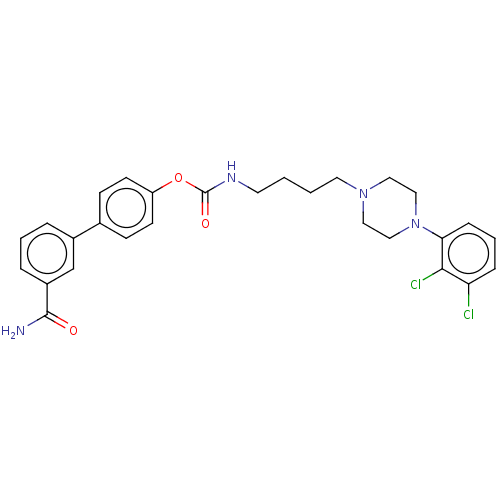 (CHEMBL4092052) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
TBA | Assay Description Partial agonist activity at human dopamine D3 receptor expressed in CHO cells assessed as inhibition of cAMP accumulation by flourescence assay | Citation and Details Article DOI: 10.1016/j.ejmech.2019.111975 BindingDB Entry DOI: 10.7270/Q261142R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50564050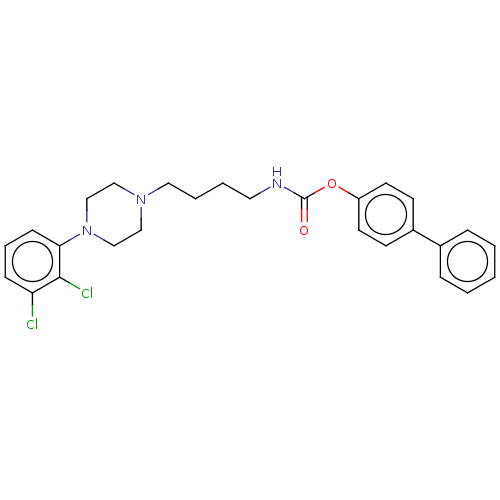 (CHEMBL4117318) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 18 | n/a | n/a | n/a | n/a |
TBA | Assay Description Partial agonist activity at human dopamine D3 receptor expressed in CHO cells assessed as inhibition of cAMP accumulation by flourescence assay | Citation and Details Article DOI: 10.1016/j.ejmech.2019.111975 BindingDB Entry DOI: 10.7270/Q261142R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236352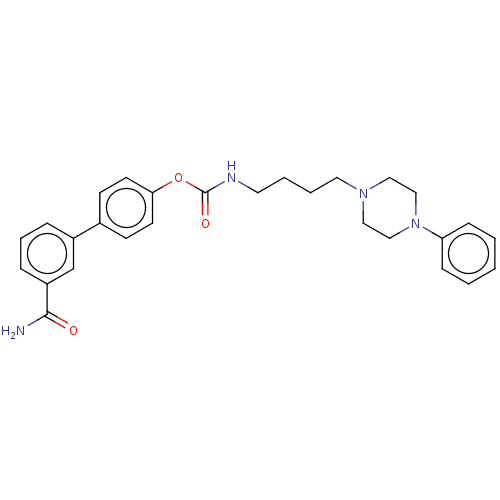 (CHEMBL4090581) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a |
TBA | Assay Description Partial agonist activity at human dopamine D3 receptor expressed in CHO cells assessed as inhibition of cAMP accumulation by flourescence assay | Citation and Details Article DOI: 10.1016/j.ejmech.2019.111975 BindingDB Entry DOI: 10.7270/Q261142R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Homo sapiens (Human)) | BDBM50320996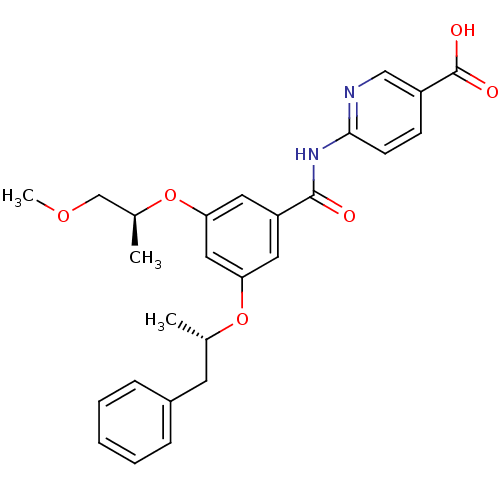 (6-(3-((S)-1-methoxypropan-2-yloxy)-5-((S)-1-phenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Displacement of TAFMT from human pancreatic N-terminal His6-tagged glucokinase isoform 1 expressed in Escherichia coli BL21(DE3) by stopped-flow fluo... | J Med Chem 59: 7167-76 (2016) Article DOI: 10.1021/acs.jmedchem.6b00632 BindingDB Entry DOI: 10.7270/Q2Z60SHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||