 Found 194 hits with Last Name = 'ponzi' and Initial = 's'
Found 194 hits with Last Name = 'ponzi' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50612449

(CHEMBL5271471)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)C1CN(C)C1)c1ncc(o1)-c1cc2ccccc2nc1OC |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50175036
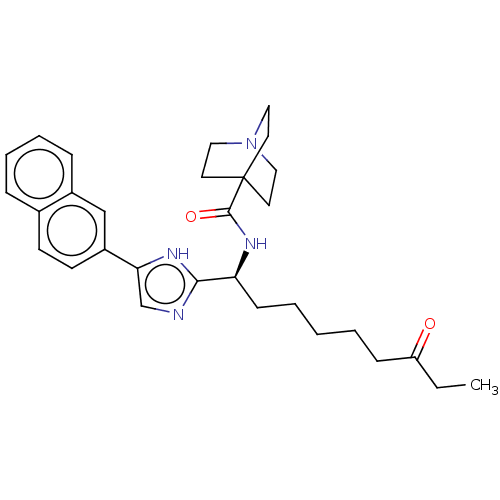
(CHEMBL3809599)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)C12CCN(CC1)CC2)c1ncc([nH]1)-c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C18H11Cl2F3N2OS/c19-13-2-1-3-14(20)12(13)8-16-24-9-15(27-16)17(26)25-11-6-4-10(5-7-11)18(21,22)23/h1-7,9H,8H2,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM Science Park
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-tagged HDAC1 expressed in insect cells preincubated for 10 mins followed by addition of FLUOR DE LYS as fluoresce... |
ACS Med Chem Lett 7: 454-9 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00468
BindingDB Entry DOI: 10.7270/Q2S184FM |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50612447

(CHEMBL5285293)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)C1CN(C)C1)c1ncc([nH]1)-c1ccc2ccccc2c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50612445

(CHEMBL5282110)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)C12CCN(CC1)CC2)c1ncc(o1)-c1cc2ccccc2nc1OC |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50612449

(CHEMBL5271471)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)C1CN(C)C1)c1ncc(o1)-c1cc2ccccc2nc1OC |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50612450
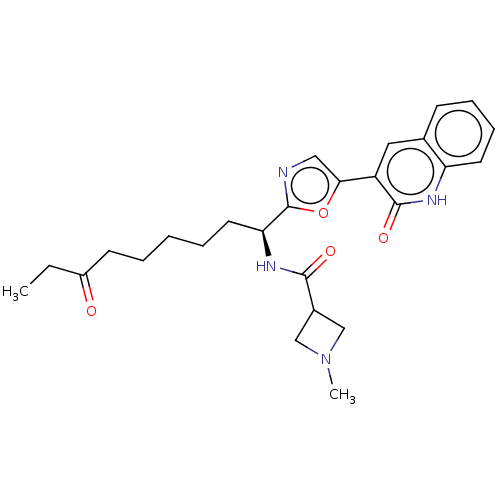
(CHEMBL5279520)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)C1CN(C)C1)c1ncc(o1)-c1cc2ccccc2[nH]c1=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50612449

