

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Madurai Kamaraj University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylcholine as substrate preincubated for 15 mins followed by substrate addition measured every minute by... | Bioorg Med Chem Lett 27: 3071-3075 (2017) Article DOI: 10.1016/j.bmcl.2017.05.050 BindingDB Entry DOI: 10.7270/Q2BG2RDS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50242974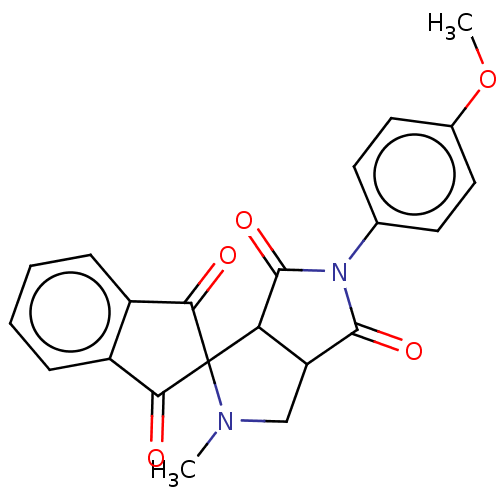 (CHEMBL4072825) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
Madurai Kamaraj University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylcholine as substrate preincubated for 15 mins followed by substrate addition measured every minute by... | Bioorg Med Chem Lett 27: 3071-3075 (2017) Article DOI: 10.1016/j.bmcl.2017.05.050 BindingDB Entry DOI: 10.7270/Q2BG2RDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50558500 (CHEMBL4752440) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine chloride as substrate preincubated with enzyme for 15 mins followed by substrate addition... | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.044 BindingDB Entry DOI: 10.7270/Q2571GQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50558514 (CHEMBL4747183) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine chloride as substrate preincubated with enzyme for 15 mins followed by substrate addition... | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.044 BindingDB Entry DOI: 10.7270/Q2571GQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 2.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50242995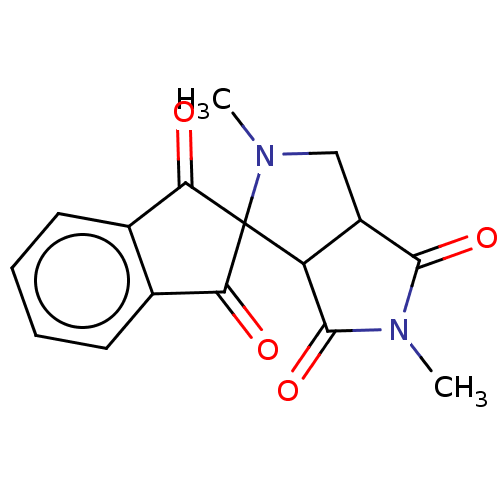 (CHEMBL4088091) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Madurai Kamaraj University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylcholine as substrate preincubated for 15 mins followed by substrate addition measured every minute by... | Bioorg Med Chem Lett 27: 3071-3075 (2017) Article DOI: 10.1016/j.bmcl.2017.05.050 BindingDB Entry DOI: 10.7270/Q2BG2RDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50558508 (CHEMBL4794820) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine chloride as substrate preincubated with enzyme for 15 mins followed by substrate addition... | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.044 BindingDB Entry DOI: 10.7270/Q2571GQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50433622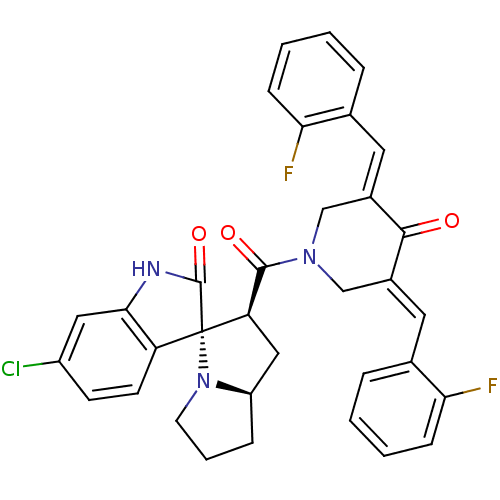 (CHEMBL2380672) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine chloride as substrate incubated for 15 mins prior to substrate addition measured after 30... | Bioorg Med Chem Lett 23: 2979-83 (2013) Article DOI: 10.1016/j.bmcl.2013.03.027 BindingDB Entry DOI: 10.7270/Q2057H97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50430681 (CHEMBL2332979) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50430652 (CHEMBL2332975) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine chloride substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50433617 (CHEMBL2380677) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine chloride as substrate incubated for 15 mins prior to substrate addition measured after 30... | Bioorg Med Chem Lett 23: 2979-83 (2013) Article DOI: 10.1016/j.bmcl.2013.03.027 BindingDB Entry DOI: 10.7270/Q2057H97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50430680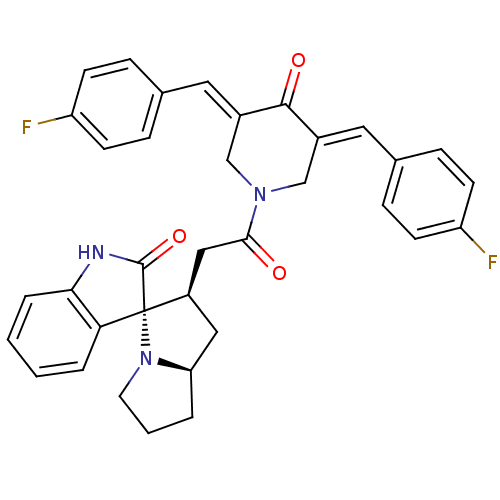 (CHEMBL2332980) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.99E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine chloride substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50430683 (CHEMBL2332977) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50242971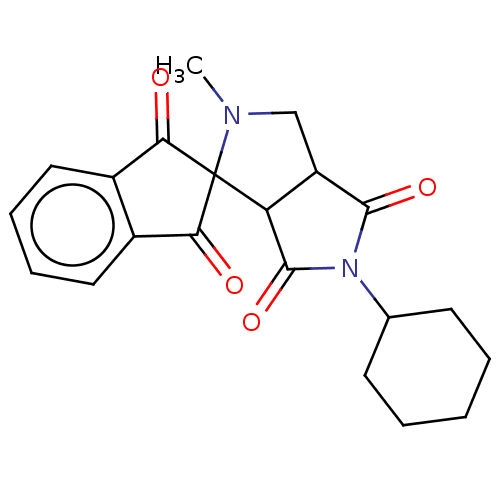 (CHEMBL4089033) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Madurai Kamaraj University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylcholine as substrate preincubated for 15 mins followed by substrate addition measured every minute by... | Bioorg Med Chem Lett 27: 3071-3075 (2017) Article DOI: 10.1016/j.bmcl.2017.05.050 BindingDB Entry DOI: 10.7270/Q2BG2RDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50430651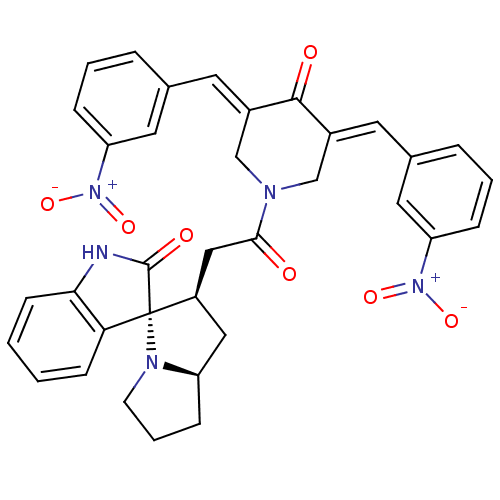 (CHEMBL2332976) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.99E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50430668 (CHEMBL2332981) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine chloride substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50558516 (CHEMBL4797045) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine chloride as substrate preincubated with enzyme for 15 mins followed by substrate addition... | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.044 BindingDB Entry DOI: 10.7270/Q2571GQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50242973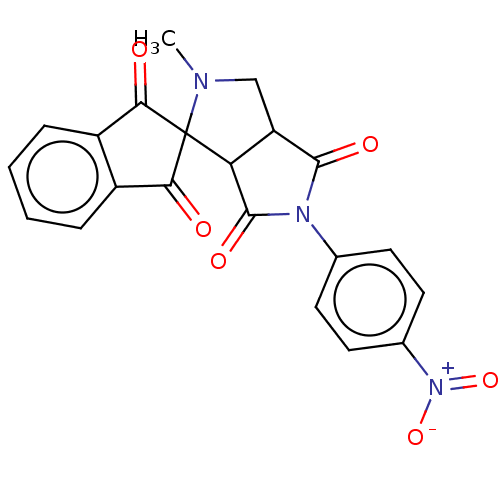 (CHEMBL4104459) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Madurai