 Found 56 hits with Last Name = 'jatheendranath' and Initial = 's'
Found 56 hits with Last Name = 'jatheendranath' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50020412

(CHEMBL3289811)Show SMILES Nc1nc2ccc(OC3CCOC3)nc2n1CC(O)c1ccc(cc1Cl)C(F)(F)F Show InChI InChI=1S/C19H18ClF3N4O3/c20-13-7-10(19(21,22)23)1-2-12(13)15(28)8-27-17-14(25-18(27)24)3-4-16(26-17)30-11-5-6-29-9-11/h1-4,7,11,15,28H,5-6,8-9H2,(H2,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50020412

(CHEMBL3289811)Show SMILES Nc1nc2ccc(OC3CCOC3)nc2n1CC(O)c1ccc(cc1Cl)C(F)(F)F Show InChI InChI=1S/C19H18ClF3N4O3/c20-13-7-10(19(21,22)23)1-2-12(13)15(28)8-27-17-14(25-18(27)24)3-4-16(26-17)30-11-5-6-29-9-11/h1-4,7,11,15,28H,5-6,8-9H2,(H2,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50020404

(CHEMBL3289803)Show SMILES Nc1nc2ccc(cc2n1CC(O)c1ccc(cc1Cl)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C17H12ClF6N3O/c18-11-5-8(16(19,20)21)1-3-10(11)14(28)7-27-13-6-9(17(22,23)24)2-4-12(13)26-15(27)25/h1-6,14,28H,7H2,(H2,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50020413
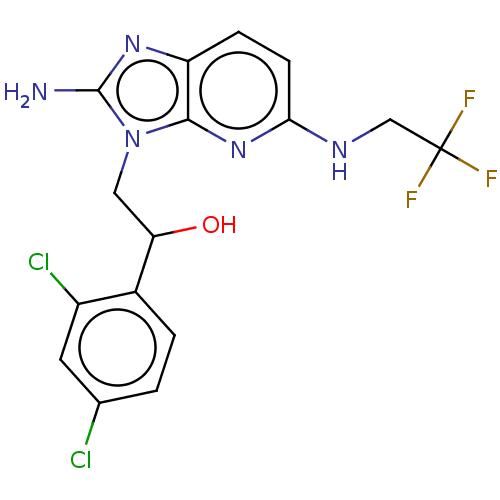
(CHEMBL3289807)Show SMILES Nc1nc2ccc(NCC(F)(F)F)nc2n1CC(O)c1ccc(Cl)cc1Cl Show InChI InChI=1S/C16H14Cl2F3N5O/c17-8-1-2-9(10(18)5-8)12(27)6-26-14-11(24-15(26)22)3-4-13(25-14)23-7-16(19,20)21/h1-5,12,27H,6-7H2,(H2,22,24)(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50020414

(CHEMBL3289797)Show InChI InChI=1S/C14H15Cl2N3O/c15-9-3-4-10(11(16)5-9)13(20)7-19-6-12(8-1-2-8)18-14(19)17/h3-6,8,13,20H,1-2,7H2,(H2,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50020411