(CHEMBL5271471)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)C1CN(C)C1)c1ncc(o1)-c1cc2ccccc2nc1OC |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50612452
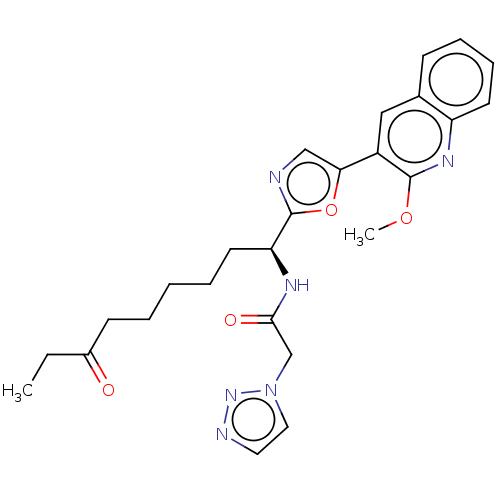
(CHEMBL5285418)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)Cn1ccnn1)c1ncc(o1)-c1cc2ccccc2nc1OC |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50406432
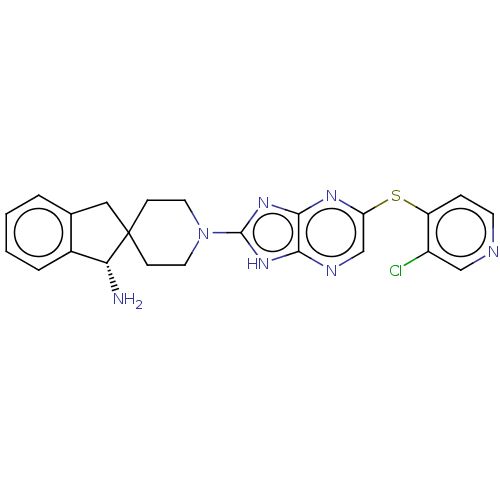
(CHEMBL5288562)Show InChI InChI=1S/C11H17NO5S2/c1-11(2,13)7-8-18(14,15)9-3-5-10(6-4-9)19(12,16)17/h3-6,13H,7-8H2,1-2H3,(H2,12,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibition of human dihydrofolate reductase |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50258549
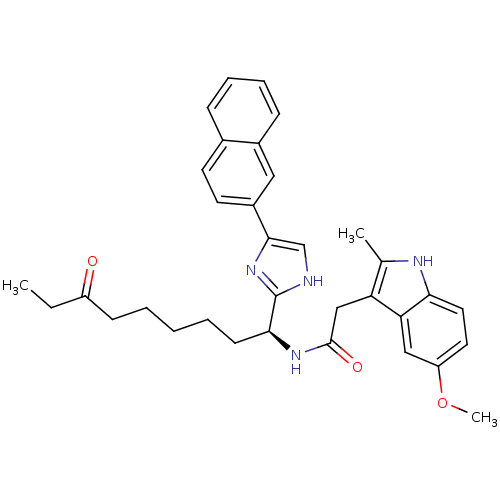
((S)-2-(5-methoxy-2-methyl-1H-indol-3-yl)-N-(1-(5-(...)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)Cc1c(C)[nH]c2ccc(OC)cc12)c1nc(c[nH]1)-c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C34H38N4O3/c1-4-26(39)12-6-5-7-13-31(34-35-21-32(38-34)25-15-14-23-10-8-9-11-24(23)18-25)37-33(40)20-28-22(2)36-30-17-16-27(41-3)19-29(28)30/h8-11,14-19,21,31,36H,4-7,12-13,20H2,1-3H3,(H,35,38)(H,37,40)/t31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50612453
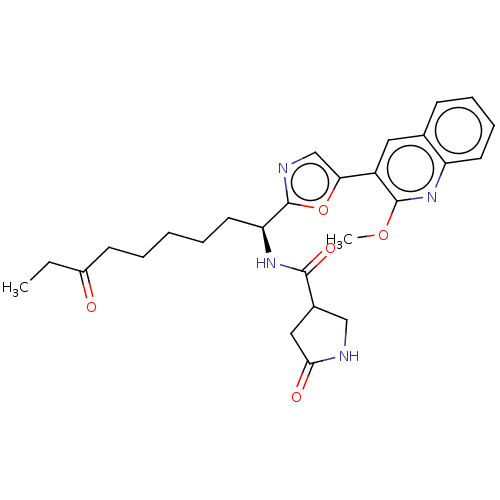
(CHEMBL5285433)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)C1CNC(=O)C1)c1ncc(o1)-c1cc2ccccc2nc1OC |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50608555
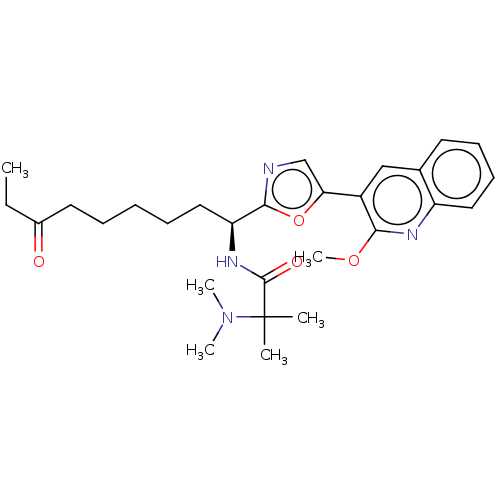
(CHEMBL5269165)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)C(C)(C)N(C)C)c1ncc(o1)-c1cc2ccccc2nc1OC |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50612445

(CHEMBL5282110)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)C12CCN(CC1)CC2)c1ncc(o1)-c1cc2ccccc2nc1OC |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50612445

(CHEMBL5282110)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)C12CCN(CC1)CC2)c1ncc(o1)-c1cc2ccccc2nc1OC |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50608555

(CHEMBL5269165)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)C(C)(C)N(C)C)c1ncc(o1)-c1cc2ccccc2nc1OC |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50608555

(CHEMBL5269165)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)C(C)(C)N(C)C)c1ncc(o1)-c1cc2ccccc2nc1OC |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50406430
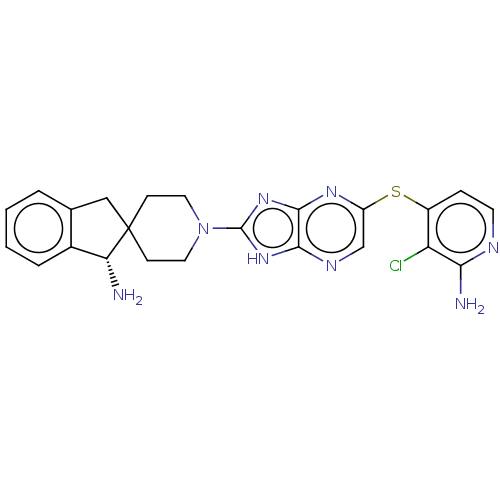
(CHEMBL5274747)Show InChI InChI=1S/C7H11NO3S3/c8-14(10,11)7-3-2-6(13-7)12-5-1-4-9/h2-3,9H,1,4-5H2,(H2,8,10,11) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of dihydrofolate reductase in Candida albicans (in vitro). |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50406429

