

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P2Y purinoceptor 14 (Homo sapiens (Human)) | BDBM50512416 (CHEMBL4447162) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of human P2Y14R | J Med Chem 63: 9563-9589 (2020) Article DOI: 10.1021/acs.jmedchem.0c00745 BindingDB Entry DOI: 10.7270/Q20R9SZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Rattus norvegicus) | BDBM50456168 (CHEMBL1800685) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Antagonist activity at P2Y14R in rat C6 cells assessed as suppression of UDP-glucose-mediated inhibition of forskolin-stimulated [3H]cyclic-AMP accum... | J Med Chem 61: 4860-4882 (2018) Article DOI: 10.1021/acs.jmedchem.8b00168 BindingDB Entry DOI: 10.7270/Q2X069NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50456166 (CHEMBL4216870) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]Rauwolscine from human adrenergic alpha2A receptor expressed in MDCK cell membranes after 90 mins by scintillation counting metho... | J Med Chem 61: 4860-4882 (2018) Article DOI: 10.1021/acs.jmedchem.8b00168 BindingDB Entry DOI: 10.7270/Q2X069NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50456166 (CHEMBL4216870) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 365 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [125I]-Iodoaminopotentidine from human histamine H2 receptor expressed in HEK293T cell membranes after 90 mins by scintillation count... | J Med Chem 61: 4860-4882 (2018) Article DOI: 10.1021/acs.jmedchem.8b00168 BindingDB Entry DOI: 10.7270/Q2X069NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50543252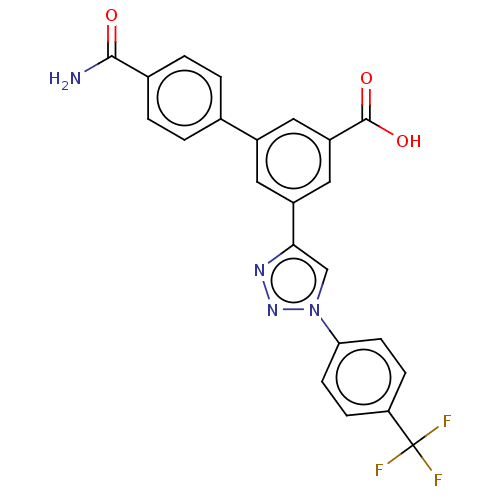 (CHEMBL4642215) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to sigma 1 receptor (unknown origin) | J Med Chem 63: 9563-9589 (2020) Article DOI: 10.1021/acs.jmedchem.0c00745 BindingDB Entry DOI: 10.7270/Q20R9SZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Homo sapiens (Human)) | BDBM50541998 (CHEMBL4642592) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to TSPO receptor (unknown origin) | J Med Chem 63: 9563-9589 (2020) Article DOI: 10.1021/acs.jmedchem.0c00745 BindingDB Entry DOI: 10.7270/Q20R9SZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50456162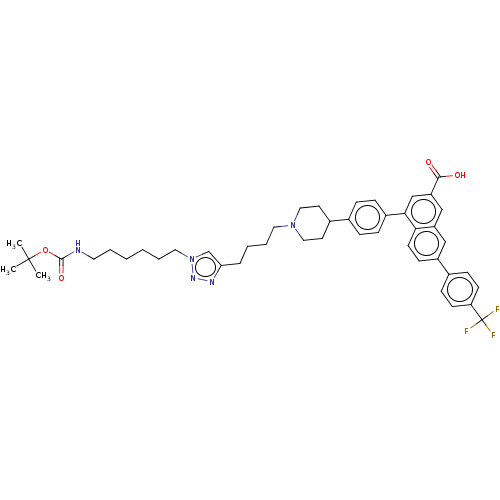 (CHEMBL4217398) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 829 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [125I]-Iodoaminopotentidine from human histamine H2 receptor expressed in HEK293T cell membranes after 90 mins by scintillation count... | J Med Chem 61: 4860-4882 (2018) Article DOI: 10.1021/acs.jmedchem.8b00168 BindingDB Entry DOI: 10.7270/Q2X069NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50456169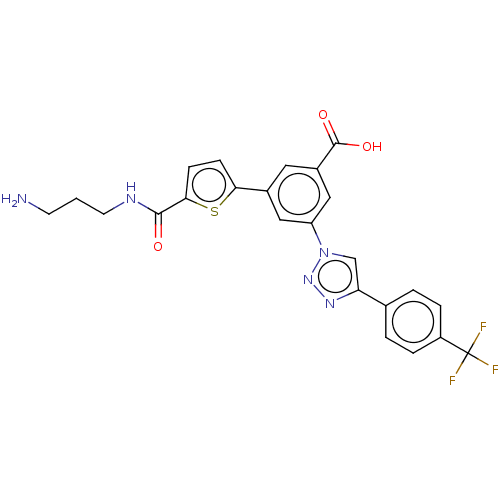 (CHEMBL4212721) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 941 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]-DADLE from human delta opioid receptor expressed in HEK293 cells after 90 mins by scintillation counting method | J Med Chem 61: 4860-4882 (2018) Article DOI: 10.1021/acs.jmedchem.8b00168 BindingDB Entry DOI: 10.7270/Q2X069NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50541996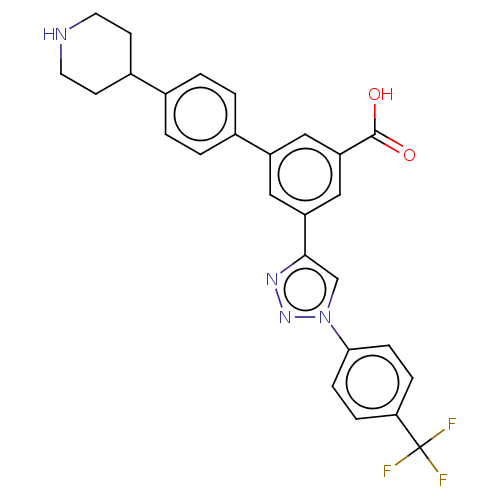 (CHEMBL4633982) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to adrenergic alpha2A receptor (unknown origin) | J Med Chem 63: 9563-9589 (2020) Article DOI: 10.1021/acs.jmedchem.0c00745 BindingDB Entry DOI: 10.7270/Q20R9SZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2B adrenergic receptor (Homo