 Found 238 hits with Last Name = 'tsutsumi' and Initial = 's'
Found 238 hits with Last Name = 'tsutsumi' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50401285
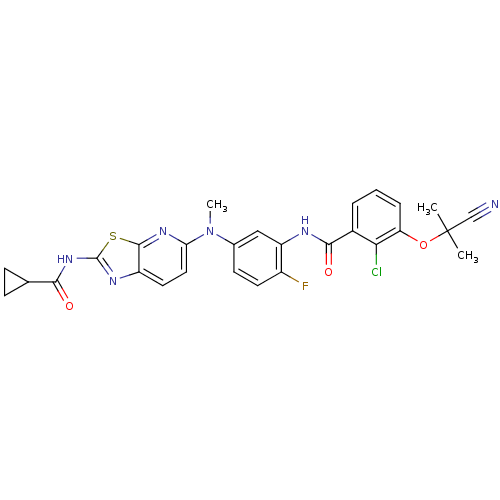
(CHEMBL2204532)Show SMILES CN(c1ccc(F)c(NC(=O)c2cccc(OC(C)(C)C#N)c2Cl)c1)c1ccc2nc(NC(=O)C3CC3)sc2n1 Show InChI InChI=1S/C28H24ClFN6O3S/c1-28(2,14-31)39-21-6-4-5-17(23(21)29)25(38)32-20-13-16(9-10-18(20)30)36(3)22-12-11-19-26(34-22)40-27(33-19)35-24(37)15-7-8-15/h4-6,9-13,15H,7-8H2,1-3H3,(H,32,38)(H,33,35,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 in HUVEC assessed as reduction of VGF-stimulated cell proliferation after 5 days |
Bioorg Med Chem 20: 5600-15 (2012)
Article DOI: 10.1016/j.bmc.2012.07.032
BindingDB Entry DOI: 10.7270/Q2765GH8 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50291167
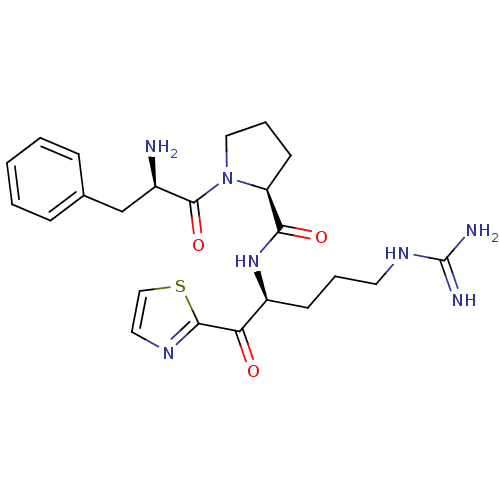
(1-((S)-Oxo-3-(R)-2-amino-1-phenyl-propyl)-pyrrolid...)Show SMILES N[C@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)c1nccs1 Show InChI InChI=1S/C23H31N7O3S/c24-16(14-15-6-2-1-3-7-15)22(33)30-12-5-9-18(30)20(32)29-17(8-4-10-28-23(25)26)19(31)21-27-11-13-34-21/h1-3,6-7,11,13,16-18H,4-5,8-10,12,14,24H2,(H,29,32)(H4,25,26,28)/t16-,17+,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 7: 533-538 (1997)
Article DOI: 10.1016/S0960-894X(97)00057-7
BindingDB Entry DOI: 10.7270/Q28S4QF2 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM50291175
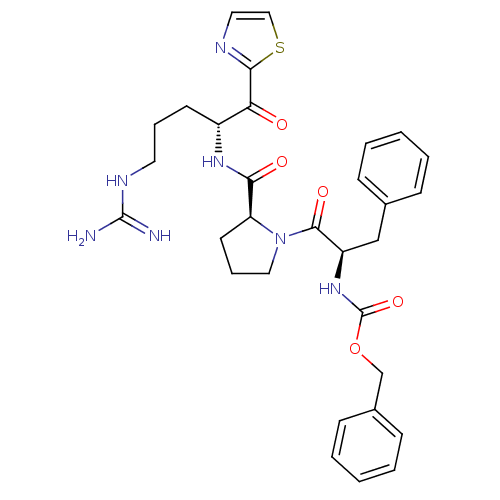
(((R)-1-Benzyl-2-{(S)-2-[(R)-4-guanidino-1-(thiazol...)Show SMILES NC(=N)NCCC[C@@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H](Cc1ccccc1)NC(=O)OCc1ccccc1)C(=O)c1nccs1 Show InChI InChI=1S/C31H37N7O5S/c32-30(33)35-15-7-13-23(26(39)28-34-16-18-44-28)36-27(40)25-14-8-17-38(25)29(41)24(19-21-9-3-1-4-10-21)37-31(42)43-20-22-11-5-2-6-12-22/h1-6,9-12,16,18,23-25H,7-8,13-15,17,19-20H2,(H,36,40)(H,37,42)(H4,32,33,35)/t23-,24-,25+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of trypsin |
Bioorg Med Chem Lett 7: 533-538 (1997)
Article DOI: 10.1016/S0960-894X(97)00057-7
BindingDB Entry DOI: 10.7270/Q28S4QF2 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50291175