Kamaraj University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylcholine as substrate preincubated for 15 mins followed by substrate addition measured every minute by... | Bioorg Med Chem Lett 27: 3071-3075 (2017) Article DOI: 10.1016/j.bmcl.2017.05.050 BindingDB Entry DOI: 10.7270/Q2BG2RDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50430679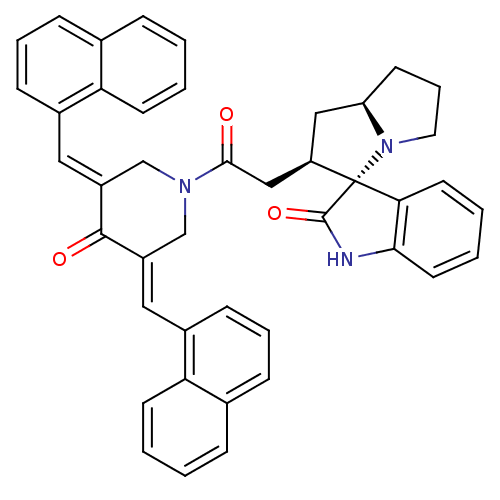 (CHEMBL2332545) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50430672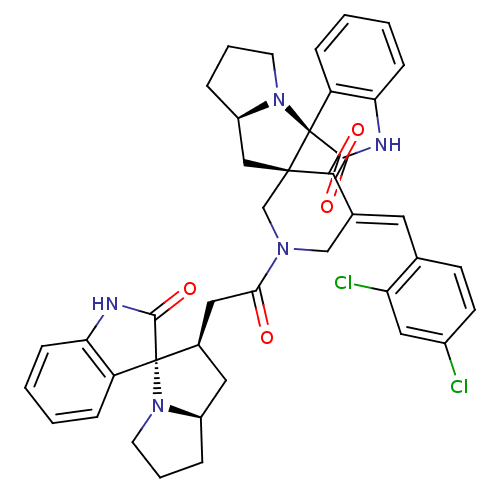 (CHEMBL2332531) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50558502 (CHEMBL4760400) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine chloride as substrate preincubated with enzyme for 15 mins followed by substrate addition... | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.044 BindingDB Entry DOI: 10.7270/Q2571GQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50242979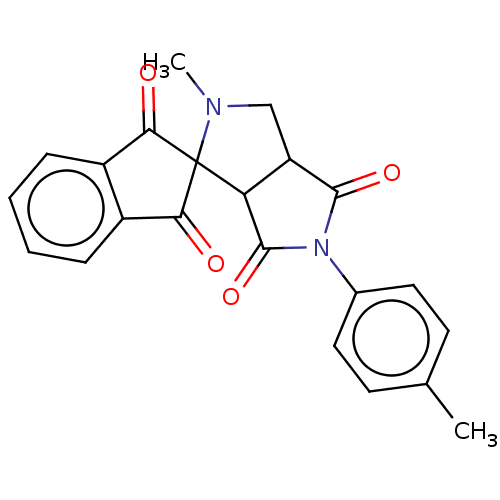 (CHEMBL4090159) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Madurai Kamaraj University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylcholine as substrate preincubated for 15 mins followed by substrate addition measured every minute by... | Bioorg Med Chem Lett 27: 3071-3075 (2017) Article DOI: 10.1016/j.bmcl.2017.05.050 BindingDB Entry DOI: 10.7270/Q2BG2RDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50433624 (CHEMBL2380670) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine chloride as substrate incubated for 15 mins prior to substrate addition measured after 30... | Bioorg Med Chem Lett 23: 2979-83 (2013) Article DOI: 10.1016/j.bmcl.2013.03.027 BindingDB Entry DOI: 10.7270/Q2057H97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50430670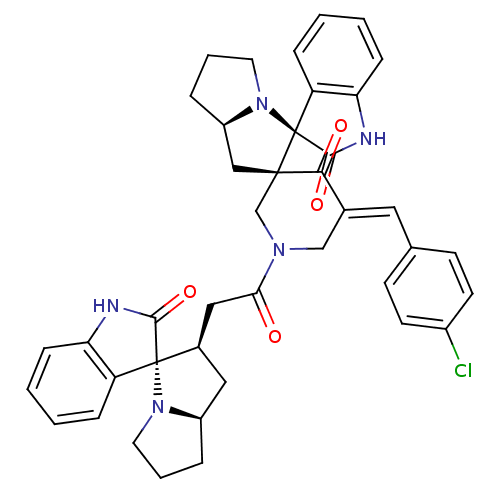 (CHEMBL2332541) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50430669 (CHEMBL2332540) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine chloride substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50430665 (CHEMBL2332539) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50430674 (CHEMBL2332533) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine chloride substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50430654 (CHEMBL2332550) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine chloride substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50430672 (CHEMBL2332531) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine chloride substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50433616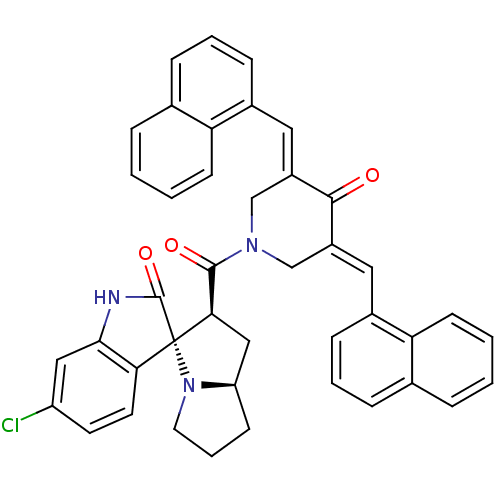 (CHEMBL2380667) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine chloride as substrate incubated for 15 mins prior to substrate addition measured after 30... | Bioorg Med Chem Lett 23: 2979-83 (2013) Article DOI: 10.1016/j.bmcl.2013.03.027 BindingDB Entry DOI: 10.7270/Q2057H97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50430662 (CHEMBL2332537) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50558511 (CHEMBL4754607) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine chloride as substrate preincubated with enzyme for 15 mins followed by substrate addition... | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.044 BindingDB Entry DOI: 10.7270/Q2571GQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50430670 (CHEMBL2332541) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine chloride substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50558517 (CHEMBL4800067) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine chloride as substrate preincubated with enzyme for 15 mins followed by substrate addition... | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.044 BindingDB Entry DOI: 10.7270/Q2571GQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50242992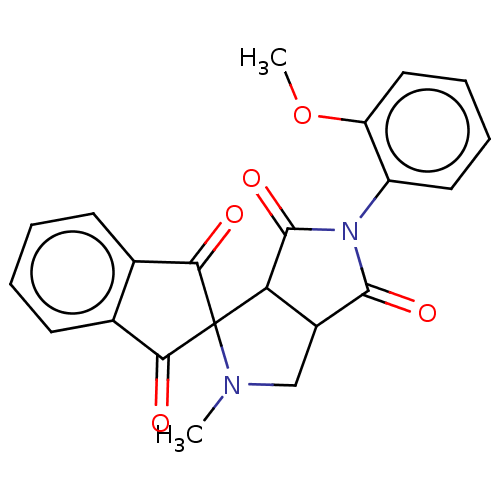 (CHEMBL4066805) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Madurai Kamaraj University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylcholine as substrate preincubated for 15 mins followed by substrate addition measured every minute by... | Bioorg Med Chem Lett 27: 3071-3075 (2017) Article DOI: 10.1016/j.bmcl.2017.05.050 BindingDB Entry DOI: 10.7270/Q2BG2RDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50430676 (CHEMBL2332542) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50430675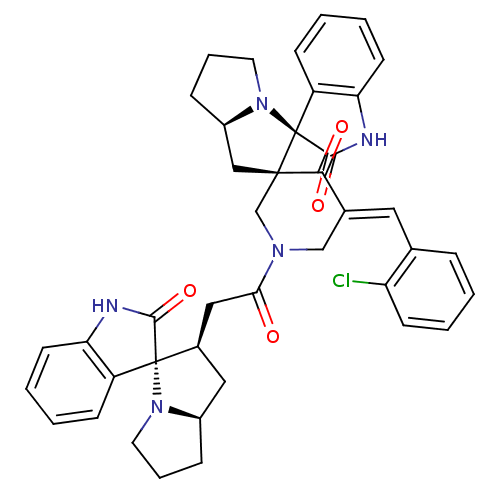 (CHEMBL2332534) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine chloride substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50430679 (CHEMBL2332545) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine chloride substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50430663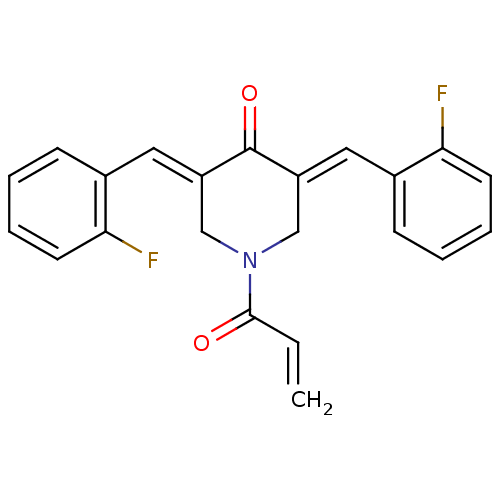 (CHEMBL2332536) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50430653 (CHEMBL2332974) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50242993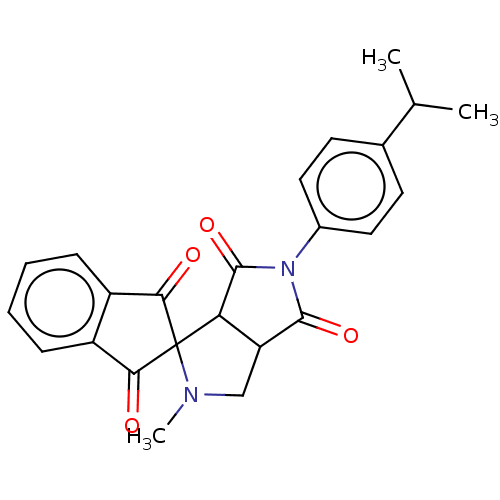 (CHEMBL4093532) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Madurai Kamaraj University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylcholine as substrate preincubated for 15 mins followed by substrate addition measured every minute by... | Bioorg Med Chem Lett 27: 3071-3075 (2017) Article DOI: 10.1016/j.bmcl.2017.05.050 BindingDB Entry DOI: 10.7270/Q2BG2RDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50558499 (CHEMBL4761620) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine chloride as substrate preincubated with enzyme for 15 mins followed by substrate addition... | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.044 BindingDB Entry DOI: 10.7270/Q2571GQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50430655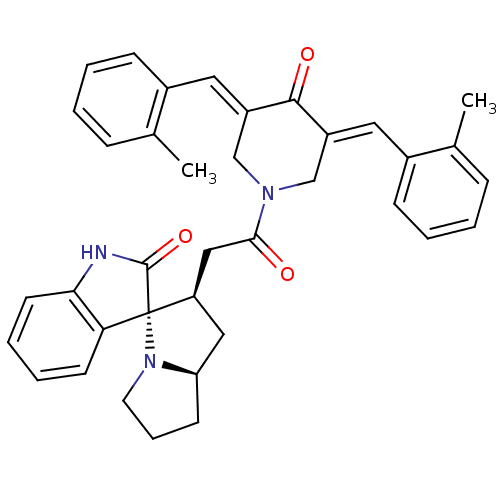 (CHEMBL2332549) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50558503 (CHEMBL4754171) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine chloride as substrate preincubated with enzyme for 15 mins followed by substrate addition... | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.044 BindingDB Entry DOI: 10.7270/Q2571GQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50430677 (CHEMBL2332543) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine chloride substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50430675 (CHEMBL2332534) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50430657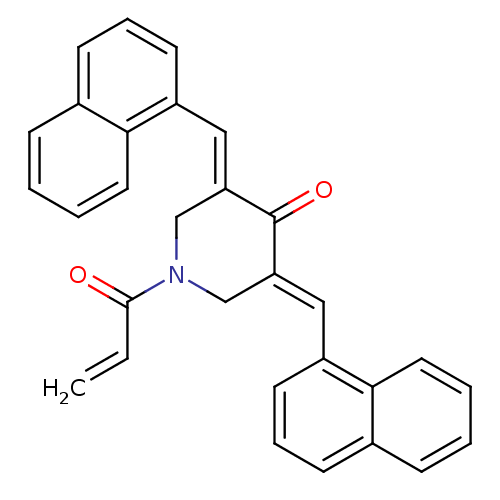 (CHEMBL2332547) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50433625 (CHEMBL2380669) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine chloride as substrate incubated for 15 mins prior to substrate addition measured after 30... | Bioorg Med Chem Lett 23: 2979-83 (2013) Article DOI: 10.1016/j.bmcl.2013.03.027 BindingDB Entry DOI: 10.7270/Q2057H97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50430658 (CHEMBL350032) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50433626 (CHEMBL2380668) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.54E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine chloride as substrate incubated for 15 mins prior to substrate addition measured after 30... | Bioorg Med Chem Lett 23: 2979-83 (2013) Article DOI: 10.1016/j.bmcl.2013.03.027 BindingDB Entry DOI: 10.7270/Q2057H97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 133 total ) | Next | Last >> |