(CHEMBL5276842)Show InChI InChI=1S/C8H11NO6S3/c1-6(10)15-4-5-17(11,12)7-2-3-8(16-7)18(9,13)14/h2-3H,4-5H2,1H3,(H2,9,13,14) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibition of human dihydrofolate reductase |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50406431

(CHEMBL5278011)Show InChI InChI=1S/C8H10FNO5S2/c9-7-5-6(17(10,14)15)1-2-8(7)16(12,13)4-3-11/h1-2,5,11H,3-4H2,(H2,10,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of dihydrofolate reductase in Candida albicans (in vitro). |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50612451

(CHEMBL5273223)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)C1CN(C)C1)c1ncc(o1)-c1ccc2ccccc2c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50612448
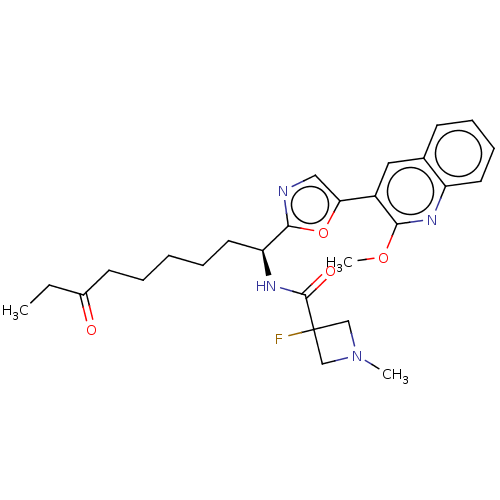
(CHEMBL5268361)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)C1(F)CN(C)C1)c1ncc(o1)-c1cc2ccccc2nc1OC |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50612444
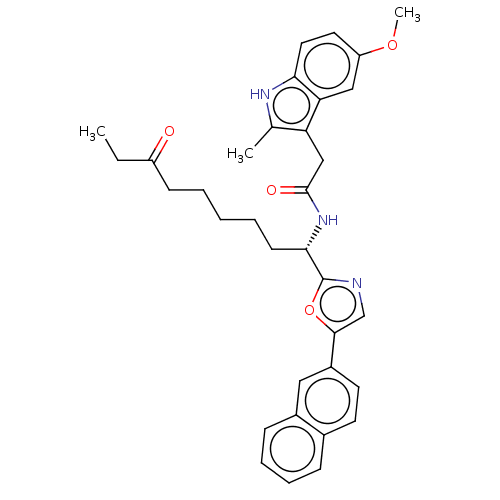
(CHEMBL5285266)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)Cc1c(C)[nH]c2ccc(OC)cc12)c1ncc(o1)-c1ccc2ccccc2c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50175039