(((R)-1-Benzyl-2-{(S)-2-[(R)-4-guanidino-1-(thiazol...)Show SMILES NC(=N)NCCC[C@@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H](Cc1ccccc1)NC(=O)OCc1ccccc1)C(=O)c1nccs1 Show InChI InChI=1S/C31H37N7O5S/c32-30(33)35-15-7-13-23(26(39)28-34-16-18-44-28)36-27(40)25-14-8-17-38(25)29(41)24(19-21-9-3-1-4-10-21)37-31(42)43-20-22-11-5-2-6-12-22/h1-6,9-12,16,18,23-25H,7-8,13-15,17,19-20H2,(H,36,40)(H,37,42)(H4,32,33,35)/t23-,24-,25+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 7: 533-538 (1997)
Article DOI: 10.1016/S0960-894X(97)00057-7
BindingDB Entry DOI: 10.7270/Q28S4QF2 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50291173
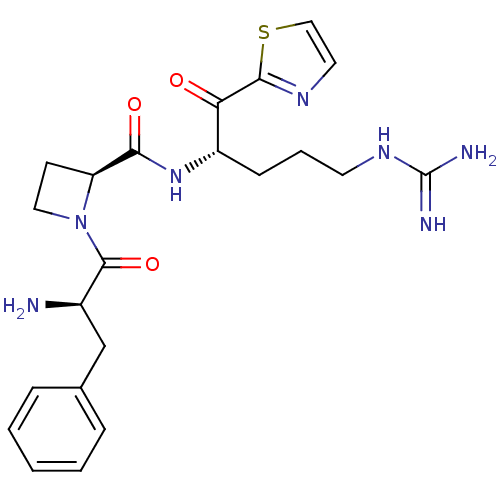
(1-((S)-2-Amino-3-(R)-phenyl-propionyl)-azetidine-2...)Show SMILES N[C@H](Cc1ccccc1)C(=O)N1CC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)c1nccs1 Show InChI InChI=1S/C22H29N7O3S/c23-15(13-14-5-2-1-3-6-14)21(32)29-11-8-17(29)19(31)28-16(7-4-9-27-22(24)25)18(30)20-26-10-12-33-20/h1-3,5-6,10,12,15-17H,4,7-9,11,13,23H2,(H,28,31)(H4,24,25,27)/t15-,16+,17+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 7: 533-538 (1997)
Article DOI: 10.1016/S0960-894X(97)00057-7
BindingDB Entry DOI: 10.7270/Q28S4QF2 |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Sus scrofa) | BDBM50284127
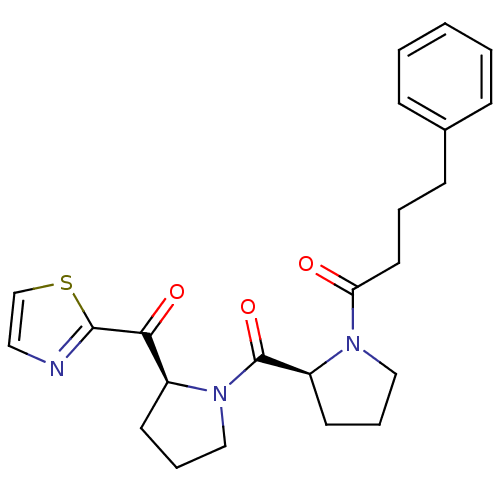
(4-Phenyl-1-{(S)-2-[(S)-2-(thiazole-2-carbonyl)-pyr...)Show SMILES O=C(CCCc1ccccc1)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)c1nccs1 Show InChI InChI=1S/C23H27N3O3S/c27-20(12-4-9-17-7-2-1-3-8-17)25-14-6-11-19(25)23(29)26-15-5-10-18(26)21(28)22-24-13-16-30-22/h1-3,7-8,13,16,18-19H,4-6,9-12,14-15H2/t18-,19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Prolyl endopeptidase (PEP) from pig kidney using Z-Gly-Pro-p-nitroanilide as substrate |
Bioorg Med Chem Lett 4: 831-834 (1994)
Article DOI: 10.1016/S0960-894X(01)80857-X
BindingDB Entry DOI: 10.7270/Q28W3D8R |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Sus scrofa) | BDBM50037610
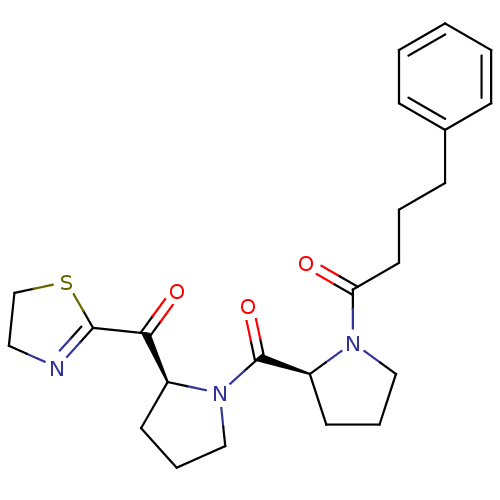
(1-{(S)-2-[(S)-2-(4,5-Dihydro-thiazole-2-carbonyl)-...)Show SMILES O=C(CCCc1ccccc1)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)C1=NCCS1 |t:28| Show InChI InChI=1S/C23H29N3O3S/c27-20(12-4-9-17-7-2-1-3-8-17)25-14-6-11-19(25)23(29)26-15-5-10-18(26)21(28)22-24-13-16-30-22/h1-3,7-8,18-19H,4-6,9-16H2/t18-,19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against prolyl endopeptidase (PEP) |
J Med Chem 37: 3492-502 (1994)
BindingDB Entry DOI: 10.7270/Q2RB73N9 |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Sus scrofa) | BDBM50037612
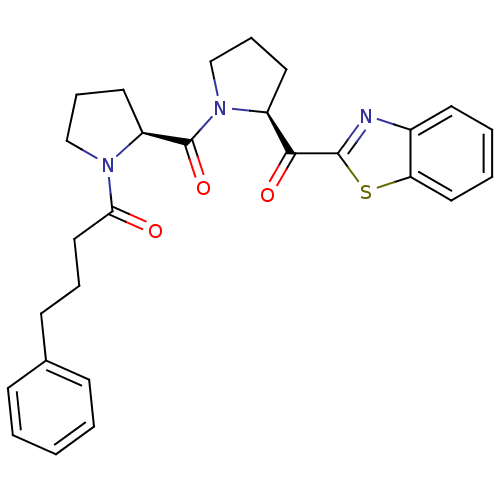
(1-{(S)-2-[(S)-2-(Benzothiazole-2-carbonyl)-pyrroli...)Show SMILES O=C(CCCc1ccccc1)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)c1nc2ccccc2s1 Show InChI InChI=1S/C27H29N3O3S/c31-24(16-6-11-19-9-2-1-3-10-19)29-17-8-14-22(29)27(33)30-18-7-13-21(30)25(32)26-28-20-12-4-5-15-23(20)34-26/h1-5,9-10,12,15,21-22H,6-8,11,13-14,16-18H2/t21-,22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against prolyl endopeptidase (PEP) |
J Med Chem 37: 3492-502 (1994)
BindingDB Entry DOI: 10.7270/Q2RB73N9 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM50291167

(1-((S)-Oxo-3-(R)-2-amino-1-phenyl-propyl)-pyrrolid...)Show SMILES N[C@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)c1nccs1 Show InChI InChI=1S/C23H31N7O3S/c24-16(14-15-6-2-1-3-7-15)22(33)30-12-5-9-18(30)20(32)29-17(8-4-10-28-23(25)26)19(31)21-27-11-13-34-21/h1-3,6-7,11,13,16-18H,4-5,8-10,12,14,24H2,(H,29,32)(H4,25,26,28)/t16-,17+,18+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of trypsin |
Bioorg Med Chem Lett 7: 533-538 (1997)
Article DOI: 10.1016/S0960-894X(97)00057-7
BindingDB Entry DOI: 10.7270/Q28S4QF2 |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Sus scrofa) | BDBM50291163
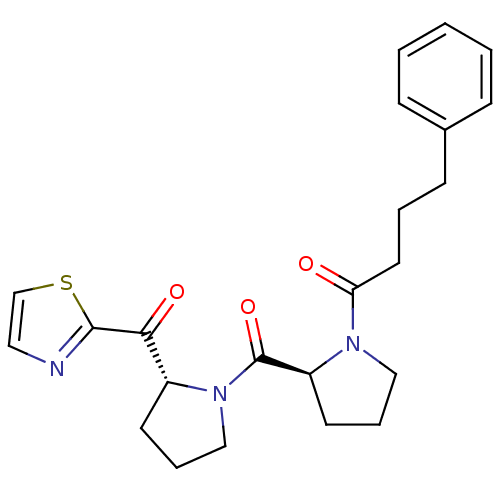
(4-Phenyl-1-{(S)-2-[(R)-2-(thiazole-2-carbonyl)-pyr...)Show SMILES O=C(CCCc1ccccc1)N1CCC[C@H]1C(=O)N1CCC[C@@H]1C(=O)c1nccs1 Show InChI InChI=1S/C23H27N3O3S/c27-20(12-4-9-17-7-2-1-3-8-17)25-14-6-11-19(25)23(29)26-15-5-10-18(26)21(28)22-24-13-16-30-22/h1-3,7-8,13,16,18-19H,4-6,9-12,14-15H2/t18-,19+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The compound was tested for the inhibitory activity against prolyl endo peptidase(PEP) enzyme |
Bioorg Med Chem Lett 7: 533-538 (1997)
Article DOI: 10.1016/S0960-894X(97)00057-7
BindingDB Entry DOI: 10.7270/Q28S4QF2 |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Sus scrofa) | BDBM50284127