sapiens (Human)) | BDBM50456166 (CHEMBL4216870) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 984 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]Rauwolscine from human adrenergic alpha2B receptor expressed in HEK293T cell membranes after 90 mins by scintillation counting me... | J Med Chem 61: 4860-4882 (2018) Article DOI: 10.1021/acs.jmedchem.8b00168 BindingDB Entry DOI: 10.7270/Q2X069NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1B) dopamine receptor (Homo sapiens (Human)) | BDBM50456166 (CHEMBL4216870) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]SCH23390 from human dopamine D5 receptor expressed in HEK293T cell membranes after 90 mins by scintillation counting method | J Med Chem 61: 4860-4882 (2018) Article DOI: 10.1021/acs.jmedchem.8b00168 BindingDB Entry DOI: 10.7270/Q2X069NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50456166 (CHEMBL4216870) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]-WIN35428 from human DAT after 90 mins by scintillation counting method | J Med Chem 61: 4860-4882 (2018) Article DOI: 10.1021/acs.jmedchem.8b00168 BindingDB Entry DOI: 10.7270/Q2X069NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50543267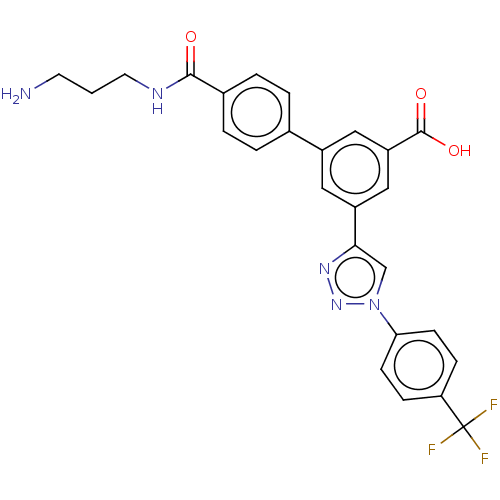 (CHEMBL4647020) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to sigma 1 receptor (unknown origin) | J Med Chem 63: 9563-9589 (2020) Article DOI: 10.1021/acs.jmedchem.0c00745 BindingDB Entry DOI: 10.7270/Q20R9SZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1B adrenergic receptor (Homo sapiens (Human)) | BDBM50456166 (CHEMBL4216870) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]-Prazosin from human adrenergic alpha1B receptor after 90 mins by scintillation counting method | J Med Chem 61: 4860-4882 (2018) Article DOI: 10.1021/acs.jmedchem.8b00168 BindingDB Entry DOI: 10.7270/Q2X069NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma intracellular receptor 2 (Homo sapiens (Human)) | BDBM50456166 (CHEMBL4216870) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]-DTG from human sigma2 receptor expressed in HEK293T cell membranes after 90 mins by scintillation counting method | J Med Chem 61: 4860-4882 (2018) Article DOI: 10.1021/acs.jmedchem.8b00168 BindingDB Entry DOI: 10.7270/Q2X069NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1D adrenergic receptor (Homo sapiens (Human)) | BDBM50456166 (CHEMBL4216870) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]-Prazosin from human adrenergic alpha1D receptor after 90 mins by scintillation counting method | J Med Chem 61: 4860-4882 (2018) Article DOI: 10.1021/acs.jmedchem.8b00168 BindingDB Entry DOI: 10.7270/Q2X069NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50456191 (CHEMBL4211068) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]-DADLE from human delta opioid receptor expressed in HEK293 cells after 90 mins by scintillation counting method | J Med Chem 61: 4860-4882 (2018) Article DOI: 10.1021/acs.jmedchem.8b00168 BindingDB Entry DOI: 10.7270/Q2X069NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50541996 (CHEMBL4633982) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to sigma 1 receptor (unknown origin) | J Med Chem 63: 9563-9589 (2020) Article DOI: 10.1021/acs.jmedchem.0c00745 BindingDB Entry DOI: 10.7270/Q20R9SZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50456166 (CHEMBL4216870) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]N-methylspiperone from human dopamine D3 receptor expressed in HEK293T cell membranes after 90 