(CHEMBL3808639)Show SMILES CNC(=O)CCCCC[C@H](NC(=O)c1cncs1)c1ncc([nH]1)-c1cc2ccccc2nc1OC |r| Show InChI InChI=1S/C17H13Cl2N3O2S/c1-24-15-6-5-10(8-20-15)21-17(23)14-9-25-16(22-14)7-11-12(18)3-2-4-13(11)19/h2-6,8-9H,7H2,1H3,(H,21,23) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50612443
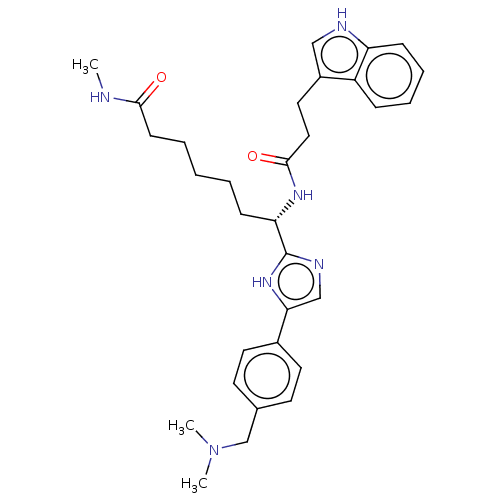
(CHEMBL5283156)Show SMILES CNC(=O)CCCCC[C@H](NC(=O)CCc1c[nH]c2ccccc12)c1ncc([nH]1)-c1ccc(CN(C)C)cc1 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50162108
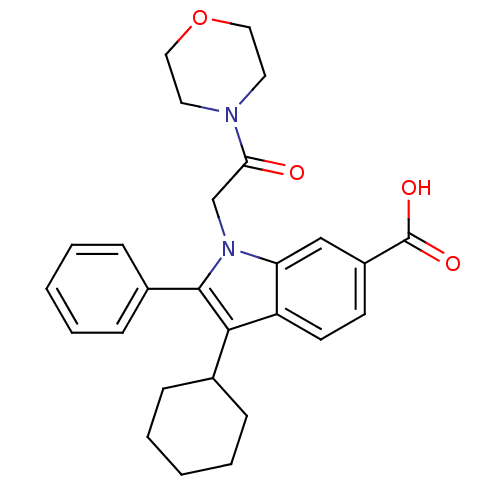
(3-CYCLOHEXYL-1-(2-MORPHOLIN-4-YL-2-OXOETHYL)-2-PHE...)Show SMILES OC(=O)c1ccc2c(C3CCCCC3)c(-c3ccccc3)n(CC(=O)N3CCOCC3)c2c1 Show InChI InChI=1S/C27H30N2O4/c30-24(28-13-15-33-16-14-28)18-29-23-17-21(27(31)32)11-12-22(23)25(19-7-3-1-4-8-19)26(29)20-9-5-2-6-10-20/h2,5-6,9-12,17,19H,1,3-4,7-8,13-16,18H2,(H,31,32) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
MRL Rome
Curated by ChEMBL
| Assay Description
Inhibition of HCV BK NS5B deltaC55 RNA dependent RNA polymerase |
Bioorg Med Chem Lett 16: 4026-30 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.012
BindingDB Entry DOI: 10.7270/Q2416WP6 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50612441
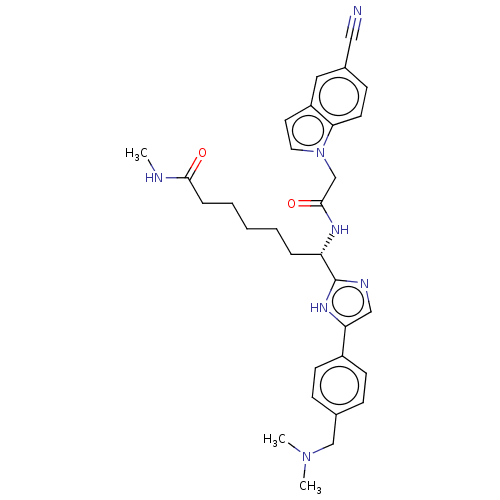
(CHEMBL5268555)Show SMILES CNC(=O)CCCCC[C@H](NC(=O)Cn1ccc2cc(ccc12)C#N)c1ncc([nH]1)-c1ccc(CN(C)C)cc1 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50612446
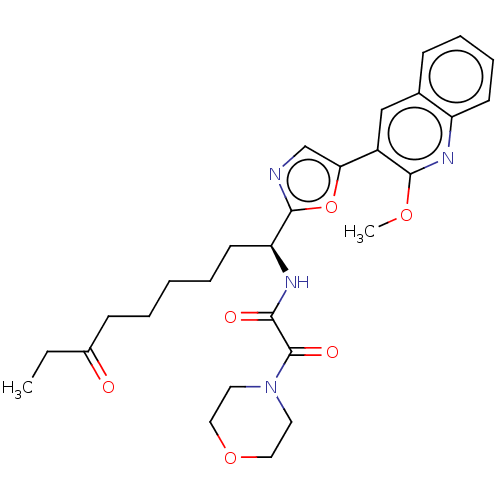
(CHEMBL5270878)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)C(=O)N1CCOCC1)c1ncc(o1)-c1cc2ccccc2nc1OC |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Sterol 14-alpha demethylase
(Trypanosoma cruzi) | BDBM50545214