(4-Phenyl-1-{(S)-2-[(S)-2-(thiazole-2-carbonyl)-pyr...)Show SMILES O=C(CCCc1ccccc1)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)c1nccs1 Show InChI InChI=1S/C23H27N3O3S/c27-20(12-4-9-17-7-2-1-3-8-17)25-14-6-11-19(25)23(29)26-15-5-10-18(26)21(28)22-24-13-16-30-22/h1-3,7-8,13,16,18-19H,4-6,9-12,14-15H2/t18-,19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against prolyl endopeptidase (PEP) |
J Med Chem 37: 3492-502 (1994)
BindingDB Entry DOI: 10.7270/Q2RB73N9 |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Sus scrofa) | BDBM50291168
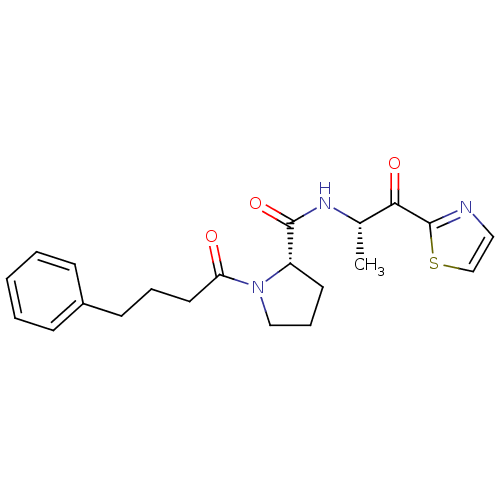
((S)-1-(4-Phenyl-butyryl)-pyrrolidine-2-carboxylic ...)Show SMILES C[C@H](NC(=O)[C@@H]1CCCN1C(=O)CCCc1ccccc1)C(=O)c1nccs1 Show InChI InChI=1S/C21H25N3O3S/c1-15(19(26)21-22-12-14-28-21)23-20(27)17-10-6-13-24(17)18(25)11-5-9-16-7-3-2-4-8-16/h2-4,7-8,12,14-15,17H,5-6,9-11,13H2,1H3,(H,23,27)/t15-,17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The compound was tested for the inhibitory activity against prolyl endo peptidase(PEP) enzyme |
Bioorg Med Chem Lett 7: 533-538 (1997)
Article DOI: 10.1016/S0960-894X(97)00057-7
BindingDB Entry DOI: 10.7270/Q28S4QF2 |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Sus scrofa) | BDBM50037605
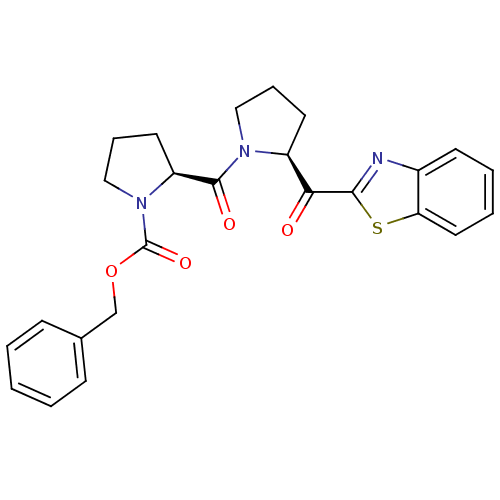
((S)-2-[(S)-2-(Benzothiazole-2-carbonyl)-pyrrolidin...)Show SMILES O=C(OCc1ccccc1)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)c1nc2ccccc2s1 Show InChI InChI=1S/C25H25N3O4S/c29-22(23-26-18-10-4-5-13-21(18)33-23)19-11-6-14-27(19)24(30)20-12-7-15-28(20)25(31)32-16-17-8-2-1-3-9-17/h1-5,8-10,13,19-20H,6-7,11-12,14-16H2/t19-,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against prolyl endopeptidase (PEP) |
J Med Chem 37: 3492-502 (1994)
BindingDB Entry DOI: 10.7270/Q2RB73N9 |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Sus scrofa) | BDBM50037611
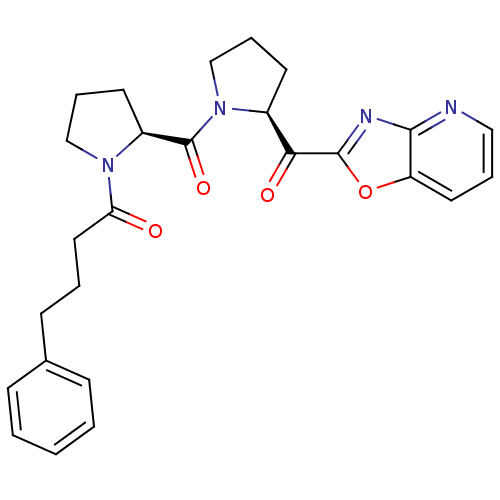
(1-{(S)-2-[(S)-2-(Oxazolo[4,5-b]pyridine-2-carbonyl...)Show SMILES O=C(CCCc1ccccc1)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)c1nc2ncccc2o1 Show InChI InChI=1S/C26H28N4O4/c31-22(14-4-10-18-8-2-1-3-9-18)29-16-7-12-20(29)26(33)30-17-6-11-19(30)23(32)25-28-24-21(34-25)13-5-15-27-24/h1-3,5,8-9,13,15,19-20H,4,6-7,10-12,14,16-17H2/t19-,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against prolyl endopeptidase (PEP) |
J Med Chem 37: 3492-502 (1994)
BindingDB Entry DOI: 10.7270/Q2RB73N9 |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Sus scrofa) | BDBM50037606
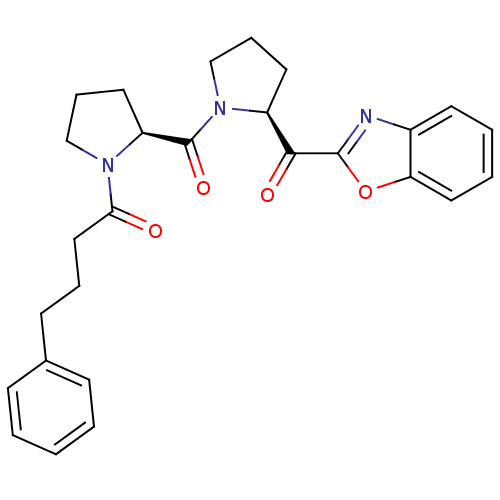
(1-{(S)-2-[(S)-2-(Benzooxazole-2-carbonyl)-pyrrolid...)Show SMILES O=C(CCCc1ccccc1)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)c1nc2ccccc2o1 Show InChI InChI=1S/C27H29N3O4/c31-24(16-6-11-19-9-2-1-3-10-19)29-17-8-14-22(29)27(33)30-18-7-13-21(30)25(32)26-28-20-12-4-5-15-23(20)34-26/h1-5,9-10,12,15,21-22H,6-8,11,13-14,16-18H2/t21-,22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against prolyl endopeptidase (PEP) |
J Med Chem 37: 3492-502 (1994)
BindingDB Entry DOI: 10.7270/Q2RB73N9 |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Sus scrofa) | BDBM50037597
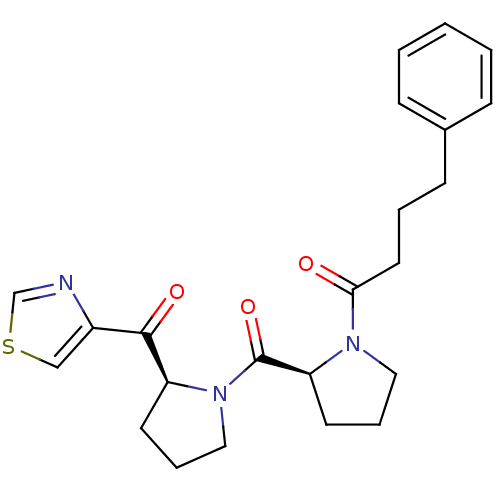
(4-Phenyl-1-{(S)-2-[(S)-2-(thiazole-4-carbonyl)-pyr...)Show SMILES O=C(CCCc1ccccc1)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)c1cscn1 Show InChI InChI=1S/C23H27N3O3S/c27-21(12-4-9-17-7-2-1-3-8-17)25-13-6-11-20(25)23(29)26-14-5-10-19(26)22(28)18-15-30-16-24-18/h1-3,7-8,15-16,19-20H,4-6,9-14H2/t19-,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Prolyl endopeptidase (PEP) from pig kidney using Z-Gly-Pro-p-nitroanilide as substrate |
Bioorg Med Chem Lett 4: 831-834 (1994)
Article DOI: 10.1016/S0960-894X(01)80857-X
BindingDB Entry DOI: 10.7270/Q28W3D8R |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Sus scrofa) | BDBM50037597