(CHEMBL3289813)Show SMILES CC(Oc1ccc2nc(N)n(CC(O)c3ccc(cc3Cl)C(F)(F)F)c2n1)C(F)(F)F Show InChI InChI=1S/C18H15ClF6N4O2/c1-8(17(20,21)22)31-14-5-4-12-15(28-14)29(16(26)27-12)7-13(30)10-3-2-9(6-11(10)19)18(23,24)25/h2-6,8,13,30H,7H2,1H3,(H2,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50020415
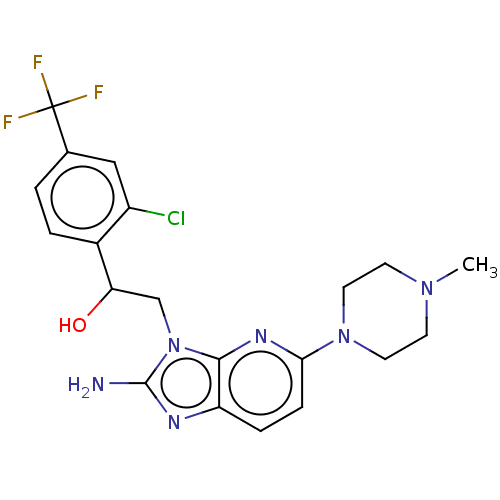
(CHEMBL3289806)Show SMILES CN1CCN(CC1)c1ccc2nc(N)n(CC(O)c3ccc(cc3Cl)C(F)(F)F)c2n1 Show InChI InChI=1S/C20H22ClF3N6O/c1-28-6-8-29(9-7-28)17-5-4-15-18(27-17)30(19(25)26-15)11-16(31)13-3-2-12(10-14(13)21)20(22,23)24/h2-5,10,16,31H,6-9,11H2,1H3,(H2,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50020408

(CHEMBL3289805)Show InChI InChI=1S/C14H12Cl2N4O/c15-9-4-1-3-8(12(9)16)11(21)7-20-13-10(19-14(20)17)5-2-6-18-13/h1-6,11,21H,7H2,(H2,17,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50019514

(CHEMBL3290772)Show SMILES Cn1ncc(C(=O)N2CCC(CC2)Nc2cc(=O)n(C)c3ccccc23)c1Cl Show InChI InChI=1S/C20H22ClN5O2/c1-24-17-6-4-3-5-14(17)16(11-18(24)27)23-13-7-9-26(10-8-13)20(28)15-12-22-25(2)19(15)21/h3-6,11-13,23H,7-10H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of adenosine A1 receptor (unknown origin) |
J Med Chem 57: 5419-34 (2014)
Article DOI: 10.1021/jm5005978
BindingDB Entry DOI: 10.7270/Q25Q4XNM |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50019516

(CHEMBL3291063)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)Nc1n[nH]c3ccc(F)cc13)N2C(=O)c1cnn(C)c1Cl |r,TLB:21:20:2.3:7.6.8,9:7:20:2.3| Show InChI InChI=1S/C19H20ClFN6O/c1-26-17(20)15(9-22-26)19(28)27-12-3-4-13(27)8-11(7-12)23-18-14-6-10(21)2-5-16(14)24-25-18/h2,5-6,9,11-13H,3-4,7-8H2,1H3,(H2,23,24,25)/t11-,12+,13- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of adenosine A1 receptor (unknown origin) |
J Med Chem 57: 5419-34 (2014)
Article DOI: 10.1021/jm5005978
BindingDB Entry DOI: 10.7270/Q25Q4XNM |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50019515

(CHEMBL3291062)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)Nc1cc(=O)n(C)c3cc(F)c(F)cc13)N2C(=O)c1noc(C)c1C |r,TLB:25:24:6.7.8:2.3,9:7:24:2.3| Show InChI InChI=1S/C23H24F2N4O3/c1-11-12(2)32-27-22(11)23(31)29-14-4-5-15(29)7-13(6-14)26-19-10-21(30)28(3)20-9-18(25)17(24)8-16(19)20/h8-10,13-15,26H,4-7H2,1-3H3/t13-,14+,15- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of adenosine A1 receptor (unknown origin) |
J Med Chem 57: 5419-34 (2014)
Article DOI: 10.1021/jm5005978
BindingDB Entry DOI: 10.7270/Q25Q4XNM |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50019519
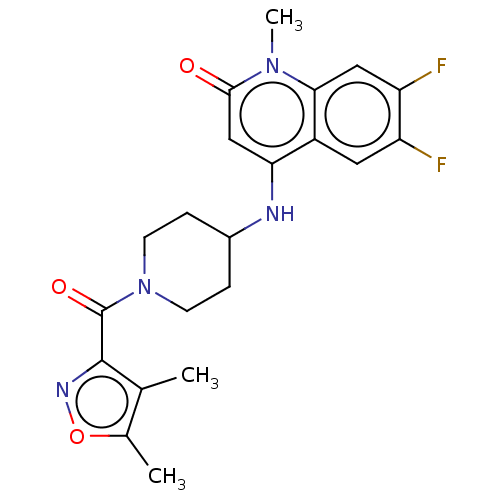
(CHEMBL3291056)Show SMILES Cc1onc(C(=O)N2CCC(CC2)Nc2cc(=O)n(C)c3cc(F)c(F)cc23)c1C Show InChI InChI=1S/C21H22F2N4O3/c1-11-12(2)30-25-20(11)21(29)27-6-4-13(5-7-27)24-17-10-19(28)26(3)18-9-16(23)15(22)8-14(17)18/h8-10,13,24H,4-7H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of adenosine A1 receptor (unknown origin) |
J Med Chem 57: 5419-34 (2014)
Article DOI: 10.1021/jm5005978
BindingDB Entry DOI: 10.7270/Q25Q4XNM |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50020408