(CHEMBL4633298)Show SMILES CCC(=O)CCCCCC(Oc1ccc2ccccc2c1)c1ncc(Cc2ccncc2)[nH]1 Show InChI InChI=1S/C28H31N3O2/c1-2-25(32)10-4-3-5-11-27(33-26-13-12-22-8-6-7-9-23(22)19-26)28-30-20-24(31-28)18-21-14-16-29-17-15-21/h6-9,12-17,19-20,27H,2-5,10-11,18H2,1H3,(H,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM Spa
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma cruzi CYP51 |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127052
BindingDB Entry DOI: 10.7270/Q2C82DW5 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50612440
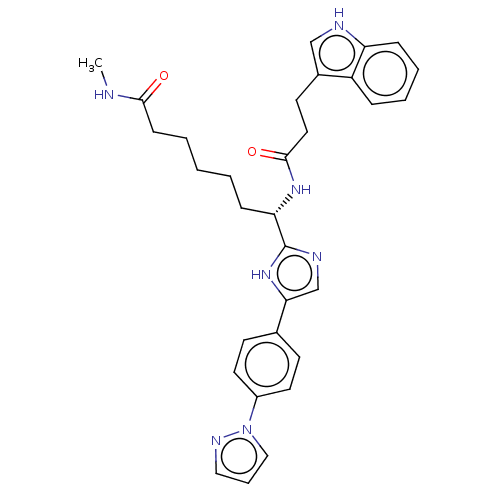
(CHEMBL5276254)Show SMILES CNC(=O)CCCCC[C@H](NC(=O)CCc1c[nH]c2ccccc12)c1ncc([nH]1)-c1ccc(cc1)-n1cccn1 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50406376
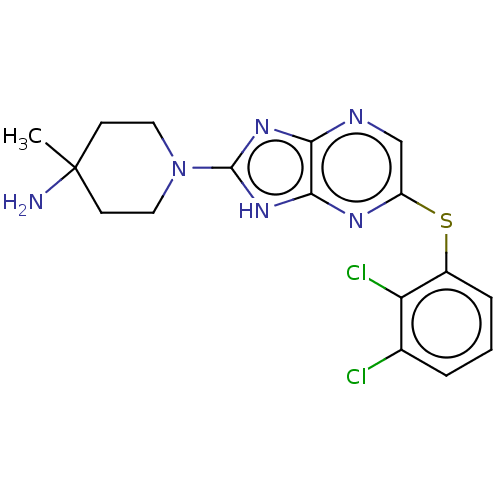
(CHEMBL5289332)Show SMILES NCCCC[C@H](OP(O)(=O)CCCCc1ccccc1)C(=O)N1C[C@@H](F)C[C@H]1C(O)=O Show InChI InChI=1S/C21H32FN2O6P/c22-17-14-18(21(26)27)24(15-17)20(25)19(11-4-6-12-23)30-31(28,29)13-7-5-10-16-8-2-1-3-9-16/h1-3,8-9,17-19H,4-7,10-15,23H2,(H,26,27)(H,28,29)/t17-,18-,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of dihydrofolate reductase in Candida albicans (in vitro). |
Citation and Details
|
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50162094
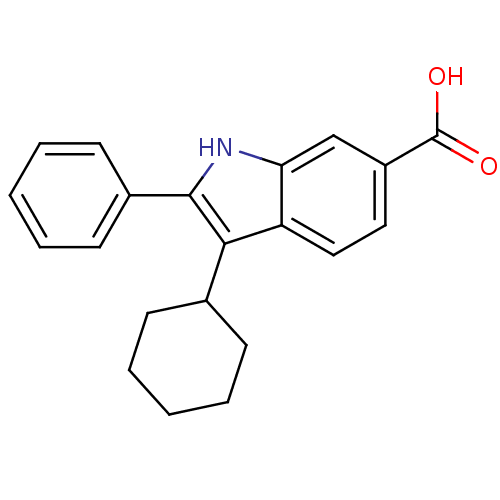
(3-Cyclohexyl-2-phenyl-1H-indole-6-carboxylic acid ...)Show SMILES OC(=O)c1ccc2c(C3CCCCC3)c([nH]c2c1)-c1ccccc1 Show InChI InChI=1S/C21H21NO2/c23-21(24)16-11-12-17-18(13-16)22-20(15-9-5-2-6-10-15)19(17)14-7-3-1-4-8-14/h2,5-6,9-14,22H,1,3-4,7-8H2,(H,23,24) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
MRL Rome
Curated by ChEMBL
| Assay Description
Inhibition of HCV BK NS5B deltaC55 RNA dependent RNA polymerase |
Bioorg Med Chem Lett 16: 4026-30 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.012
BindingDB Entry DOI: 10.7270/Q2416WP6 |
More data for this
Ligand-Target Pair | |
Sterol 14-alpha demethylase
(Trypanosoma cruzi) | BDBM50545214

(CHEMBL4633298)Show SMILES CCC(=O)CCCCCC(Oc1ccc2ccccc2c1)c1ncc(Cc2ccncc2)[nH]1 Show InChI InChI=1S/C28H31N3O2/c1-2-25(32)10-4-3-5-11-27(33-26-13-12-22-8-6-7-9-23(22)19-26)28-30-20-24(31-28)18-21-14-16-29-17-15-21/h6-9,12-17,19-20,27H,2-5,10-11,18H2,1H3,(H,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM Spa
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma cruzi CYP51 |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127052
BindingDB Entry DOI: 10.7270/Q2C82DW5 |
More data for this
Ligand-Target Pair | |
Sterol 14-alpha demethylase
(Trypanosoma cruzi) | BDBM50545214