(4-Phenyl-1-{(S)-2-[(S)-2-(thiazole-4-carbonyl)-pyr...)Show SMILES O=C(CCCc1ccccc1)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)c1cscn1 Show InChI InChI=1S/C23H27N3O3S/c27-21(12-4-9-17-7-2-1-3-8-17)25-13-6-11-20(25)23(29)26-14-5-10-19(26)22(28)18-15-30-16-24-18/h1-3,7-8,15-16,19-20H,4-6,9-14H2/t19-,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against prolyl endopeptidase (PEP) |
J Med Chem 37: 3492-502 (1994)
BindingDB Entry DOI: 10.7270/Q2RB73N9 |
More data for this
Ligand-Target Pair | |
Bifunctional methylenetetrahydrofolate dehydrogenase/cyclohydrolase, mitochondrial
(Homo sapiens (Human)) | BDBM50509364

(CHEMBL4463968)Show SMILES C[C@H]1CN(CCN1C)c1ccc2c3CCN(Cc3c(=O)oc2c1C)C(=O)c1ccc(NS(C)(=O)=O)c(OC(F)(F)F)c1 |r| Show InChI InChI=1S/C28H31F3N4O6S/c1-16-14-34(12-11-33(16)3)23-8-6-20-19-9-10-35(15-21(19)27(37)40-25(20)17(23)2)26(36)18-5-7-22(32-42(4,38)39)24(13-18)41-28(29,30)31/h5-8,13,16,32H,9-12,14-15H2,1-4H3/t16-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant MTHFD2 (unknown origin) using THF as substrate incubated for 30 mins in presence of NAD |
J Med Chem 62: 10204-10220 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01113
BindingDB Entry DOI: 10.7270/Q2BC42TM |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Sus scrofa) | BDBM50037600
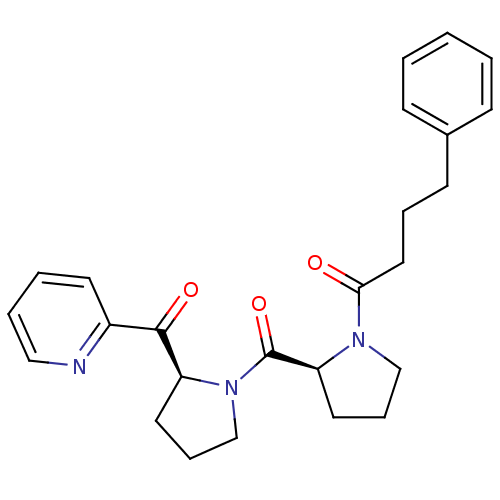
(4-Phenyl-1-{(S)-2-[(S)-2-(pyridine-2-carbonyl)-pyr...)Show SMILES O=C(CCCc1ccccc1)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)c1ccccn1 Show InChI InChI=1S/C25H29N3O3/c29-23(15-6-11-19-9-2-1-3-10-19)27-17-8-14-22(27)25(31)28-18-7-13-21(28)24(30)20-12-4-5-16-26-20/h1-5,9-10,12,16,21-22H,6-8,11,13-15,17-18H2/t21-,22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against prolyl endopeptidase (PEP) |
J Med Chem 37: 3492-502 (1994)
BindingDB Entry DOI: 10.7270/Q2RB73N9 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50401285

(CHEMBL2204532)Show SMILES CN(c1ccc(F)c(NC(=O)c2cccc(OC(C)(C)C#N)c2Cl)c1)c1ccc2nc(NC(=O)C3CC3)sc2n1 Show InChI InChI=1S/C28H24ClFN6O3S/c1-28(2,14-31)39-21-6-4-5-17(23(21)29)25(38)32-20-13-16(9-10-18(20)30)36(3)22-12-11-19-26(34-22)40-27(33-19)35-24(37)15-7-8-15/h4-6,9-13,15H,7-8H2,1-3H3,(H,32,38)(H,33,35,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 |
Bioorg Med Chem 20: 5600-15 (2012)
Article DOI: 10.1016/j.bmc.2012.07.032
BindingDB Entry DOI: 10.7270/Q2765GH8 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM99471