(CHEMBL3289805)Show InChI InChI=1S/C14H12Cl2N4O/c15-9-4-1-3-8(12(9)16)11(21)7-20-13-10(19-14(20)17)5-2-6-18-13/h1-6,11,21H,7H2,(H2,17,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50020416
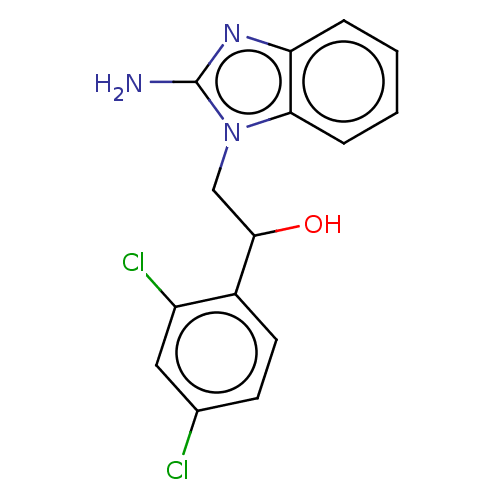
(CHEMBL3289799)Show InChI InChI=1S/C15H13Cl2N3O/c16-9-5-6-10(11(17)7-9)14(21)8-20-13-4-2-1-3-12(13)19-15(20)18/h1-7,14,21H,8H2,(H2,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50020404

(CHEMBL3289803)Show SMILES Nc1nc2ccc(cc2n1CC(O)c1ccc(cc1Cl)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C17H12ClF6N3O/c18-11-5-8(16(19,20)21)1-3-10(11)14(28)7-27-13-6-9(17(22,23)24)2-4-12(13)26-15(27)25/h1-6,14,28H,7H2,(H2,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | >1.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50020415

(CHEMBL3289806)Show SMILES CN1CCN(CC1)c1ccc2nc(N)n(CC(O)c3ccc(cc3Cl)C(F)(F)F)c2n1 Show InChI InChI=1S/C20H22ClF3N6O/c1-28-6-8-29(9-7-28)17-5-4-15-18(27-17)30(19(25)26-15)11-16(31)13-3-2-12(10-14(13)21)20(22,23)24/h2-5,10,16,31H,6-9,11H2,1H3,(H2,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50020412

(CHEMBL3289811)Show SMILES Nc1nc2ccc(OC3CCOC3)nc2n1CC(O)c1ccc(cc1Cl)C(F)(F)F Show InChI InChI=1S/C19H18ClF3N4O3/c20-13-7-10(19(21,22)23)1-2-12(13)15(28)8-27-17-14(25-18(27)24)3-4-16(26-17)30-11-5-6-29-9-11/h1-4,7,11,15,28H,5-6,8-9H2,(H2,24,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50020415