(CHEMBL4633298)Show SMILES CCC(=O)CCCCCC(Oc1ccc2ccccc2c1)c1ncc(Cc2ccncc2)[nH]1 Show InChI InChI=1S/C28H31N3O2/c1-2-25(32)10-4-3-5-11-27(33-26-13-12-22-8-6-7-9-23(22)19-26)28-30-20-24(31-28)18-21-14-16-29-17-15-21/h6-9,12-17,19-20,27H,2-5,10-11,18H2,1H3,(H,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM Spa
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma cruzi CYP51 |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127052
BindingDB Entry DOI: 10.7270/Q2C82DW5 |
More data for this
Ligand-Target Pair | |
Sterol 14-alpha demethylase
(Trypanosoma cruzi) | BDBM50545219
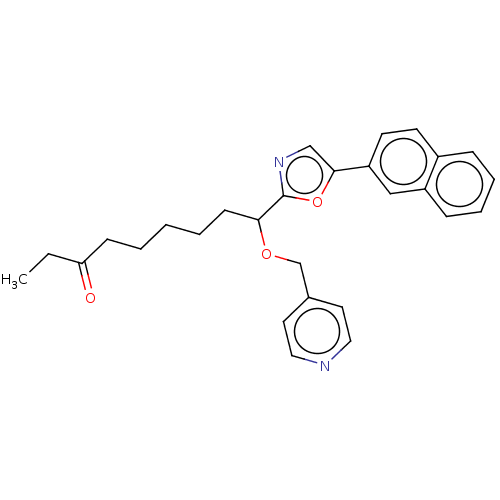
(CHEMBL4646274)Show SMILES CCC(=O)CCCCCC(OCc1ccncc1)c1ncc(o1)-c1ccc2ccccc2c1 Show InChI InChI=1S/C28H30N2O3/c1-2-25(31)10-4-3-5-11-26(32-20-21-14-16-29-17-15-21)28-30-19-27(33-28)24-13-12-22-8-6-7-9-23(22)18-24/h6-9,12-19,26H,2-5,10-11,20H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM Spa
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma cruzi CYP51 |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127052
BindingDB Entry DOI: 10.7270/Q2C82DW5 |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50187149
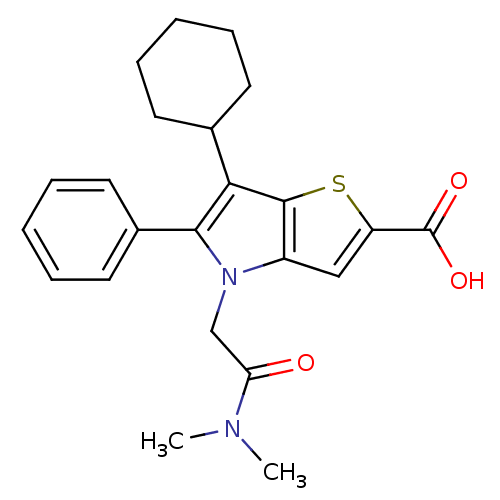
(6-cyclohexyl-4-(2-(dimethylamino)-2-oxoethyl)-5-ph...)Show SMILES CN(C)C(=O)Cn1c(c(C2CCCCC2)c2sc(cc12)C(O)=O)-c1ccccc1 Show InChI InChI=1S/C23H26N2O3S/c1-24(2)19(26)14-25-17-13-18(23(27)28)29-22(17)20(15-9-5-3-6-10-15)21(25)16-11-7-4-8-12-16/h4,7-8,11-13,15H,3,5-6,9-10,14H2,1-2H3,(H,27,28) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
MRL Rome
Curated by ChEMBL
| Assay Description
Inhibition of HCV BK NS5B deltaC55 RNA dependent RNA polymerase |
Bioorg Med Chem Lett 16: 4026-30 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.012
BindingDB Entry DOI: 10.7270/Q2416WP6 |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50162102
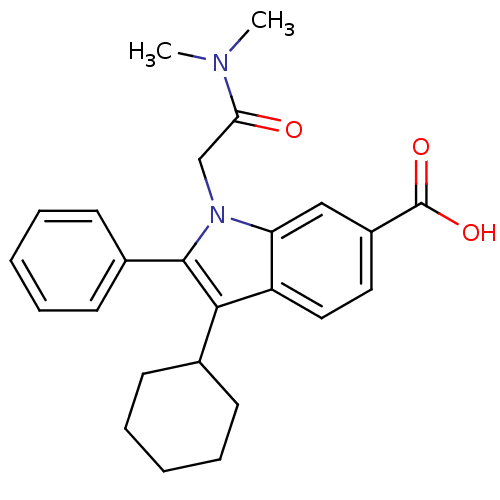
(3-Cyclohexyl-1-dimethylcarbamoylmethyl-2-phenyl-1H...)Show SMILES CN(C)C(=O)Cn1c(c(C2CCCCC2)c2ccc(cc12)C(O)=O)-c1ccccc1 Show InChI InChI=1S/C25H28N2O3/c1-26(2)22(28)16-27-21-15-19(25(29)30)13-14-20(21)23(17-9-5-3-6-10-17)24(27)18-11-7-4-8-12-18/h4,7-8,11-15,17H,3,5-6,9-10,16H2,1-2H3,(H,29,30) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
MRL Rome
Curated by ChEMBL
| Assay Description
Inhibition of HCV BK NS5B deltaC55 RNA dependent RNA polymerase |
Bioorg Med Chem Lett 16: 4026-30 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.012
BindingDB Entry DOI: 10.7270/Q2416WP6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50406428
(CHEMBL5289545)Show InChI InChI=1S/C10H15NO5S2/c11-18(15,16)10-5-3-9(4-6-10)17(13,14)8-2-1-7-12/h3-6,12H,1-2,7-8H2,(H2,11,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibition of human dihydrofolate reductase |
Citation and Details
|
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50187141
(6-cyclohexyl-5-phenyl-4H-thieno[3,2-b]pyrrole-2-ca...)Show InChI InChI=1S/C19H19NO2S/c21-19(22)15-11-14-18(23-15)16(12-7-3-1-4-8-12)17(20-14)13-9-5-2-6-10-13/h2,5-6,9-12,20H,1,3-4,7-8H2,(H,21,22) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
MRL Rome
Curated by ChEMBL
| Assay Description
Inhibition of HCV BK NS5B deltaC55 RNA dependent RNA polymerase |
Bioorg Med Chem Lett 16: 4026-30 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.012
BindingDB Entry DOI: 10.7270/Q2416WP6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM38019