(US8497274, 32)Show SMILES Fc1ccc(Oc2ccc3nc(NC(=O)C4CC4)sc3c2C#N)cc1NC(=O)Cc1cccc(c1)C(F)(F)F Show InChI InChI=1S/C27H18F4N4O3S/c28-19-7-6-17(12-21(19)33-23(36)11-14-2-1-3-16(10-14)27(29,30)31)38-22-9-8-20-24(18(22)13-32)39-26(34-20)35-25(37)15-4-5-15/h1-3,6-10,12,15H,4-5,11H2,(H,33,36)(H,34,35,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal FLAG-tagged wild type BRAF (unknown origin) expressed in baculovirus system using GST-MEK1(K96R) as substrate after 20 mins |
J Med Chem 56: 6478-94 (2013)
Article DOI: 10.1021/jm400778d
BindingDB Entry DOI: 10.7270/Q2W95BPS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prolyl endopeptidase
(Sus scrofa) | BDBM50037609
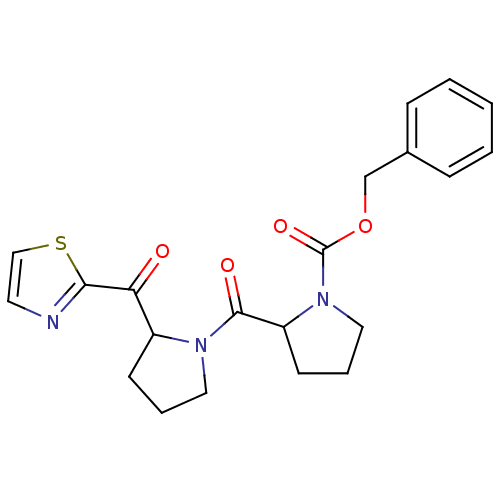
(2-[2-(Thiazole-2-carbonyl)-pyrrolidine-1-carbonyl]...)Show SMILES O=C(OCc1ccccc1)N1CCCC1C(=O)N1CCCC1C(=O)c1nccs1 Show InChI InChI=1S/C21H23N3O4S/c25-18(19-22-10-13-29-19)16-8-4-11-23(16)20(26)17-9-5-12-24(17)21(27)28-14-15-6-2-1-3-7-15/h1-3,6-7,10,13,16-17H,4-5,8-9,11-12,14H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against prolyl endopeptidase (PEP) |
J Med Chem 37: 3492-502 (1994)
BindingDB Entry DOI: 10.7270/Q2RB73N9 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50291166
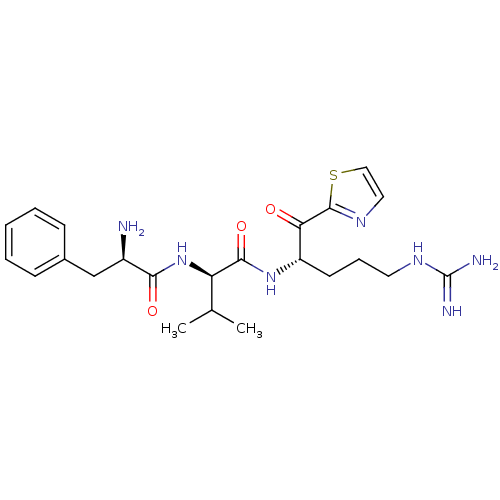
((R)-2-((R)-2-Amino-3-phenyl-propionylamino)-N-[(S)...)Show SMILES CC(C)[C@@H](NC(=O)[C@H](N)Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)c1nccs1 Show InChI InChI=1S/C23H33N7O3S/c1-14(2)18(30-20(32)16(24)13-15-7-4-3-5-8-15)21(33)29-17(9-6-10-28-23(25)26)19(31)22-27-11-12-34-22/h3-5,7-8,11-12,14,16-18H,6,9-10,13,24H2,1-2H3,(H,29,33)(H,30,32)(H4,25,26,28)/t16-,17+,18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 7: 533-538 (1997)
Article DOI: 10.1016/S0960-894X(97)00057-7
BindingDB Entry DOI: 10.7270/Q28S4QF2 |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Sus scrofa) | BDBM50284126
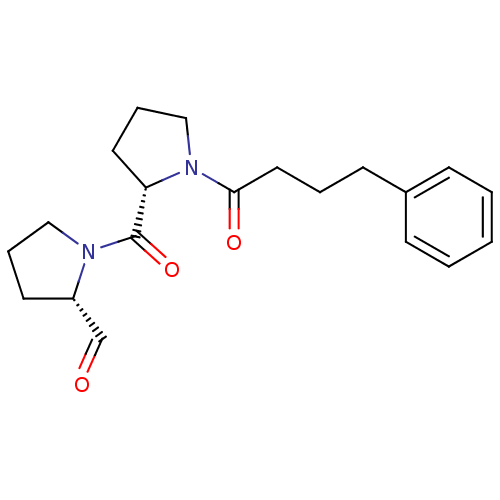
((S)-1-((S)-1-(4-phenylbutanoyl)pyrrolidine-2-carbo...)Show SMILES O=C[C@@H]1CCCN1C(=O)[C@@H]1CCCN1C(=O)CCCc1ccccc1 Show InChI InChI=1S/C20H26N2O3/c23-15-17-10-5-13-21(17)20(25)18-11-6-14-22(18)19(24)12-4-9-16-7-2-1-3-8-16/h1-3,7-8,15,17-18H,4-6,9-14H2/t17-,18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Prolyl endopeptidase (PEP) from pig kidney using Z-Gly-Pro-p-nitroanilide as substrate |
Bioorg Med Chem Lett 4: 831-834 (1994)
Article DOI: 10.1016/S0960-894X(01)80857-X
BindingDB Entry DOI: 10.7270/Q28W3D8R |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Sus scrofa) | BDBM50037602
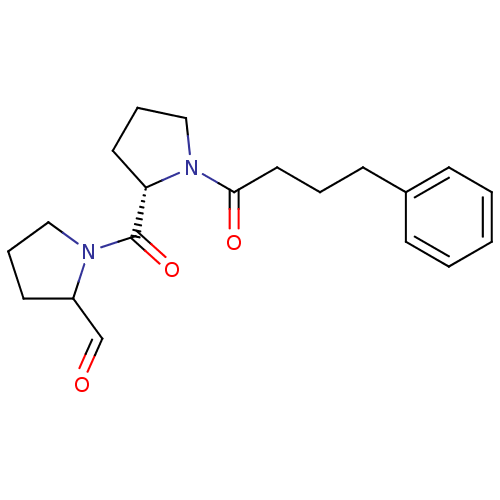
(1-[(S)-1-(4-Phenyl-butyryl)-pyrrolidine-2-carbonyl...)Show SMILES O=CC1CCCN1C(=O)[C@@H]1CCCN1C(=O)CCCc1ccccc1 Show InChI InChI=1S/C20H26N2O3/c23-15-17-10-5-13-21(17)20(25)18-11-6-14-22(18)19(24)12-4-9-16-7-2-1-3-8-16/h1-3,7-8,15,17-18H,4-6,9-14H2/t17?,18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against prolyl endopeptidase (PEP) |
J Med Chem 37: 3492-502 (1994)
BindingDB Entry DOI: 10.7270/Q2RB73N9 |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Sus scrofa) | BDBM50037604
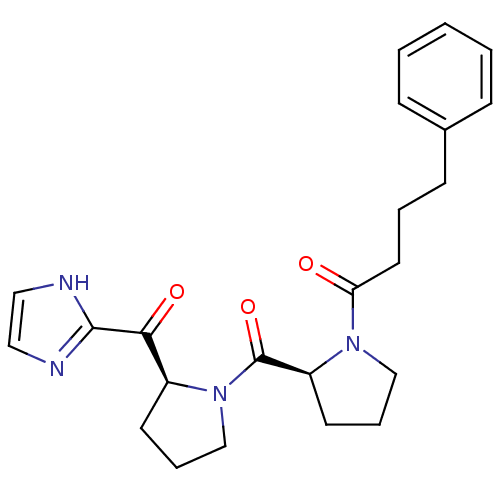
(1-{(S)-2-[(S)-2-(1H-Imidazole-2-carbonyl)-pyrrolid...)Show SMILES O=C(CCCc1ccccc1)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)c1ncc[nH]1 Show InChI InChI=1S/C23H28N4O3/c28-20(12-4-9-17-7-2-1-3-8-17)26-15-6-11-19(26)23(30)27-16-5-10-18(27)21(29)22-24-13-14-25-22/h1-3,7-8,13-14,18-19H,4-6,9-12,15-16H2,(H,24,25)/t18-,19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against prolyl endopeptidase (PEP) |
J Med Chem 37: 3492-502 (1994)
BindingDB Entry DOI: 10.7270/Q2RB73N9 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM50291173

