

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50044676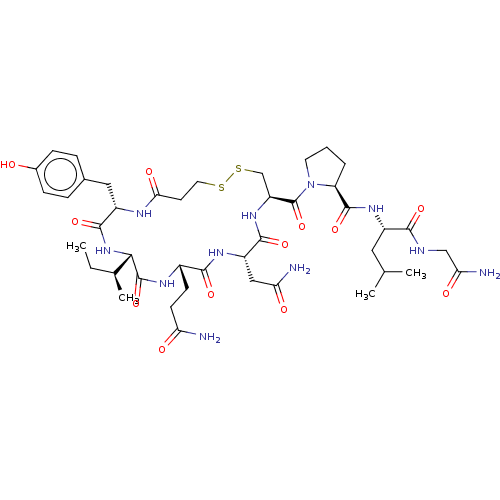 (CHEMBL439044) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]8-arginine-vasopressin from human oxytocin receptor expressed in CHO cell membrane incubated for 1 hr by liquid scintillation cou... | Bioorg Med Chem 24: 3513-20 (2016) Article DOI: 10.1016/j.bmc.2016.05.062 BindingDB Entry DOI: 10.7270/Q2WH2RXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50205990 (CHEMBL395429 | OXYTOCIN) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]8-arginine-vasopressin from human oxytocin receptor expressed in CHO cell membrane incubated for 1 hr by liquid scintillation cou... | Bioorg Med Chem 24: 3513-20 (2016) Article DOI: 10.1016/j.bmc.2016.05.062 BindingDB Entry DOI: 10.7270/Q2WH2RXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50180014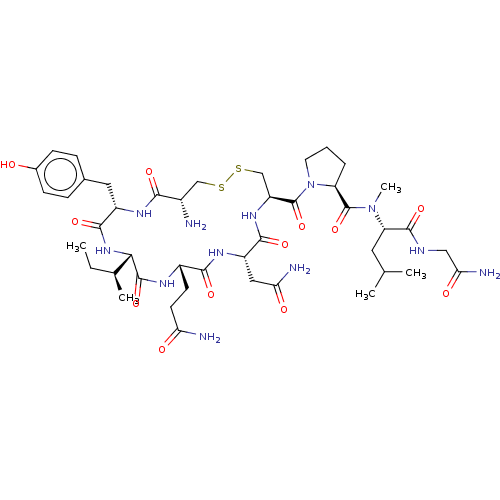 (CHEMBL3814744) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]8-arginine-vasopressin from human oxytocin receptor expressed in CHO cell membrane incubated for 1 hr by liquid scintillation cou... | Bioorg Med Chem 24: 3513-20 (2016) Article DOI: 10.1016/j.bmc.2016.05.062 BindingDB Entry DOI: 10.7270/Q2WH2RXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50180155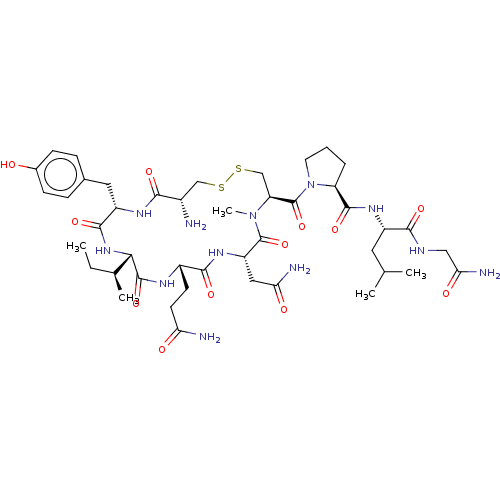 (CHEMBL3813894) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]8-arginine-vasopressin from human oxytocin receptor expressed in CHO cell membrane incubated for 1 hr by liquid scintillation cou... | Bioorg Med Chem 24: 3513-20 (2016) Article DOI: 10.1016/j.bmc.2016.05.062 BindingDB Entry DOI: 10.7270/Q2WH2RXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50180132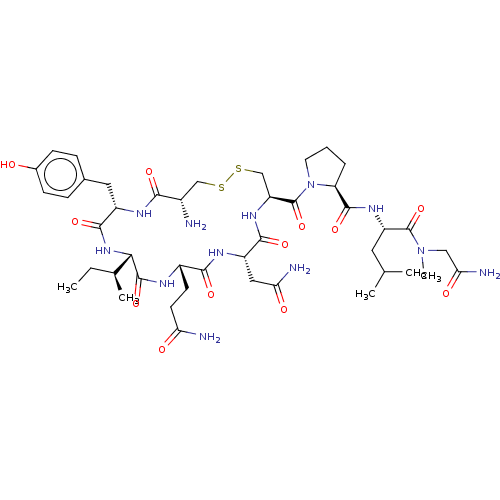 (CHEMBL3814633) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]8-arginine-vasopressin