(CHEMBL3289806)Show SMILES CN1CCN(CC1)c1ccc2nc(N)n(CC(O)c3ccc(cc3Cl)C(F)(F)F)c2n1 Show InChI InChI=1S/C20H22ClF3N6O/c1-28-6-8-29(9-7-28)17-5-4-15-18(27-17)30(19(25)26-15)11-16(31)13-3-2-12(10-14(13)21)20(22,23)24/h2-5,10,16,31H,6-9,11H2,1H3,(H2,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50019515

(CHEMBL3291062)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)Nc1cc(=O)n(C)c3cc(F)c(F)cc13)N2C(=O)c1noc(C)c1C |r,TLB:25:24:6.7.8:2.3,9:7:24:2.3| Show InChI InChI=1S/C23H24F2N4O3/c1-11-12(2)32-27-22(11)23(31)29-14-4-5-15(29)7-13(6-14)26-19-10-21(30)28(3)20-9-18(25)17(24)8-16(19)20/h8-10,13-15,26H,4-7H2,1-3H3/t13-,14+,15- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by medium-throughput electrophysiology |
J Med Chem 57: 5419-34 (2014)
Article DOI: 10.1021/jm5005978
BindingDB Entry DOI: 10.7270/Q25Q4XNM |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50020412

(CHEMBL3289811)Show SMILES Nc1nc2ccc(OC3CCOC3)nc2n1CC(O)c1ccc(cc1Cl)C(F)(F)F Show InChI InChI=1S/C19H18ClF3N4O3/c20-13-7-10(19(21,22)23)1-2-12(13)15(28)8-27-17-14(25-18(27)24)3-4-16(26-17)30-11-5-6-29-9-11/h1-4,7,11,15,28H,5-6,8-9H2,(H2,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.66E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50020411

(CHEMBL3289813)Show SMILES CC(Oc1ccc2nc(N)n(CC(O)c3ccc(cc3Cl)C(F)(F)F)c2n1)C(F)(F)F Show InChI InChI=1S/C18H15ClF6N4O2/c1-8(17(20,21)22)31-14-5-4-12-15(28-14)29(16(26)27-12)7-13(30)10-3-2-9(6-11(10)19)18(23,24)25/h2-6,8,13,30H,7H2,1H3,(H2,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50019511

(CHEMBL3290766)Show SMILES Cn1ncc(C(=O)N2CCC(CC2)Nc2cc(=O)[nH]c3ccccc23)c1Cl Show InChI InChI=1S/C19H20ClN5O2/c1-24-18(20)14(11-21-24)19(27)25-8-6-12(7-9-25)22-16-10-17(26)23-15-5-3-2-4-13(15)16/h2-5,10-12H,6-9H2,1H3,(H2,22,23,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of mu opioid receptor (unknown origin) |
J Med Chem 57: 5419-34 (2014)
Article DOI: 10.1021/jm5005978
BindingDB Entry DOI: 10.7270/Q25Q4XNM |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50020417

(CHEMBL3289794)Show InChI InChI=1S/C11H11Cl2N3O/c12-8-3-1-2-7(10(8)13)9(17)6-16-5-4-15-11(16)14/h1-5,9,17H,6H2,(H2,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50020411