(1-((S)-2-Amino-3-(R)-phenyl-propionyl)-azetidine-2...)Show SMILES N[C@H](Cc1ccccc1)C(=O)N1CC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)c1nccs1 Show InChI InChI=1S/C22H29N7O3S/c23-15(13-14-5-2-1-3-6-14)21(32)29-11-8-17(29)19(31)28-16(7-4-9-27-22(24)25)18(30)20-26-10-12-33-20/h1-3,5-6,10,12,15-17H,4,7-9,11,13,23H2,(H,28,31)(H4,24,25,27)/t15-,16+,17+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of trypsin |
Bioorg Med Chem Lett 7: 533-538 (1997)
Article DOI: 10.1016/S0960-894X(97)00057-7
BindingDB Entry DOI: 10.7270/Q28S4QF2 |
More data for this
Ligand-Target Pair | |
Bifunctional methylenetetrahydrofolate dehydrogenase/cyclohydrolase, mitochondrial
(Homo sapiens (Human)) | BDBM50509346

(CHEMBL4470947)Show SMILES C[C@H]1CN(CCN1C)c1ccc2c3CCN(Cc3c(=O)oc2c1C)C(=O)c1ccc(NS(C)(=O)=O)c(Cl)c1 |r| Show InChI InChI=1S/C27H31ClN4O5S/c1-16-14-31(12-11-30(16)3)24-8-6-20-19-9-10-32(15-21(19)27(34)37-25(20)17(24)2)26(33)18-5-7-23(22(28)13-18)29-38(4,35)36/h5-8,13,16,29H,9-12,14-15H2,1-4H3/t16-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant MTHFD2 (unknown origin) using THF as substrate incubated for 30 mins in presence of NAD |
J Med Chem 62: 10204-10220 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01113
BindingDB Entry DOI: 10.7270/Q2BC42TM |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Sus scrofa) | BDBM50088247

(CHEMBL303966 | Oxo-{(S)-1-[(S)-1-(4-phenyl-butyryl...)Show SMILES CCOC(=O)C(=O)[C@@H]1CCCN1C(=O)[C@@H]1CCCN1C(=O)CCCc1ccccc1 Show InChI InChI=1S/C23H30N2O5/c1-2-30-23(29)21(27)18-12-7-16-25(18)22(28)19-13-8-15-24(19)20(26)14-6-11-17-9-4-3-5-10-17/h3-5,9-10,18-19H,2,6-8,11-16H2,1H3/t18-,19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Prolyl endopeptidase (PEP) from pig kidney using Z-Gly-Pro-p-nitroanilide as substrate |
Bioorg Med Chem Lett 4: 831-834 (1994)
Article DOI: 10.1016/S0960-894X(01)80857-X
BindingDB Entry DOI: 10.7270/Q28W3D8R |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50401288
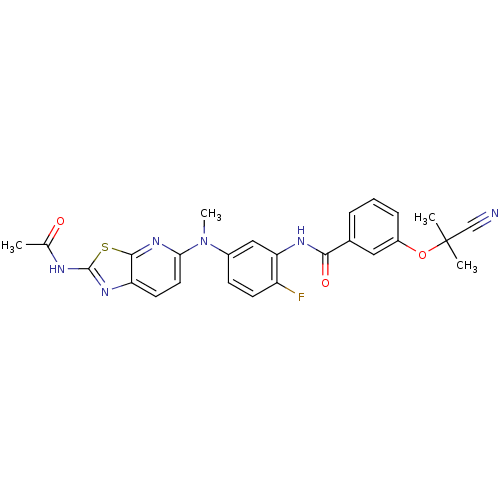
(CHEMBL2204530)Show SMILES CN(c1ccc(F)c(NC(=O)c2cccc(OC(C)(C)C#N)c2)c1)c1ccc2nc(NC(C)=O)sc2n1 Show InChI InChI=1S/C26H23FN6O3S/c1-15(34)29-25-31-20-10-11-22(32-24(20)37-25)33(4)17-8-9-19(27)21(13-17)30-23(35)16-6-5-7-18(12-16)36-26(2,3)14-28/h5-13H,1-4H3,(H,30,35)(H,29,31,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 in HUVEC assessed as reduction of VGF-stimulated cell proliferation after 5 days |
Bioorg Med Chem 20: 5600-15 (2012)
Article DOI: 10.1016/j.bmc.2012.07.032
BindingDB Entry DOI: 10.7270/Q2765GH8 |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Sus scrofa) | BDBM50037607
(CHEMBL333638 | Oxo-{1-[(S)-1-(4-phenyl-butyryl)-py...)Show SMILES CCOC(=O)C(=O)C1CCCN1C(=O)[C@@H]1CCCN1C(=O)CCCc1ccccc1 Show InChI InChI=1S/C23H30N2O5/c1-2-30-23(29)21(27)18-12-7-16-25(18)22(28)19-13-8-15-24(19)20(26)14-6-11-17-9-4-3-5-10-17/h3-5,9-10,18-19H,2,6-8,11-16H2,1H3/t18?,19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 10.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against prolyl endopeptidase (PEP) |
J Med Chem 37: 3492-502 (1994)
BindingDB Entry DOI: 10.7270/Q2RB73N9 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50401287
(CHEMBL2204531)Show SMILES CN(c1ccc(F)c(NC(=O)c2cccc(OC(C)(C)C#N)c2Cl)c1)c1ccc2nc(NC(C)=O)sc2n1 Show InChI InChI=1S/C26H22ClFN6O3S/c1-14(35)30-25-32-18-10-11-21(33-24(18)38-25)34(4)15-8-9-17(28)19(12-15)31-23(36)16-6-5-7-20(22(16)27)37-26(2,3)13-29/h5-12H,1-4H3,(H,31,36)(H,30,32,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 in HUVEC assessed as reduction of VGF-stimulated cell proliferation after 5 days |
Bioorg Med Chem 20: 5600-15 (2012)
Article DOI: 10.1016/j.bmc.2012.07.032
BindingDB Entry DOI: 10.7270/Q2765GH8 |
More data for this
Ligand-Target Pair | |
Bifunctional methylenetetrahydrofolate dehydrogenase/cyclohydrolase, mitochondrial
(Homo sapiens (Human)) | BDBM50509358

(CHEMBL4534641)Show SMILES OC(=O)C(F)(F)F.CN1CCN(CC1)c1ccc2c3CCN(Cc3c(=O)oc2c1C)C(=O)c1ccc2NS(=O)(=O)Cc2c1 Show InChI InChI=1S/C26H28N4O5S/c1-16-23(29-11-9-28(2)10-12-29)6-4-20-19-7-8-30(14-21(19)26(32)35-24(16)20)25(31)17-3-5-22-18(13-17)15-36(33,34)27-22/h3-6,13,27H,7-12,14-15H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant MTHFD2 (unknown origin) using THF as substrate incubated for 30 mins in presence of NAD |
J Med Chem 62: 10204-10220 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01113
BindingDB Entry DOI: 10.7270/Q2BC42TM |
More data for this
Ligand-Target Pair | |
Bifunctional methylenetetrahydrofolate dehydrogenase/cyclohydrolase, mitochondrial
(Homo sapiens (Human)) | BDBM50509362