mins by scintillation counting met... | J Med Chem 61: 4860-4882 (2018) Article DOI: 10.1021/acs.jmedchem.8b00168 BindingDB Entry DOI: 10.7270/Q2X069NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50541998 (CHEMBL4642592) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to 5HT1D receptor (unknown origin) | J Med Chem 63: 9563-9589 (2020) Article DOI: 10.1021/acs.jmedchem.0c00745 BindingDB Entry DOI: 10.7270/Q20R9SZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50456166 (CHEMBL4216870) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]-Prazosin from human adrenergic alpha1A receptor after 90 mins by scintillation counting method | J Med Chem 61: 4860-4882 (2018) Article DOI: 10.1021/acs.jmedchem.8b00168 BindingDB Entry DOI: 10.7270/Q2X069NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50456166 (CHEMBL4216870) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.87E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]-Nisoxetine from human NET expressed in HEK293 cell membranes after 90 mins by scintillation counting method | J Med Chem 61: 4860-4882 (2018) Article DOI: 10.1021/acs.jmedchem.8b00168 BindingDB Entry DOI: 10.7270/Q2X069NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50456162 (CHEMBL4217398) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]-Citalopram from human SERT expressed in HEK293 cell membranes after 90 mins by scintillation counting method | J Med Chem 61: 4860-4882 (2018) Article DOI: 10.1021/acs.jmedchem.8b00168 BindingDB Entry DOI: 10.7270/Q2X069NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50456162 (CHEMBL4217398) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]-CGP12177 from human adrenergic beta-3 receptor expressed in HEK Flp-In cell membranes after 90 mins by scintillation counting me... | J Med Chem 61: 4860-4882 (2018) Article DOI: 10.1021/acs.jmedchem.8b00168 BindingDB Entry DOI: 10.7270/Q2X069NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50543252 (CHEMBL4642215) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to DOR (unknown origin) | J Med Chem 63: 9563-9589 (2020) Article DOI: 10.1021/acs.jmedchem.0c00745 BindingDB Entry DOI: 10.7270/Q20R9SZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50543253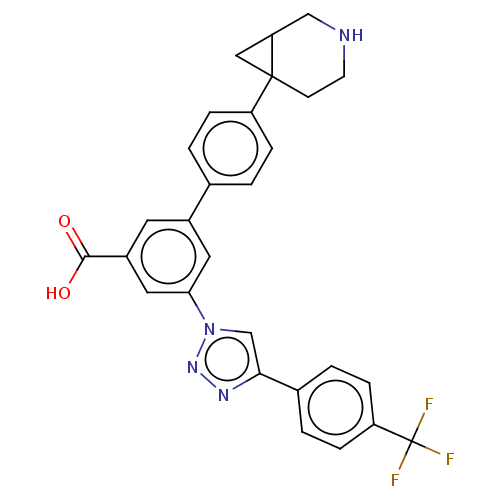 (CHEMBL4637158) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to DOR (unknown origin) | J Med Chem 63: 9563-9589 (2020) Article DOI: 10.1021/acs.jmedchem.0c00745 BindingDB Entry DOI: 10.7270/Q20R9SZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM50541996 (CHEMBL4633982) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to adrenergic alpha2C receptor (unknown origin) | J Med Chem 63: 9563-9589 (2020) Article DOI: 10.1021/acs.jmedchem.0c00745 BindingDB Entry DOI: 10.7270/Q20R9SZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50456168 (CHEMBL1800685) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 2.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to DOR (unknown origin) | J Med Chem 63: 9563-9589 (2020) Article DOI: 10.1021/acs.jmedchem.0c00745 BindingDB Entry DOI: 10.7270/Q20R9SZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50456162 (CHEMBL4217398) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]-alpha-methylhistamine from human histamine H3 receptor expressed in HEK Flp-In cell membranes after 90 mins by scintillation cou... | J Med Chem 61: 4860-4882 (2018) Article DOI: 10.1021/acs.jmedchem.8b00168 BindingDB Entry DOI: 10.7270/Q2X069NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50456162 (CHEMBL4217398) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]-LSD from human 5-HT2B receptor expressed in HEK293 cell membranes after 90 mins by scintillation counting method | J Med Chem 61: 4860-4882 (2018) Article DOI: 10.1021/acs.jmedchem.8b00168 BindingDB Entry DOI: 10.7270/Q2X069NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50543267 (CHEMBL4647020) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to DOR (unknown origin) | J Med Chem 63: 9563-9589 (2020) Article DOI: 10.1021/acs.jmedchem.0c00745 BindingDB Entry DOI: 10.7270/Q20R9SZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50456169 (CHEMBL4212721) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to DOR (unknown origin) | J Med Chem 63: 9563-9589 (2020) Article DOI: 10.1021/acs.jmedchem.0c00745 BindingDB Entry DOI: 10.7270/Q20R9SZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50456178 (CHEMBL4214536) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]-DADLE from human delta opioid receptor expressed in HEK293 cells after 90 mins by scintillation counting method | J Med Chem 61: 4860-4882 (2018) Article DOI: 10.1021/acs.jmedchem.8b00168 BindingDB Entry DOI: 10.7270/Q2X069NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50541996 (CHEMBL4633982) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to DOR (unknown origin) | J Med Chem 63: 9563-9589 (2020) Article DOI: 10.1021/acs.jmedchem.0c00745 BindingDB Entry DOI: 10.7270/Q20R9SZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50541996 (CHEMBL4633982) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to DOR (unknown origin) | J Med Chem 63: 9563-9589 (2020) Article DOI: 10.1021/acs.jmedchem.0c00745 BindingDB Entry DOI: 10.7270/Q20R9SZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50456162 (CHEMBL4217398) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]-U69593 from human KOR expressed in HEK293 cell membranes after 90 mins by scintillation counting method | J Med Chem 61: 4860-4882 (2018) Article DOI: 10.1021/acs.jmedchem.8b00168 BindingDB Entry DOI: 10.7270/Q2X069NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50541998 (CHEMBL4642592) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to DRD3 (unknown origin) | J Med Chem 63: 9563-9589 (2020) Article DOI: 10.1021/acs.jmedchem.0c00745 BindingDB Entry DOI: 10.7270/Q20R9SZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50456169 (CHEMBL4212721) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to 5HT1D receptor (unknown origin) | J Med Chem 63: 9563-9589 (2020) Article DOI: 10.1021/acs.jmedchem.0c00745 BindingDB Entry DOI: 10.7270/Q20R9SZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1B) dopamine receptor (Homo sapiens (Human)) | BDBM50541998 (CHEMBL4642592) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to dopamine D5 receptor (unknown origin) | J Med Chem 63: 9563-9589 (2020) Article DOI: 10.1021/acs.jmedchem.0c00745 BindingDB Entry DOI: 10.7270/Q20R9SZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50456162 (CHEMBL4217398) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]Rauwolscine from human adrenergic alpha2A receptor expressed in MDCK cell membranes after 90 mins by scintillation counting metho... | J Med Chem 61: 4860-4882 (2018) Article DOI: 10.1021/acs.jmedchem.8b00168 BindingDB Entry DOI: 10.7270/Q2X069NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2B adrenergic receptor (Homo sapiens (Human)) | BDBM50541996 (CHEMBL4633982) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to