(CHEMBL3289813)Show SMILES CC(Oc1ccc2nc(N)n(CC(O)c3ccc(cc3Cl)C(F)(F)F)c2n1)C(F)(F)F Show InChI InChI=1S/C18H15ClF6N4O2/c1-8(17(20,21)22)31-14-5-4-12-15(28-14)29(16(26)27-12)7-13(30)10-3-2-9(6-11(10)19)18(23,24)25/h2-6,8,13,30H,7H2,1H3,(H2,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.29E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50020418
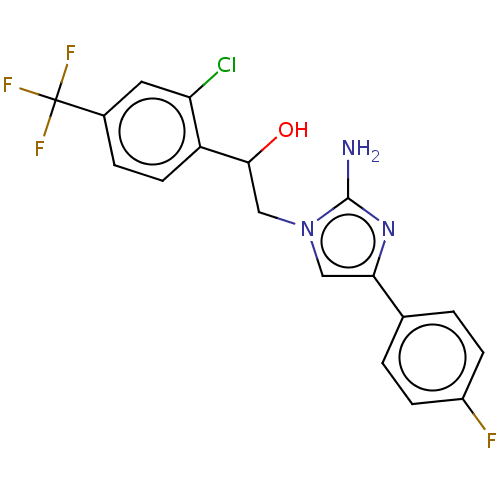
(CHEMBL3289798)Show SMILES Nc1nc(cn1CC(O)c1ccc(cc1Cl)C(F)(F)F)-c1ccc(F)cc1 Show InChI InChI=1S/C18H14ClF4N3O/c19-14-7-11(18(21,22)23)3-6-13(14)16(27)9-26-8-15(25-17(26)24)10-1-4-12(20)5-2-10/h1-8,16,27H,9H2,(H2,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50020404

(CHEMBL3289803)Show SMILES Nc1nc2ccc(cc2n1CC(O)c1ccc(cc1Cl)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C17H12ClF6N3O/c18-11-5-8(16(19,20)21)1-3-10(11)14(28)7-27-13-6-9(17(22,23)24)2-4-12(13)26-15(27)25/h1-6,14,28H,7H2,(H2,25,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50020415

(CHEMBL3289806)Show SMILES CN1CCN(CC1)c1ccc2nc(N)n(CC(O)c3ccc(cc3Cl)C(F)(F)F)c2n1 Show InChI InChI=1S/C20H22ClF3N6O/c1-28-6-8-29(9-7-28)17-5-4-15-18(27-17)30(19(25)26-15)11-16(31)13-3-2-12(10-14(13)21)20(22,23)24/h2-5,10,16,31H,6-9,11H2,1H3,(H2,25,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.79E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50020404

(CHEMBL3289803)Show SMILES Nc1nc2ccc(cc2n1CC(O)c1ccc(cc1Cl)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C17H12ClF6N3O/c18-11-5-8(16(19,20)21)1-3-10(11)14(28)7-27-13-6-9(17(22,23)24)2-4-12(13)26-15(27)25/h1-6,14,28H,7H2,(H2,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.79E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50020411

(CHEMBL3289813)Show SMILES CC(Oc1ccc2nc(N)n(CC(O)c3ccc(cc3Cl)C(F)(F)F)c2n1)C(F)(F)F Show InChI InChI=1S/C18H15ClF6N4O2/c1-8(17(20,21)22)31-14-5-4-12-15(28-14)29(16(26)27-12)7-13(30)10-3-2-9(6-11(10)19)18(23,24)25/h2-6,8,13,30H,7H2,1H3,(H2,26,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.83E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50020404

(CHEMBL3289803)Show SMILES Nc1nc2ccc(cc2n1CC(O)c1ccc(cc1Cl)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C17H12ClF6N3O/c18-11-5-8(16(19,20)21)1-3-10(11)14(28)7-27-13-6-9(17(22,23)24)2-4-12(13)26-15(27)25/h1-6,14,28H,7H2,(H2,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.94E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50020412

(CHEMBL3289811)Show SMILES Nc1nc2ccc(OC3CCOC3)nc2n1CC(O)c1ccc(cc1Cl)C(F)(F)F Show InChI InChI=1S/C19H18ClF3N4O3/c20-13-7-10(19(21,22)23)1-2-12(13)15(28)8-27-17-14(25-18(27)24)3-4-16(26-17)30-11-5-6-29-9-11/h1-4,7,11,15,28H,5-6,8-9H2,(H2,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50020408

(CHEMBL3289805)Show InChI InChI=1S/C14H12Cl2N4O/c15-9-4-1-3-8(12(9)16)11(21)7-20-13-10(19-14(20)17)5-2-6-18-13/h1-6,11,21H,7H2,(H2,17,19) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50020408