(CHEMBL4483030)Show SMILES CN1CCN(CC1)c1ccc2c3CCN(Cc3c(=O)oc2c1C)C(=O)c1ccc(NS(C)(=O)=O)c(OC(F)(F)F)c1 Show InChI InChI=1S/C27H29F3N4O6S/c1-16-22(33-12-10-32(2)11-13-33)7-5-19-18-8-9-34(15-20(18)26(36)39-24(16)19)25(35)17-4-6-21(31-41(3,37)38)23(14-17)40-27(28,29)30/h4-7,14,31H,8-13,15H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant MTHFD2 (unknown origin) using THF as substrate incubated for 30 mins in presence of NAD |
J Med Chem 62: 10204-10220 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01113
BindingDB Entry DOI: 10.7270/Q2BC42TM |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50401285

(CHEMBL2204532)Show SMILES CN(c1ccc(F)c(NC(=O)c2cccc(OC(C)(C)C#N)c2Cl)c1)c1ccc2nc(NC(=O)C3CC3)sc2n1 Show InChI InChI=1S/C28H24ClFN6O3S/c1-28(2,14-31)39-21-6-4-5-17(23(21)29)25(38)32-20-13-16(9-10-18(20)30)36(3)22-12-11-19-26(34-22)40-27(33-19)35-24(37)15-7-8-15/h4-6,9-13,15H,7-8H2,1-3H3,(H,32,38)(H,33,35,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of c-RAF |
Bioorg Med Chem 20: 5600-15 (2012)
Article DOI: 10.1016/j.bmc.2012.07.032
BindingDB Entry DOI: 10.7270/Q2765GH8 |
More data for this
Ligand-Target Pair | |
Bifunctional methylenetetrahydrofolate dehydrogenase/cyclohydrolase, mitochondrial
(Homo sapiens (Human)) | BDBM50509354

(CHEMBL4542665)Show SMILES CN1CCN(CC1)c1ccc2c3CCN(Cc3c(=O)oc2c1C)C(=O)c1ccc(NS(C)(=O)=O)c(Br)c1 Show InChI InChI=1S/C26H29BrN4O5S/c1-16-23(30-12-10-29(2)11-13-30)7-5-19-18-8-9-31(15-20(18)26(33)36-24(16)19)25(32)17-4-6-22(21(27)14-17)28-37(3,34)35/h4-7,14,28H,8-13,15H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant MTHFD2 (unknown origin) using THF as substrate incubated for 30 mins in presence of NAD |
J Med Chem 62: 10204-10220 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01113
BindingDB Entry DOI: 10.7270/Q2BC42TM |
More data for this
Ligand-Target Pair | |
Bifunctional methylenetetrahydrofolate dehydrogenase/cyclohydrolase, mitochondrial
(Homo sapiens (Human)) | BDBM50509356

(CHEMBL4584730)Show SMILES C[C@H]1CN(C[C@@H](C)N1C)c1ccc2c3CCN(Cc3c(=O)oc2c1C)C(=O)c1ccc(NS(C)(=O)=O)c(Cl)c1 |r| Show InChI InChI=1S/C28H33ClN4O5S/c1-16-13-33(14-17(2)31(16)4)25-9-7-21-20-10-11-32(15-22(20)28(35)38-26(21)18(25)3)27(34)19-6-8-24(23(29)12-19)30-39(5,36)37/h6-9,12,16-17,30H,10-11,13-15H2,1-5H3/t16-,17+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant MTHFD2 (unknown origin) using THF as substrate incubated for 30 mins in presence of NAD |
J Med Chem 62: 10204-10220 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01113
BindingDB Entry DOI: 10.7270/Q2BC42TM |
More data for this
Ligand-Target Pair | |
Bifunctional methylenetetrahydrofolate dehydrogenase/cyclohydrolase, mitochondrial
(Homo sapiens (Human)) | BDBM50509360
(CHEMBL4538356)Show SMILES CN1CCN(CC1)c1ccc2c3CCN(Cc3c(=O)oc2c1C)C(=O)c1ccc(NS(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C26H29FN4O5S/c1-16-23(30-12-10-29(2)11-13-30)7-5-19-18-8-9-31(15-20(18)26(33)36-24(16)19)25(32)17-4-6-22(21(27)14-17)28-37(3,34)35/h4-7,14,28H,8-13,15H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant MTHFD2 (unknown origin) using THF as substrate incubated for 30 mins in presence of NAD |
J Med Chem 62: 10204-10220 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01113
BindingDB Entry DOI: 10.7270/Q2BC42TM |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha
(Homo sapiens (Human)) | BDBM50401285

(CHEMBL2204532)Show SMILES CN(c1ccc(F)c(NC(=O)c2cccc(OC(C)(C)C#N)c2Cl)c1)c1ccc2nc(NC(=O)C3CC3)sc2n1 Show InChI InChI=1S/C28H24ClFN6O3S/c1-28(2,14-31)39-21-6-4-5-17(23(21)29)25(38)32-20-13-16(9-10-18(20)30)36(3)22-12-11-19-26(34-22)40-27(33-19)35-24(37)15-7-8-15/h4-6,9-13,15H,7-8H2,1-3H3,(H,32,38)(H,33,35,37) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of PDGFRalpha |
Bioorg Med Chem 20: 5600-15 (2012)
Article DOI: 10.1016/j.bmc.2012.07.032
BindingDB Entry DOI: 10.7270/Q2765GH8 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50401285

(CHEMBL2204532)Show SMILES CN(c1ccc(F)c(NC(=O)c2cccc(OC(C)(C)C#N)c2Cl)c1)c1ccc2nc(NC(=O)C3CC3)sc2n1 Show InChI InChI=1S/C28H24ClFN6O3S/c1-28(2,14-31)39-21-6-4-5-17(23(21)29)25(38)32-20-13-16(9-10-18(20)30)36(3)22-12-11-19-26(34-22)40-27(33-19)35-24(37)15-7-8-15/h4-6,9-13,15H,7-8H2,1-3H3,(H,32,38)(H,33,35,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha |
Bioorg Med Chem 20: 5600-15 (2012)
Article DOI: 10.1016/j.bmc.2012.07.032
BindingDB Entry DOI: 10.7270/Q2765GH8 |
More data for this
Ligand-Target Pair | |
Bifunctional methylenetetrahydrofolate dehydrogenase/cyclohydrolase, mitochondrial
(Homo sapiens (Human)) | BDBM50509353
(CHEMBL4546647)Show SMILES CN1CCN(CC1)c1ccc2c3CCN(Cc3c(=O)oc2c1C)C(=O)c1ccc(NS(C)(=O)=O)c(Cl)c1 Show InChI InChI=1S/C26H29ClN4O5S/c1-16-23(30-12-10-29(2)11-13-30)7-5-19-18-8-9-31(15-20(18)26(33)36-24(16)19)25(32)17-4-6-22(21(27)14-17)28-37(3,34)35/h4-7,14,28H,8-13,15H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant MTHFD2 (unknown origin) using THF as substrate incubated for 30 mins in presence of NAD |
J Med Chem 62: 10204-10220 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01113
BindingDB Entry DOI: 10.7270/Q2BC42TM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bifunctional methylenetetrahydrofolate dehydrogenase/cyclohydrolase, mitochondrial
(Homo sapiens (Human)) | BDBM50509350