from human oxytocin receptor expressed in CHO cell membrane incubated for 1 hr by liquid scintillation cou... | Bioorg Med Chem 24: 3513-20 (2016) Article DOI: 10.1016/j.bmc.2016.05.062 BindingDB Entry DOI: 10.7270/Q2WH2RXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50180160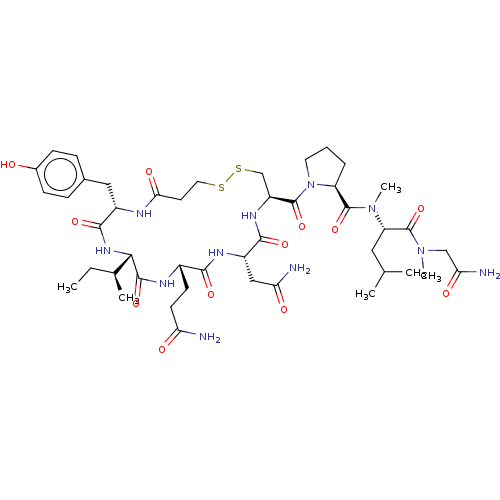 (CHEMBL3815099) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]8-arginine-vasopressin from human oxytocin receptor expressed in CHO cell membrane incubated for 1 hr by liquid scintillation cou... | Bioorg Med Chem 24: 3513-20 (2016) Article DOI: 10.1016/j.bmc.2016.05.062 BindingDB Entry DOI: 10.7270/Q2WH2RXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50179992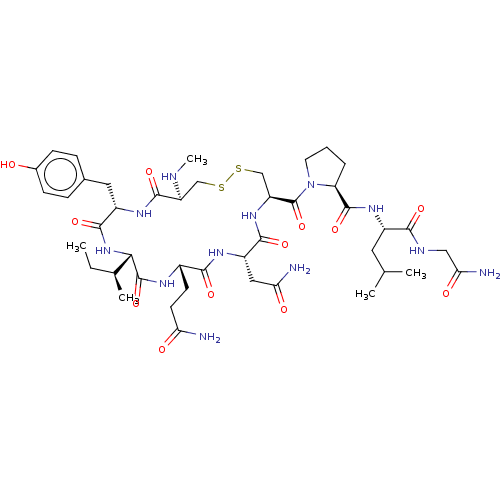 (CHEMBL3814395) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]8-arginine-vasopressin from human oxytocin receptor expressed in CHO cell membrane incubated for 1 hr by liquid scintillation cou... | Bioorg Med Chem 24: 3513-20 (2016) Article DOI: 10.1016/j.bmc.2016.05.062 BindingDB Entry DOI: 10.7270/Q2WH2RXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50179993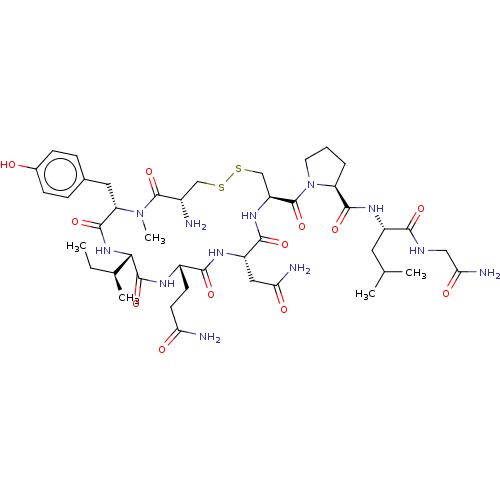 (CHEMBL3814165) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]8-arginine-vasopressin from human oxytocin receptor expressed in CHO cell membrane incubated for 1 hr by liquid scintillation cou... | Bioorg Med Chem 24: 3513-20 (2016) Article DOI: 10.1016/j.bmc.2016.05.062 BindingDB Entry DOI: 10.7270/Q2WH2RXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50180165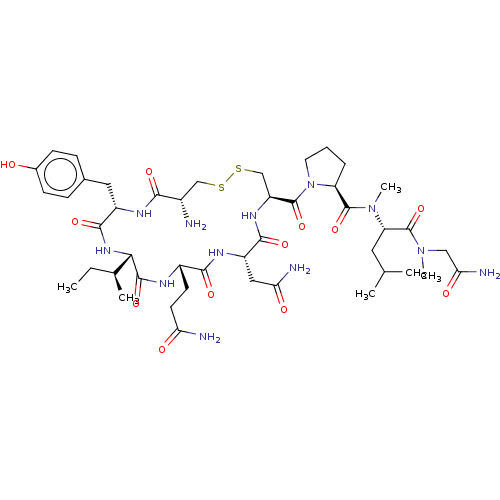 (CHEMBL3813722) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]8-arginine-vasopressin from human oxytocin receptor expressed in CHO cell membrane incubated for 1 hr by liquid scintillation cou... | Bioorg Med Chem 24: 