(CHEMBL3289805)Show InChI InChI=1S/C14H12Cl2N4O/c15-9-4-1-3-8(12(9)16)11(21)7-20-13-10(19-14(20)17)5-2-6-18-13/h1-6,11,21H,7H2,(H2,17,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50020404

(CHEMBL3289803)Show SMILES Nc1nc2ccc(cc2n1CC(O)c1ccc(cc1Cl)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C17H12ClF6N3O/c18-11-5-8(16(19,20)21)1-3-10(11)14(28)7-27-13-6-9(17(22,23)24)2-4-12(13)26-15(27)25/h1-6,14,28H,7H2,(H2,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50020411

(CHEMBL3289813)Show SMILES CC(Oc1ccc2nc(N)n(CC(O)c3ccc(cc3Cl)C(F)(F)F)c2n1)C(F)(F)F Show InChI InChI=1S/C18H15ClF6N4O2/c1-8(17(20,21)22)31-14-5-4-12-15(28-14)29(16(26)27-12)7-13(30)10-3-2-9(6-11(10)19)18(23,24)25/h2-6,8,13,30H,7H2,1H3,(H2,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50020412

(CHEMBL3289811)Show SMILES Nc1nc2ccc(OC3CCOC3)nc2n1CC(O)c1ccc(cc1Cl)C(F)(F)F Show InChI InChI=1S/C19H18ClF3N4O3/c20-13-7-10(19(21,22)23)1-2-12(13)15(28)8-27-17-14(25-18(27)24)3-4-16(26-17)30-11-5-6-29-9-11/h1-4,7,11,15,28H,5-6,8-9H2,(H2,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50020415

(CHEMBL3289806)Show SMILES CN1CCN(CC1)c1ccc2nc(N)n(CC(O)c3ccc(cc3Cl)C(F)(F)F)c2n1 Show InChI InChI=1S/C20H22ClF3N6O/c1-28-6-8-29(9-7-28)17-5-4-15-18(27-17)30(19(25)26-15)11-16(31)13-3-2-12(10-14(13)21)20(22,23)24/h2-5,10,16,31H,6-9,11H2,1H3,(H2,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50020408

(CHEMBL3289805)Show InChI InChI=1S/C14H12Cl2N4O/c15-9-4-1-3-8(12(9)16)11(21)7-20-13-10(19-14(20)17)5-2-6-18-13/h1-6,11,21H,7H2,(H2,17,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50020415

(CHEMBL3289806)Show SMILES CN1CCN(CC1)c1ccc2nc(N)n(CC(O)c3ccc(cc3Cl)C(F)(F)F)c2n1 Show InChI InChI=1S/C20H22ClF3N6O/c1-28-6-8-29(9-7-28)17-5-4-15-18(27-17)30(19(25)26-15)11-16(31)13-3-2-12(10-14(13)21)20(22,23)24/h2-5,10,16,31H,6-9,11H2,1H3,(H2,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50019516

(CHEMBL3291063)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)Nc1n[nH]c3ccc(F)cc13)N2C(=O)c1cnn(C)c1Cl |r,TLB:21:20:2.3:7.6.8,9:7:20:2.3| Show InChI InChI=1S/C19H20ClFN6O/c1-26-17(20)15(9-22-26)19(28)27-12-3-4-13(27)8-11(7-12)23-18-14-6-10(21)2-5-16(14)24-25-18/h2,5-6,9,11-13H,3-4,7-8H2,1H3,(H2,23,24,25)/t11-,12+,13- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by medium-throughput electrophysiology |
J Med Chem 57: 5419-34 (2014)
Article DOI: 10.1021/jm5005978
BindingDB Entry DOI: 10.7270/Q25Q4XNM |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50019518