(US10093646, Compound 1 | US10301278, Example 00003...)Show InChI InChI=1S/C24H34N4O2/c1-18-8-7-9-19-16-20(23(29)26-22(18)19)17-28(15-14-27-12-5-6-13-27)24(30)25-21-10-3-2-4-11-21/h7-9,16,21H,2-6,10-15,17H2,1H3,(H,25,30)(H,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
| n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of dihydrofolate reductase in Candida albicans (in vitro). |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50406374
(CHEMBL5266583)Show SMILES NCCCC[C@H](OP(O)(=O)CCCCc1ccccc1)C(=O)N1[C@H]2CCCC[C@H]2C[C@H]1C(O)=O Show InChI InChI=1S/C25H39N2O6P/c26-16-8-6-15-23(33-34(31,32)17-9-7-12-19-10-2-1-3-11-19)24(28)27-21-14-5-4-13-20(21)18-22(27)25(29)30/h1-3,10-11,20-23H,4-9,12-18,26H2,(H,29,30)(H,31,32)/t20-,21-,22-,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of dihydrofolate reductase in Candida albicans (in vitro). |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Sterol 14-alpha demethylase
(Trypanosoma cruzi) | BDBM50545216
(CHEMBL4638031)Show InChI InChI=1S/C21H19N3O/c1-15(25-14-16-8-10-22-11-9-16)21-23-13-20(24-21)19-7-6-17-4-2-3-5-18(17)12-19/h2-13,15H,14H2,1H3,(H,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM Spa
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma cruzi CYP51 |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127052
BindingDB Entry DOI: 10.7270/Q2C82DW5 |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50187135
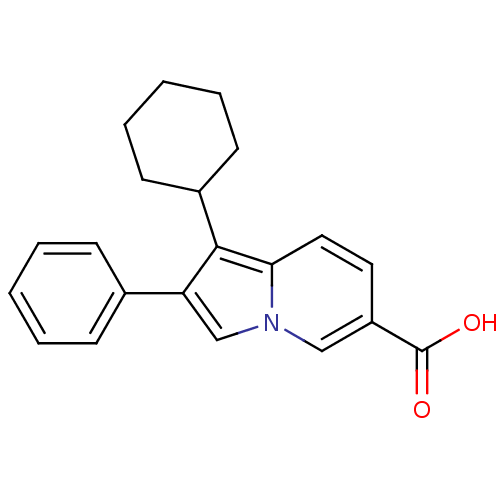
(1-cyclohexyl-2-phenylindolizine-6-carboxylic acid ...)Show InChI InChI=1S/C21H21NO2/c23-21(24)17-11-12-19-20(16-9-5-2-6-10-16)18(14-22(19)13-17)15-7-3-1-4-8-15/h1,3-4,7-8,11-14,16H,2,5-6,9-10H2,(H,23,24) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
MRL Rome
Curated by ChEMBL
| Assay Description
Inhibition of HCV BK NS5B deltaC55 RNA dependent RNA polymerase |
Bioorg Med Chem Lett 16: 4026-30 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.012
BindingDB Entry DOI: 10.7270/Q2416WP6 |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50187140