(CHEMBL4441993)Show SMILES Cl.CN(C)CCOc1ccc2c3CCN(Cc3c(=O)oc2c1C)C(=O)c1ccc(cc1)C(O)=O Show InChI InChI=1S/C25H26N2O6/c1-15-21(32-13-12-26(2)3)9-8-19-18-10-11-27(14-20(18)25(31)33-22(15)19)23(28)16-4-6-17(7-5-16)24(29)30/h4-9H,10-14H2,1-3H3,(H,29,30) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant MTHFD2 (unknown origin) using THF as substrate incubated for 30 mins in presence of NAD |
J Med Chem 62: 10204-10220 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01113
BindingDB Entry DOI: 10.7270/Q2BC42TM |
More data for this
Ligand-Target Pair | |
Bifunctional methylenetetrahydrofolate dehydrogenase/cyclohydrolase, mitochondrial
(Homo sapiens (Human)) | BDBM50509351

(CHEMBL4460077)Show SMILES OC(=O)C(F)(F)F.CN(C)CCOc1ccc2c3CCN(Cc3c(=O)oc2c1C)C(=O)c1ccc2NS(=O)(=O)Cc2c1 Show InChI InChI=1S/C25H27N3O6S/c1-15-22(33-11-10-27(2)3)7-5-19-18-8-9-28(13-20(18)25(30)34-23(15)19)24(29)16-4-6-21-17(12-16)14-35(31,32)26-21/h4-7,12,26H,8-11,13-14H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant MTHFD2 (unknown origin) using THF as substrate incubated for 30 mins in presence of NAD |
J Med Chem 62: 10204-10220 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01113
BindingDB Entry DOI: 10.7270/Q2BC42TM |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Sus scrofa) | BDBM50037603
(4-Phenyl-1-{(S)-2-[(S)-2-(pyrimidine-5-carbonyl)-p...)Show SMILES O=C(CCCc1ccccc1)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)c1cncnc1 Show InChI InChI=1S/C24H28N4O3/c29-22(12-4-9-18-7-2-1-3-8-18)27-13-6-11-21(27)24(31)28-14-5-10-20(28)23(30)19-15-25-17-26-16-19/h1-3,7-8,15-17,20-21H,4-6,9-14H2/t20-,21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against prolyl endopeptidase (PEP) |
J Med Chem 37: 3492-502 (1994)
BindingDB Entry DOI: 10.7270/Q2RB73N9 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50401286
(CHEMBL2204533)Show SMILES CC(C)(Oc1cccc(C(=O)Nc2cc(Nc3ccc4nc(NC(=O)C5CC5)sc4n3)ccc2F)c1Cl)C#N Show InChI InChI=1S/C27H22ClFN6O3S/c1-27(2,13-30)38-20-5-3-4-16(22(20)28)24(37)32-19-12-15(8-9-17(19)29)31-21-11-10-18-25(34-21)39-26(33-18)35-23(36)14-6-7-14/h3-5,8-12,14H,6-7H2,1-2H3,(H,31,34)(H,32,37)(H,33,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 in HUVEC assessed as reduction of VGF-stimulated cell proliferation after 5 days |
Bioorg Med Chem 20: 5600-15 (2012)
Article DOI: 10.1016/j.bmc.2012.07.032
BindingDB Entry DOI: 10.7270/Q2765GH8 |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM99471

(US8497274, 32)Show SMILES Fc1ccc(Oc2ccc3nc(NC(=O)C4CC4)sc3c2C#N)cc1NC(=O)Cc1cccc(c1)C(F)(F)F Show InChI InChI=1S/C27H18F4N4O3S/c28-19-7-6-17(12-21(19)33-23(36)11-14-2-1-3-16(10-14)27(29,30)31)38-22-9-8-20-24(18(22)13-32)39-26(34-20)35-25(37)15-4-5-15/h1-3,6-10,12,15H,4-5,11H2,(H,33,36)(H,34,35,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-6-tagged Aurora kinase B (unknown origin) expressed in baculovirus system after 60 mins |
J Med Chem 56: 6478-94 (2013)
Article DOI: 10.1021/jm400778d
BindingDB Entry DOI: 10.7270/Q2W95BPS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50401285

(CHEMBL2204532)Show SMILES CN(c1ccc(F)c(NC(=O)c2cccc(OC(C)(C)C#N)c2Cl)c1)c1ccc2nc(NC(=O)C3CC3)sc2n1 Show InChI InChI=1S/C28H24ClFN6O3S/c1-28(2,14-31)39-21-6-4-5-17(23(21)29)25(38)32-20-13-16(9-10-18(20)30)36(3)22-12-11-19-26(34-22)40-27(33-19)35-24(37)15-7-8-15/h4-6,9-13,15H,7-8H2,1-3H3,(H,32,38)(H,33,35,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of wild type human BRAF (445 to 726) expressed in Sf9 insect cells |
Bioorg Med Chem 20: 5600-15 (2012)
Article DOI: 10.1016/j.bmc.2012.07.032
BindingDB Entry DOI: 10.7270/Q2765GH8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Fibroblast growth factor receptor 3
(Homo sapiens (Human)) | BDBM50401285

(CHEMBL2204532)Show SMILES CN(c1ccc(F)c(NC(=O)c2cccc(OC(C)(C)C#N)c2Cl)c1)c1ccc2nc(NC(=O)C3CC3)sc2n1 Show InChI InChI=1S/C28H24ClFN6O3S/c1-28(2,14-31)39-21-6-4-5-17(23(21)29)25(38)32-20-13-16(9-10-18(20)30)36(3)22-12-11-19-26(34-22)40-27(33-19)35-24(37)15-7-8-15/h4-6,9-13,15H,7-8H2,1-3H3,(H,32,38)(H,33,35,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of FGFR3 |
Bioorg Med Chem 20: 5600-15 (2012)
Article DOI: 10.1016/j.bmc.2012.07.032
BindingDB Entry DOI: 10.7270/Q2765GH8 |
More data for this
Ligand-Target Pair | |
Bifunctional methylenetetrahydrofolate dehydrogenase/cyclohydrolase, mitochondrial
(Homo sapiens (Human)) | BDBM50509355

(CHEMBL4551634)Show SMILES CN1CCN(CC1)c1ccc2c3CCN(Cc3c(=O)oc2c1C)C(=O)c1cc(Cl)cc(NS(C)(=O)=O)c1 Show InChI InChI=1S/C26H29ClN4O5S/c1-16-23(30-10-8-29(2)9-11-30)5-4-21-20-6-7-31(15-22(20)26(33)36-24(16)21)25(32)17-12-18(27)14-19(13-17)28-37(3,34)35/h4-5,12-14,28H,6-11,15H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant MTHFD2 (unknown origin) using THF as substrate incubated for 30 mins in presence of NAD |
J Med Chem 62: 10204-10220 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01113
BindingDB Entry DOI: 10.7270/Q2BC42TM |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50401285

(CHEMBL2204532)Show SMILES CN(c1ccc(F)c(NC(=O)c2cccc(OC(C)(C)C#N)c2Cl)c1)c1ccc2nc(NC(=O)C3CC3)sc2n1 Show InChI InChI=1S/C28H24ClFN6O3S/c1-28(2,14-31)39-21-6-4-5-17(23(21)29)25(38)32-20-13-16(9-10-18(20)30)36(3)22-12-11-19-26(34-22)40-27(33-19)35-24(37)15-7-8-15/h4-6,9-13,15H,7-8H2,1-3H3,(H,32,38)(H,33,35,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of PDGFRbeta |
Bioorg Med Chem 20: 5600-15 (2012)
Article DOI: 10.1016/j.bmc.2012.07.032
BindingDB Entry DOI: 10.7270/Q2765GH8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data