3513-20 (2016) Article DOI: 10.1016/j.bmc.2016.05.062 BindingDB Entry DOI: 10.7270/Q2WH2RXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50180154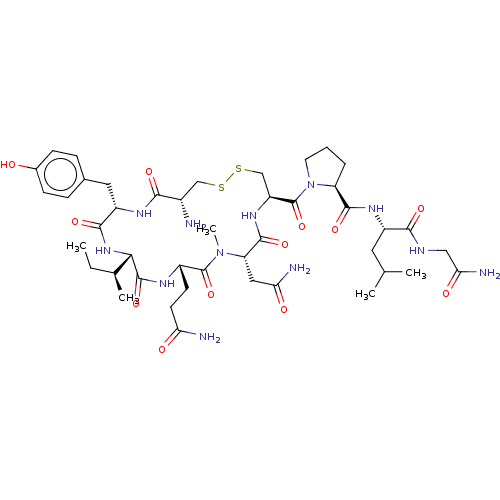 (CHEMBL3814617) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]8-arginine-vasopressin from human oxytocin receptor expressed in CHO cell membrane incubated for 1 hr by liquid scintillation cou... | Bioorg Med Chem 24: 3513-20 (2016) Article DOI: 10.1016/j.bmc.2016.05.062 BindingDB Entry DOI: 10.7270/Q2WH2RXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50180164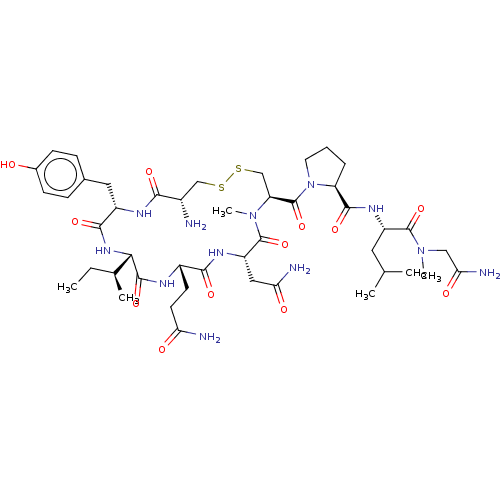 (CHEMBL3814120) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]8-arginine-vasopressin from human oxytocin receptor expressed in CHO cell membrane incubated for 1 hr by liquid scintillation cou... | Bioorg Med Chem 24: 3513-20 (2016) Article DOI: 10.1016/j.bmc.2016.05.062 BindingDB Entry DOI: 10.7270/Q2WH2RXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50180158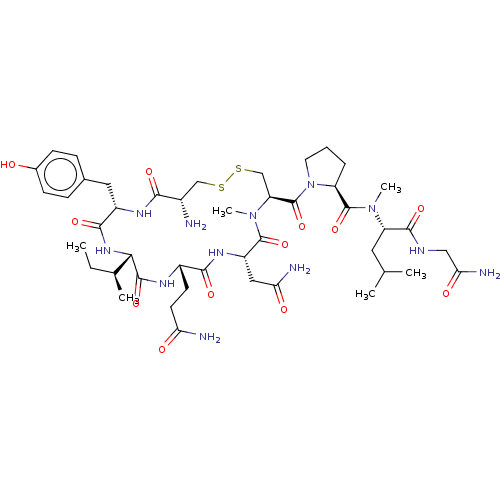 (CHEMBL3813858) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]8-arginine-vasopressin from human oxytocin receptor expressed in CHO cell membrane incubated for 1 hr by liquid scintillation cou... | Bioorg Med Chem 24: 3513-20 (2016) Article DOI: 10.1016/j.bmc.2016.05.062 BindingDB Entry DOI: 10.7270/Q2WH2RXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50180159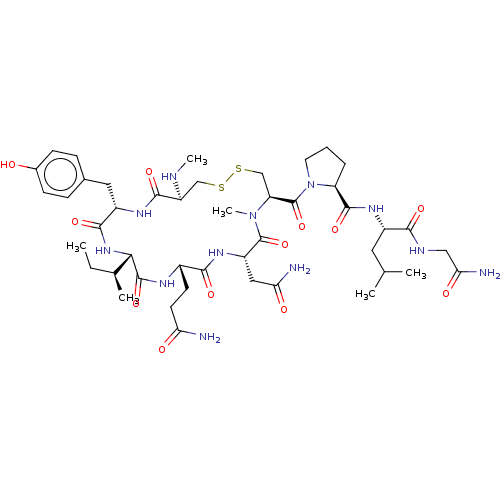 (CHEMBL3814232) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]8-arginine-vasopressin from human oxytocin receptor expressed in CHO cell membrane incubated for 1 hr by liquid scintillation cou... | Bioorg Med Chem 24: 3513-20 (2016) Article DOI: 10.1016/j.bmc.2016.05.062 BindingDB Entry DOI: 10.7270/Q2WH2RXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50179994 (CHEMBL3814126) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 113 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]8-arginine-vasopressin from human oxytocin receptor expressed in CHO cell membrane incubated for 1 hr by liquid scintillation cou... | Bioorg Med Chem 24: 3513-20 (2016) Article DOI: 10.1016/j.bmc.2016.05.062 BindingDB Entry DOI: 10.7270/Q2WH2RXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50180013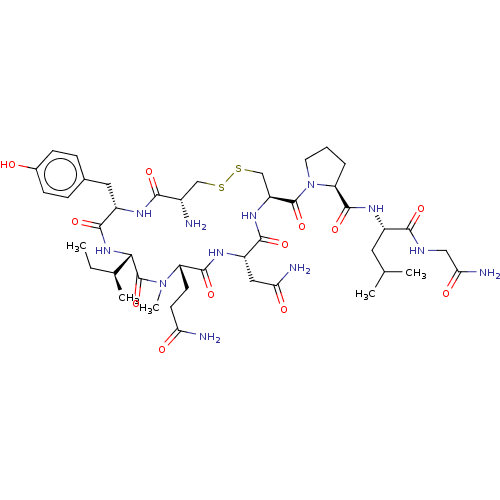 (CHEMBL3814904) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >1.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]8-arginine-vasopressin from human oxytocin receptor expressed in CHO cell membrane incubated for 1 hr by liquid scintillation cou... | Bioorg Med Chem 24: 3513-20 (2016) Article DOI: 10.1016/j.bmc.2016.05.062 BindingDB Entry DOI: 10.7270/Q2WH2RXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM490011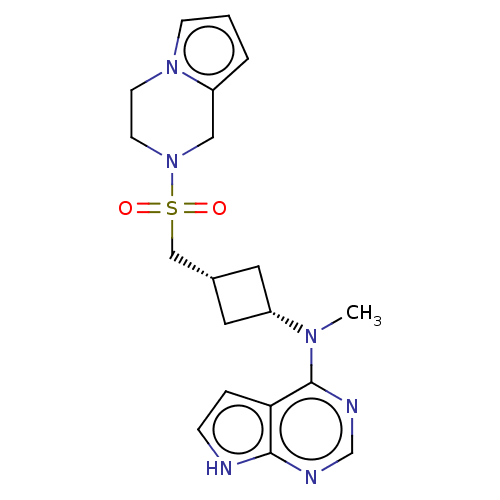 (US10966980, Example 265) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... | US Patent US10966980 (2021) BindingDB Entry DOI: 10.7270/Q29S1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM490009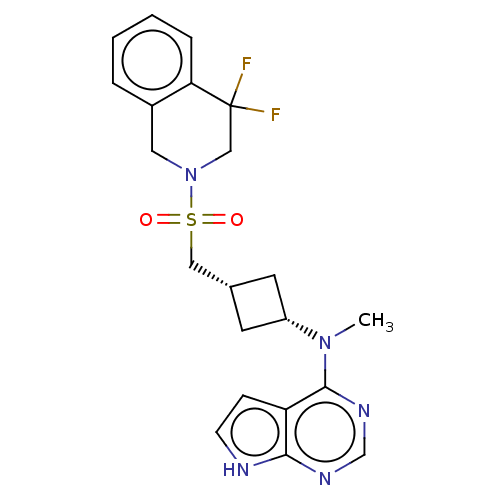 (US10966980, Example 263) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... | US Patent US10966980 (2021) BindingDB Entry DOI: 10.7270/Q29S1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM489965 (US10966980, Example 219) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... | US Patent US10966980 (2021) BindingDB Entry DOI: 10.7270/Q29S1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM489909 (US10966980, Example 163) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... | US Patent US10966980 (2021) BindingDB Entry DOI: 10.7270/Q29S1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM489982 (US10966980, Example 236) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... | US Patent US10966980 (2021) BindingDB Entry DOI: 10.7270/Q29S1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM489985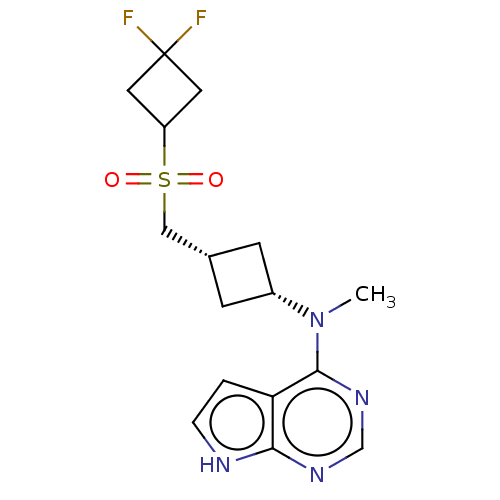 (US10966980, Example 239) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... | US Patent US10966980 (2021) BindingDB Entry DOI: 10.7270/Q29S1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM489983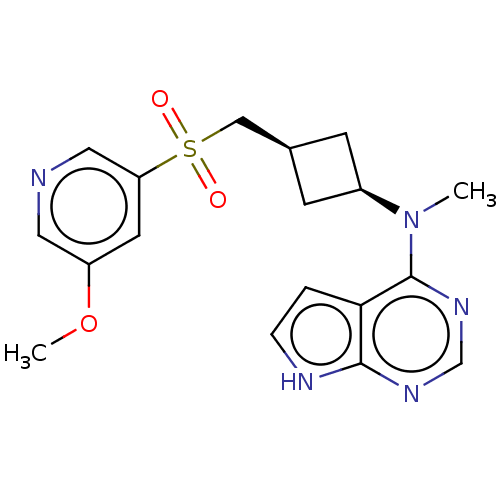 (US10966980, Example 237) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... | US Patent US10966980 (2021) BindingDB Entry DOI: 10.7270/Q29S1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM489960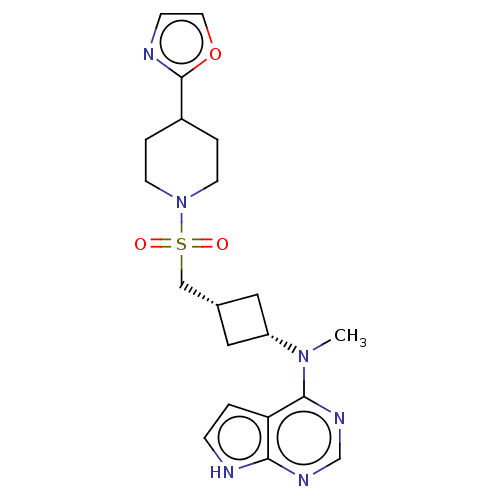 (US10966980, Example 214) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... | US Patent US10966980 (2021) BindingDB Entry DOI: 10.7270/Q29S1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM489998 (US10966980, Example 252) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... | US Patent US10966980 (2021) BindingDB Entry DOI: 10.7270/Q29S1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM489974 (US10966980, Example 228) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... | US Patent US10966980 (2021) BindingDB Entry DOI: 10.7270/Q29S1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM489940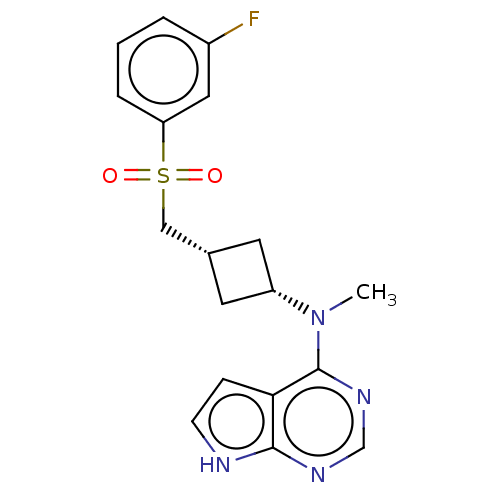 (US10966980, Example 194) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... | US Patent US10966980 (2021) BindingDB Entry DOI: 10.7270/Q29S1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM490006 (US10966980, Example 260) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... | US Patent US10966980 (2021) BindingDB Entry DOI: 10.7270/Q29S1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM489977 (US10966980, Example 231) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... | US Patent US10966980 (2021) BindingDB Entry DOI: 10.7270/Q29S1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM490010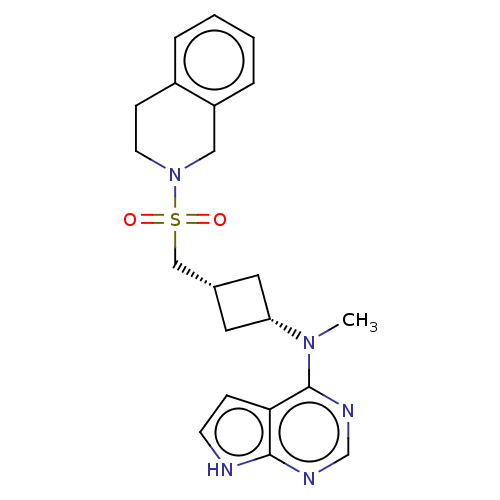 (US10966980, Example 264) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... | US Patent US10966980 (2021) BindingDB Entry DOI: 10.7270/Q29S1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM489986 (US10966980, Example 240) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... | US Patent US10966980 (2021) BindingDB Entry DOI: 10.7270/Q29S1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM490008 (US10966980, Example 262) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... | US Patent US10966980 (2021) BindingDB Entry DOI: 10.7270/Q29S1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM489922 (US10966980, Example 176) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... | US Patent US10966980 (2021) BindingDB Entry DOI: 10.7270/Q29S1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM489916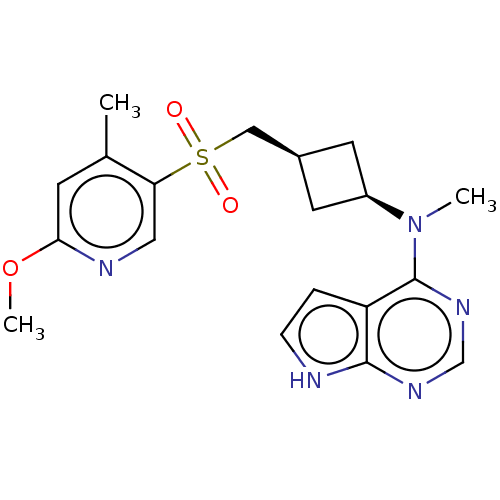 (US10966980, Example 170) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... | US Patent US10966980 (2021) BindingDB Entry DOI: 10.7270/Q29S1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM489978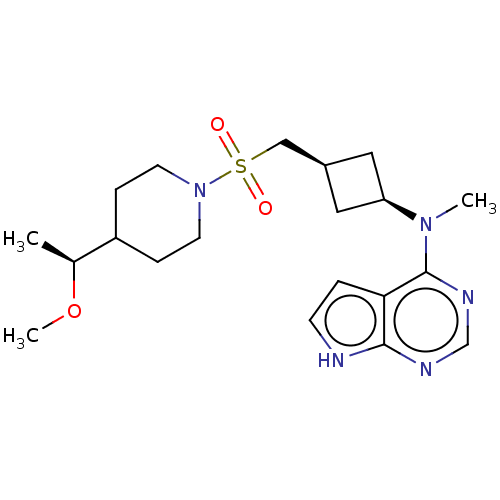 (US10966980, Example 232) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... | US Patent US10966980 (2021) BindingDB Entry DOI: 10.7270/Q29S1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM489941 (US10966980, Example 195) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... | US Patent US10966980 (2021) BindingDB Entry DOI: 10.7270/Q29S1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM489987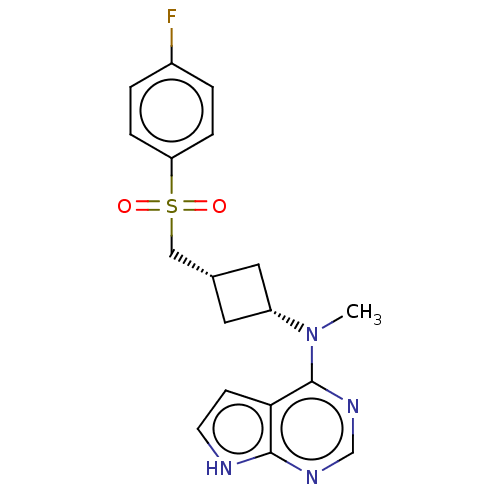 (US10966980, Example 241) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... | US Patent US10966980 (2021) BindingDB Entry DOI: 10.7270/Q29S1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM490005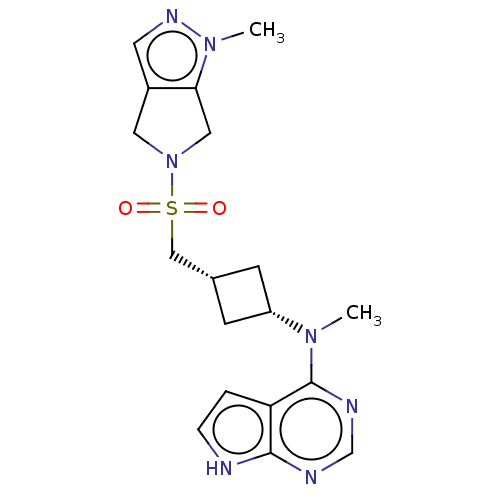 (US10966980, Example 259) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... | US Patent US10966980 (2021) BindingDB Entry DOI: 10.7270/Q29S1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM489994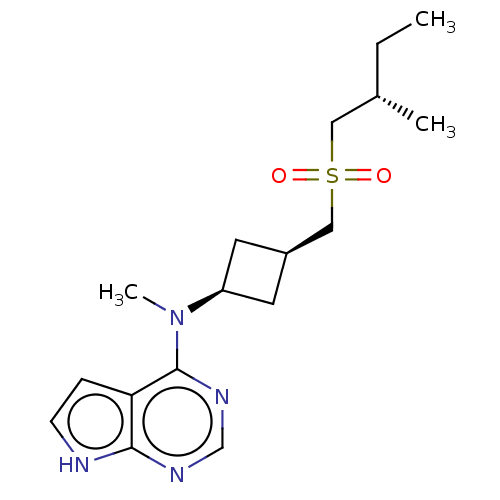 (US10966980, Example 248) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... | US Patent US10966980 (2021) BindingDB Entry DOI: 10.7270/Q29S1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM489934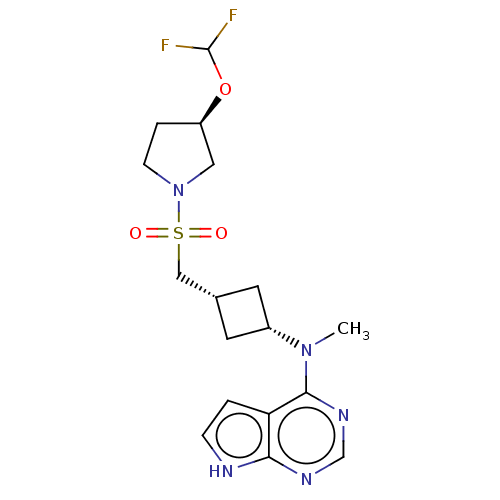 (US10966980, Example 188) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... | US Patent US10966980 (2021) BindingDB Entry DOI: 10.7270/Q29S1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM489968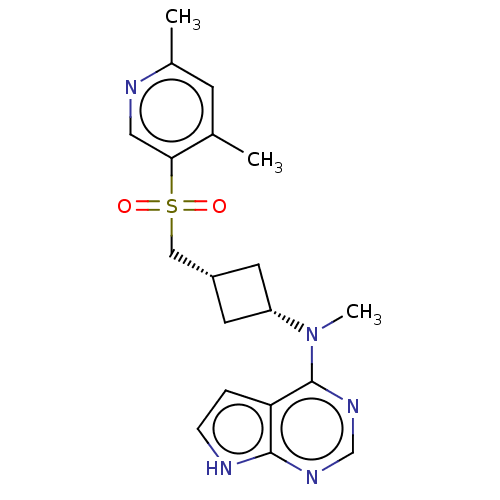 (US10966980, Example 222) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... | US Patent US10966980 (2021) BindingDB Entry DOI: 10.7270/Q29S1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM489906 (US10966980, Example 160) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... | US Patent US10966980 (2021) BindingDB Entry DOI: 10.7270/Q29S1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM489971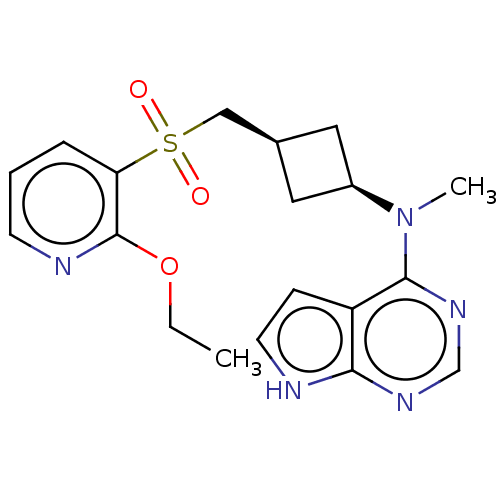 (US10966980, Example 225) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... | US Patent US10966980 (2021) BindingDB Entry DOI: 10.7270/Q29S1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM489970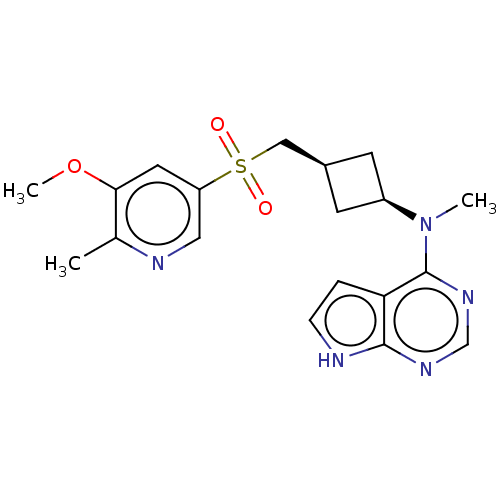 (US10966980, Example 224) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... | US Patent US10966980 (2021) BindingDB Entry DOI: 10.7270/Q29S1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM489969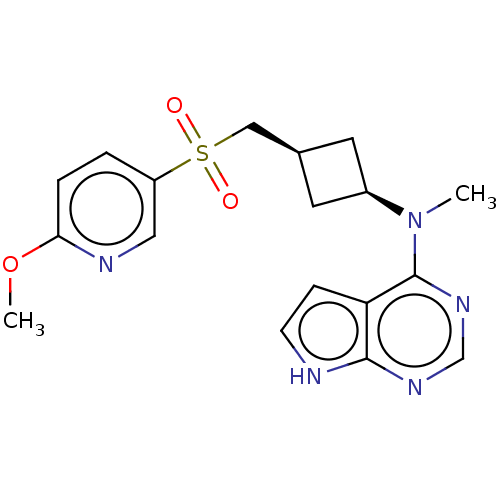 (US10966980, Example 223) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... | US Patent US10966980 (2021) BindingDB Entry DOI: 10.7270/Q29S1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM489901 (US10966980, Example 155) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... | US Patent US10966980 (2021) BindingDB Entry DOI: 10.7270/Q29S1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM489920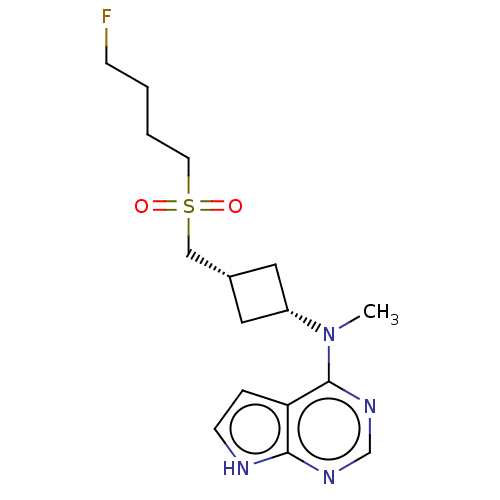 (US10966980, Example 174) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... | US Patent US10966980 (2021) BindingDB Entry DOI: 10.7270/Q29S1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM490000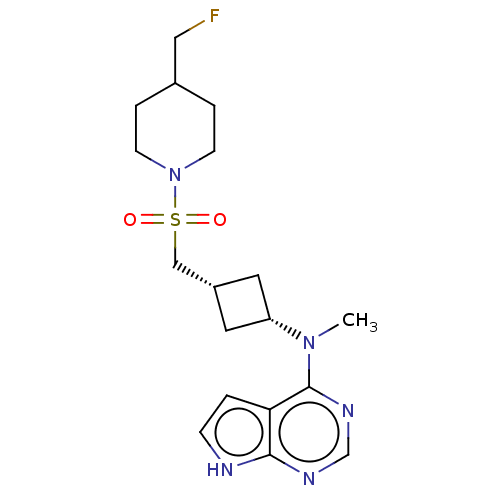 (US10966980, Example 254) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... | US Patent US10966980 (2021) BindingDB Entry DOI: 10.7270/Q29S1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM489961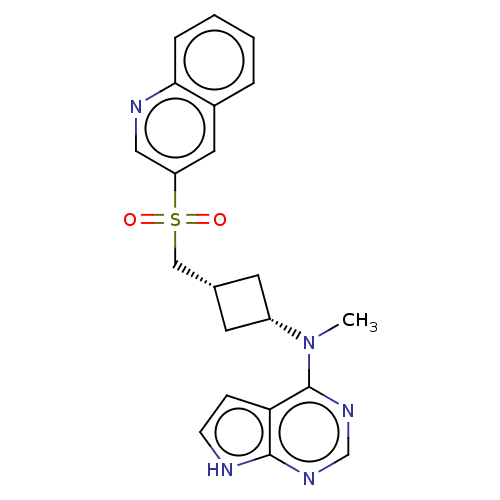 (US10966980, Example 215) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... | US Patent US10966980 (2021) BindingDB Entry DOI: 10.7270/Q29S1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM489962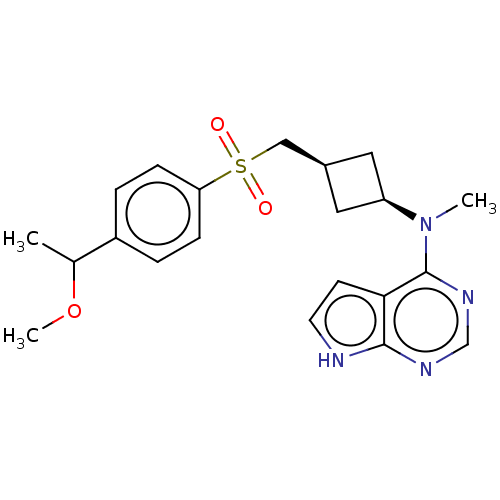 (US10966980, Example 216) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... | US Patent US10966980 (2021) BindingDB Entry DOI: 10.7270/Q29S1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM489963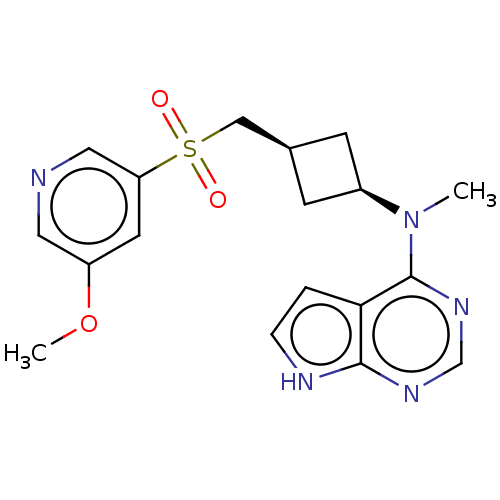 (US10966980, Example 217) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... | US Patent US10966980 (2021) BindingDB Entry DOI: 10.7270/Q29S1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 3388 total ) | Next | Last >> |