(4-cyclohexyl-5-phenyl-6H-thieno[2,3-b]pyrrole-2-ca...)Show InChI InChI=1S/C19H19NO2S/c21-19(22)15-11-14-16(12-7-3-1-4-8-12)17(20-18(14)23-15)13-9-5-2-6-10-13/h2,5-6,9-12,20H,1,3-4,7-8H2,(H,21,22) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
MRL Rome
Curated by ChEMBL
| Assay Description
Inhibition of HCV BK NS5B deltaC55 RNA dependent RNA polymerase |
Bioorg Med Chem Lett 16: 4026-30 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.012
BindingDB Entry DOI: 10.7270/Q2416WP6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM392347
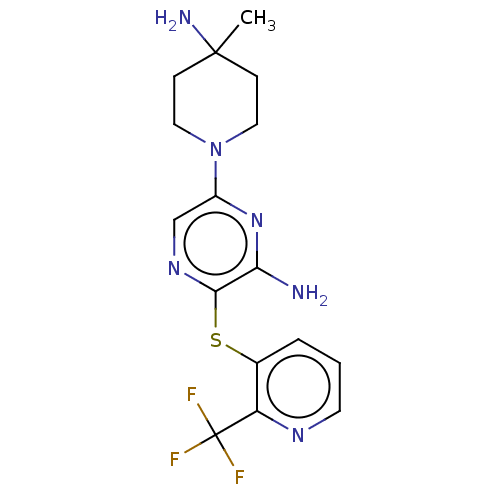
(US10301278, Example 30)Show SMILES CC1(N)CCN(CC1)c1cnc(Sc2cccnc2C(F)(F)F)c(N)n1 Show InChI InChI=1S/C16H19F3N6S/c1-15(21)4-7-25(8-5-15)11-9-23-14(13(20)24-11)26-10-3-2-6-22-12(10)16(17,18)19/h2-3,6,9H,4-5,7-8,21H2,1H3,(H2,20,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 142 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibition of human dihydrofolate reductase |
Citation and Details
|
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50187139
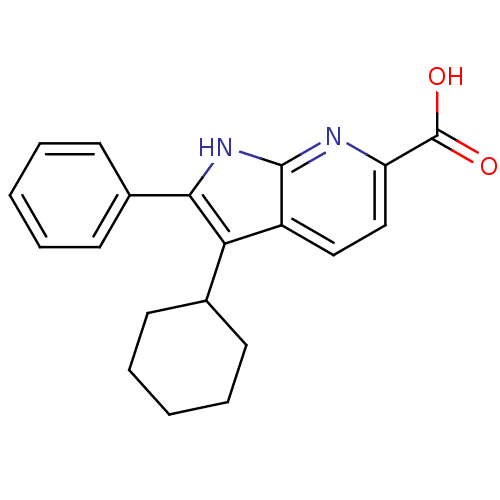
(3-cyclohexyl-2-phenyl-1H-pyrrolo[2,3-b]pyridine-6-...)Show SMILES OC(=O)c1ccc2c(C3CCCCC3)c([nH]c2n1)-c1ccccc1 Show InChI InChI=1S/C20H20N2O2/c23-20(24)16-12-11-15-17(13-7-3-1-4-8-13)18(22-19(15)21-16)14-9-5-2-6-10-14/h2,5-6,9-13H,1,3-4,7-8H2,(H,21,22)(H,23,24) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
MRL Rome
Curated by ChEMBL
| Assay Description
Inhibition of HCV BK NS5B deltaC55 RNA dependent RNA polymerase |
Bioorg Med Chem Lett 16: 4026-30 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.012
BindingDB Entry DOI: 10.7270/Q2416WP6 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50612442
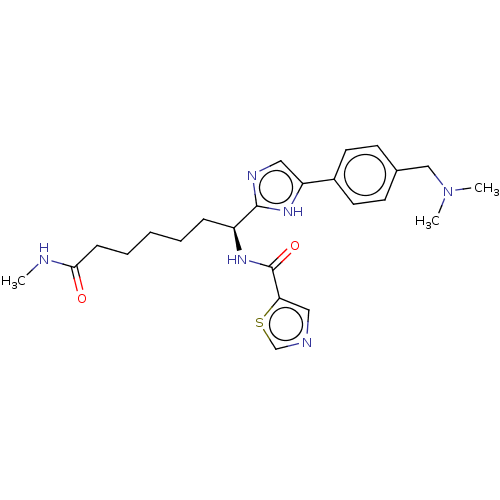
(CHEMBL5271865)Show SMILES CNC(=O)CCCCC[C@H](NC(=O)c1cncs1)c1ncc([nH]1)-c1ccc(CN(C)C)cc1 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 162 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50612449

(CHEMBL5271471)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)C1CN(C)C1)c1ncc(o1)-c1cc2ccccc2nc1OC |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 177 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50612445

(CHEMBL5282110)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)C12CCN(CC1)CC2)c1ncc(o1)-c1cc2ccccc2nc1OC |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50175039

(CHEMBL3808639)Show SMILES CNC(=O)CCCCC[C@H](NC(=O)c1cncs1)c1ncc([nH]1)-c1cc2ccccc2nc1OC |r| Show InChI InChI=1S/C17H13Cl2N3O2S/c1-24-15-6-5-10(8-20-15)21-17(23)14-9-25-16(22-14)7-11-12(18)3-2-4-13(11)19/h2-6,8-9H,7H2,1H3,(H,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| | n/a | n/a | 198 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50175039

(CHEMBL3808639)Show SMILES CNC(=O)CCCCC[C@H](NC(=O)c1cncs1)c1ncc([nH]1)-c1cc2ccccc2nc1OC |r| Show InChI InChI=1S/C17H13Cl2N3O2S/c1-24-15-6-5-10(8-20-15)21-17(23)14-9-25-16(22-14)7-11-12(18)3-2-4-13(11)19/h2-6,8-9H,7H2,1H3,(H,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM Science Park
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-tagged HDAC1 expressed in insect cells preincubated for 10 mins followed by addition of FLUOR DE LYS as fluoresce... |
ACS Med Chem Lett 7: 454-9 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00468
BindingDB Entry DOI: 10.7270/Q2S184FM |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data