(CHEMBL3291066)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)Nc1cc(=O)n(C)c3nccnc13)N2C(=O)c1noc(C)c1C |r,TLB:23:22:6.7.8:2.3,9:7:22:2.3| Show InChI InChI=1S/C21H24N6O3/c1-11-12(2)30-25-18(11)21(29)27-14-4-5-15(27)9-13(8-14)24-16-10-17(28)26(3)20-19(16)22-6-7-23-20/h6-7,10,13-15,24H,4-5,8-9H2,1-3H3/t13-,14+,15- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by medium-throughput electrophysiology |
J Med Chem 57: 5419-34 (2014)
Article DOI: 10.1021/jm5005978
BindingDB Entry DOI: 10.7270/Q25Q4XNM |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50019517

(CHEMBL3291065)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)Nc1cc(=O)n(C)c3ncccc13)N2C(=O)c1cnn(C)c1Cl |r,TLB:23:22:2.3:7.6.8,9:7:22:2.3| Show InChI InChI=1S/C21H23ClN6O2/c1-26-18(29)10-17(15-4-3-7-23-20(15)26)25-12-8-13-5-6-14(9-12)28(13)21(30)16-11-24-27(2)19(16)22/h3-4,7,10-14,25H,5-6,8-9H2,1-2H3/t12-,13+,14- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by medium-throughput electrophysiology |
J Med Chem 57: 5419-34 (2014)
Article DOI: 10.1021/jm5005978
BindingDB Entry DOI: 10.7270/Q25Q4XNM |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50019514