adrenergic alpha2B receptor (unknown origin) | J Med Chem 63: 9563-9589 (2020) Article DOI: 10.1021/acs.jmedchem.0c00745 BindingDB Entry DOI: 10.7270/Q20R9SZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50456162 (CHEMBL4217398) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]-PK11195 from rat brain TSPO after 90 mins by scintillation counting method | J Med Chem 61: 4860-4882 (2018) Article DOI: 10.1021/acs.jmedchem.8b00168 BindingDB Entry DOI: 10.7270/Q2X069NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50456166 (CHEMBL4216870) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]-Way100635 from human 5-HT1A receptor expressed in CHO cell membranes after 90 mins by scintillation counting method | J Med Chem 61: 4860-4882 (2018) Article DOI: 10.1021/acs.jmedchem.8b00168 BindingDB Entry DOI: 10.7270/Q2X069NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50456190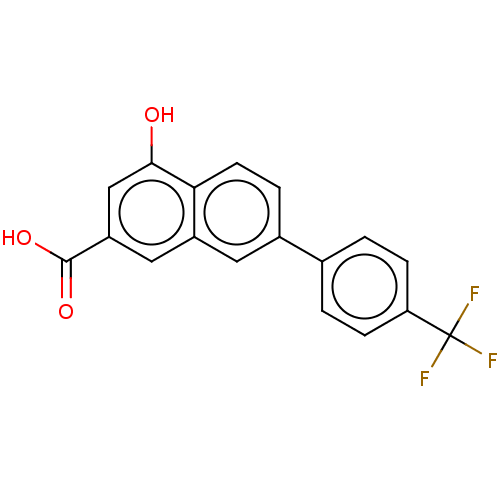 (CHEMBL4205190) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]-Nisoxetine from human NET expressed in HEK293 cell membranes after 90 mins by scintillation counting method | J Med Chem 61: 4860-4882 (2018) Article DOI: 10.1021/acs.jmedchem.8b00168 BindingDB Entry DOI: 10.7270/Q2X069NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50456168 (CHEMBL1800685) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 6.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to DRD3 (unknown origin) | J Med Chem 63: 9563-9589 (2020) Article DOI: 10.1021/acs.jmedchem.0c00745 BindingDB Entry DOI: 10.7270/Q20R9SZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50456166 (CHEMBL4216870) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]SCH23390 from human dopamine D1 receptor expressed in HEK293T cell membranes after 90 mins by scintillation counting method | J Med Chem 61: 4860-4882 (2018) Article DOI: 10.1021/acs.jmedchem.8b00168 BindingDB Entry DOI: 10.7270/Q2X069NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50456162 (CHEMBL4217398) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]-LSD from human 5-HT6 receptor expressed in HEK293 cell membranes after 90 mins by scintillation counting method | J Med Chem 61: 4860-4882 (2018) Article DOI: 10.1021/acs.jmedchem.8b00168 BindingDB Entry DOI: 10.7270/Q2X069NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50456166 (CHEMBL4216870) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]N-methylspiperone from human dopamine D2 receptor expressed in HEK293T cell membranes after 90 mins by scintillation counting met... | J Med Chem 61: 4860-4882 (2018) Article DOI: 10.1021/acs.jmedchem.8b00168 BindingDB Entry DOI: 10.7270/Q2X069NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50456166 (CHEMBL4216870) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]-Ketanserin from human 5-HT2A receptor expressed in HEK293T cell membranes after 90 mins by scintillation counting method | J Med Chem 61: 4860-4882 (2018) Article DOI: 10.1021/acs.jmedchem.8b00168 BindingDB Entry DOI: 10.7270/Q2X069NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Homo sapiens (Human)) | BDBM50456168 (CHEMBL1800685) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of MRS4174 from human P2Y14R expressed in CHO cells preincubated for 30 mins followed by MRS4174 addition measured after 30 mins by flow... | J Med Chem 61: 4860-4882 (2018) Article DOI: 10.1021/acs.jmedchem.8b00168 BindingDB Entry DOI: 10.7270/Q2X069NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 162 total ) | Next | Last >> |