(CHEMBL3290772)Show SMILES Cn1ncc(C(=O)N2CCC(CC2)Nc2cc(=O)n(C)c3ccccc23)c1Cl Show InChI InChI=1S/C20H22ClN5O2/c1-24-17-6-4-3-5-14(17)16(11-18(24)27)23-13-7-9-26(10-8-13)20(28)15-12-22-25(2)19(15)21/h3-6,11-13,23H,7-10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by medium-throughput electrophysiology |
J Med Chem 57: 5419-34 (2014)
Article DOI: 10.1021/jm5005978
BindingDB Entry DOI: 10.7270/Q25Q4XNM |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50020420
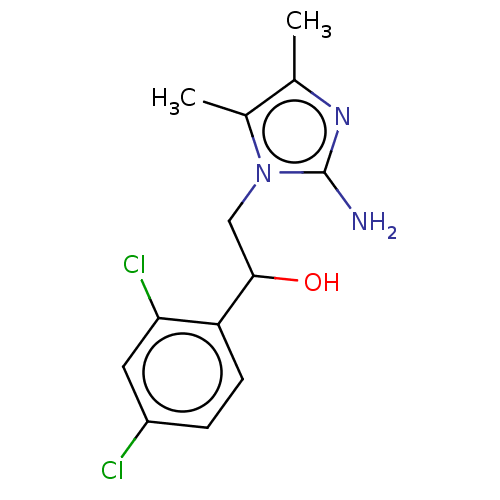
(CHEMBL3289796)Show InChI InChI=1S/C13H15Cl2N3O/c1-7-8(2)18(13(16)17-7)6-12(19)10-4-3-9(14)5-11(10)15/h3-5,12,19H,6H2,1-2H3,(H2,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50020419
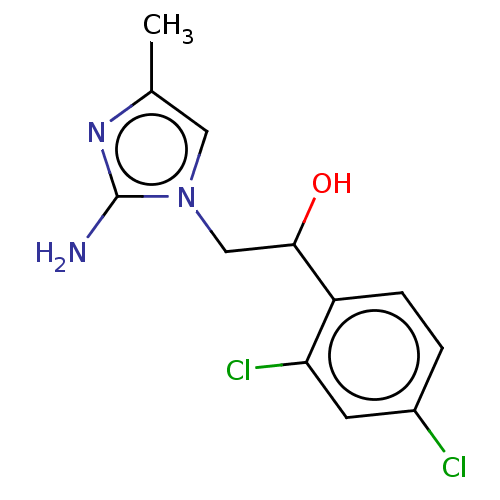
(CHEMBL3289795)Show InChI InChI=1S/C12H13Cl2N3O/c1-7-5-17(12(15)16-7)6-11(18)9-3-2-8(13)4-10(9)14/h2-5,11,18H,6H2,1H3,(H2,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50020405
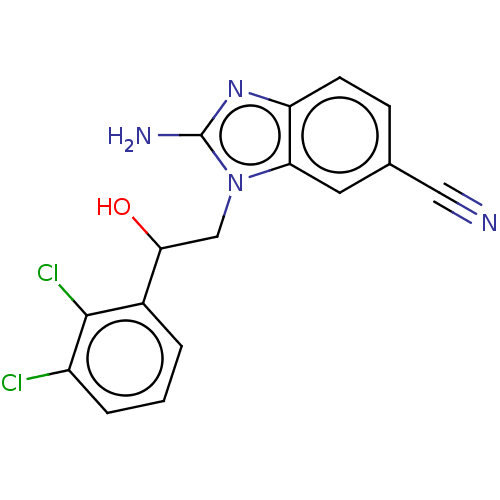
(CHEMBL3289800)Show InChI InChI=1S/C16H12Cl2N4O/c17-11-3-1-2-10(15(11)18)14(23)8-22-13-6-9(7-19)4-5-12(13)21-16(22)20/h1-6,14,23H,8H2,(H2,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50020406
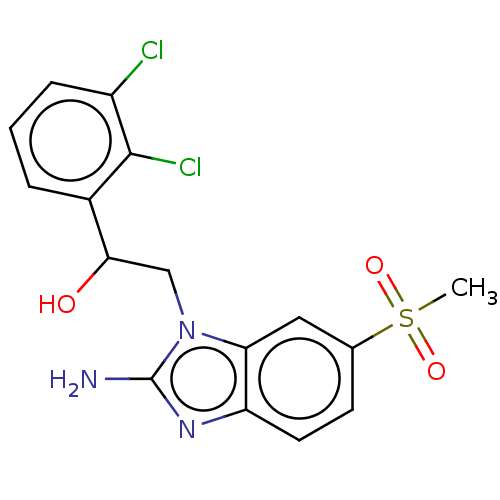
(CHEMBL3289801)Show SMILES CS(=O)(=O)c1ccc2nc(N)n(CC(O)c3cccc(Cl)c3Cl)c2c1 Show InChI InChI=1S/C16H15Cl2N3O3S/c1-25(23,24)9-5-6-12-13(7-9)21(16(19)20-12)8-14(22)10-3-2-4-11(17)15(10)18/h2-7,14,22H,8H2,1H3,(H2,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50020407

(CHEMBL3289802)Show InChI InChI=1S/C15H12Cl3N3O/c16-8-4-5-11-12(6-8)21(15(19)20-11)7-13(22)9-2-1-3-10(17)14(9)18/h1-6,13,22H,7H2,(H2,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50020408

(CHEMBL3289805)Show InChI InChI=1S/C14H12Cl2N4O/c15-9-4-1-3-8(12(9)16)11(21)7-20-13-10(19-14(20)17)5-2-6-18-13/h1-6,11,21H,7H2,(H2,17,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50020411

(CHEMBL3289813)Show SMILES CC(Oc1ccc2nc(N)n(CC(O)c3ccc(cc3Cl)C(F)(F)F)c2n1)C(F)(F)F Show InChI InChI=1S/C18H15ClF6N4O2/c1-8(17(20,21)22)31-14-5-4-12-15(28-14)29(16(26)27-12)7-13(30)10-3-2-9(6-11(10)19)18(23,24)25/h2-6,8,13,30H,7H2,1H3,(H2,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 57: 5702-13 (2014)
Article DOI: 10.1021/jm500535j
BindingDB Entry DOI: 10.7270/Q2X3501C |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data