

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50469565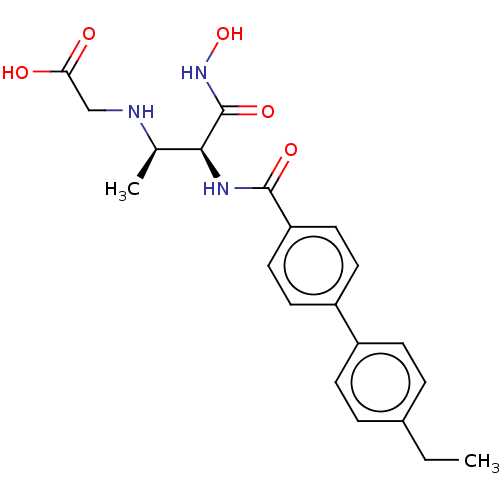 (CHEMBL4082918) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa LpxC using UDP-3-O-(R-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 30 mins followed by sub... | J Med Chem 60: 5002-5014 (2017) Article DOI: 10.1021/acs.jmedchem.7b00377 BindingDB Entry DOI: 10.7270/Q2FN18MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50106090 ((S)-3-(3,4-Dimethoxy-phenyl)-3-[2-((4-methoxy-buty...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro Very late antigen 4 cell adhesion inhibitory activity measured by binding of fluorescently labeled Ramos cells to immobilized VCAM-1. | Bioorg Med Chem Lett 11: 2955-8 (2001) BindingDB Entry DOI: 10.7270/Q20001CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50469558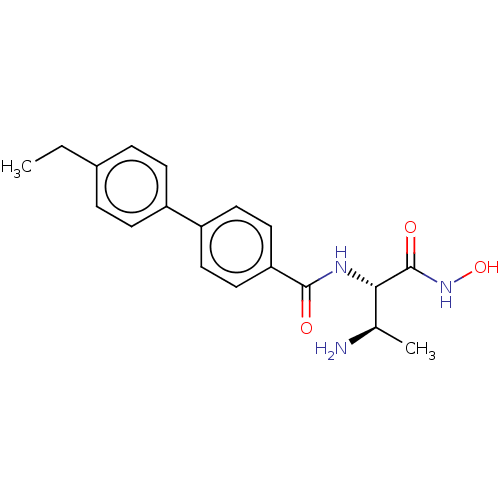 (CHEMBL4061041) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.46 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa LpxC using UDP-3-O-(R-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 30 mins followed by sub... | J Med Chem 60: 5002-5014 (2017) Article DOI: 10.1021/acs.jmedchem.7b00377 BindingDB Entry DOI: 10.7270/Q2FN18MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50106091 ((S)-3-Benzo[1,3]dioxol-5-yl-3-((S)-4-methyl-2-{2-[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro Very late antigen 4 cell adhesion inhibitory activity measured by binding of fluorescently labeled Ramos cells to immobilized VCAM-1. | Bioorg Med Chem Lett 11: 2955-8 (2001) BindingDB Entry DOI: 10.7270/Q20001CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50249583 (CHEMBL4097399) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 3.13 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa LpxC using UDP-3-O-(R-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 30 mins followed by sub... | J Med Chem 60: 5002-5014 (2017) Article DOI: 10.1021/acs.jmedchem.7b00377 BindingDB Entry DOI: 10.7270/Q2FN18MZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50469562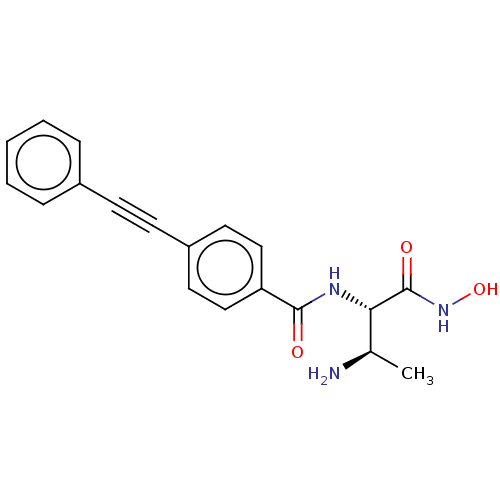 (CHEMBL4069725) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.56 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa LpxC using UDP-3-O-(R-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 30 mins followed by sub... | J Med Chem 60: 5002-5014 (2017) Article DOI: 10.1021/acs.jmedchem.7b00377 BindingDB Entry DOI: 10.7270/Q2FN18MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50469559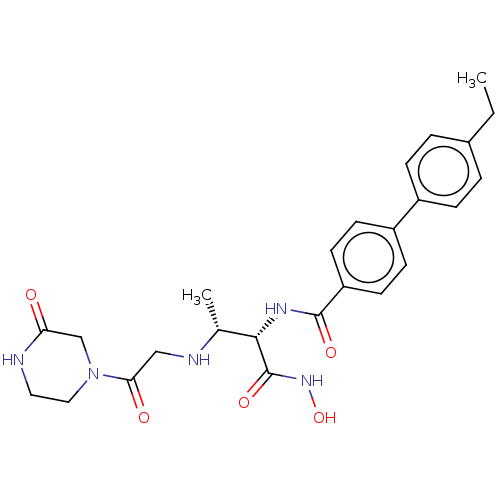 (CHEMBL4063087) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.15 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa LpxC using UDP-3-O-(R-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 30 mins followed by sub... | J Med Chem 60: 5002-5014 (2017) Article DOI: 10.1021/acs.jmedchem.7b00377 BindingDB Entry DOI: 10.7270/Q2FN18MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50469555 (CHEMBL4090716) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.19 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa LpxC using UDP-3-O-(R-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 30 mins followed by sub... | J Med Chem 60: 5002-5014 (2017) Article DOI: 10.1021/acs.jmedchem.7b00377 BindingDB Entry DOI: 10.7270/Q2FN18MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50106083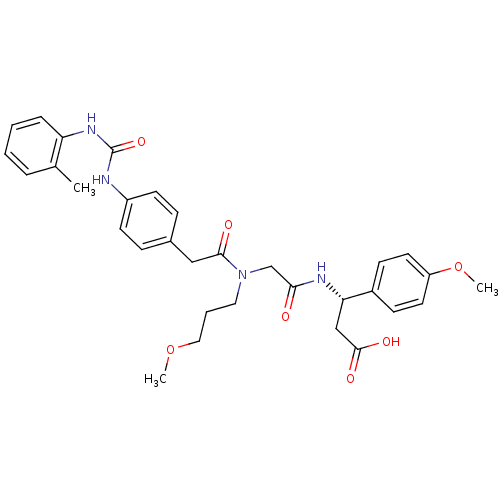 ((S)-3-(4-Methoxy-phenyl)-3-[2-((3-methoxy-propyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro Very late antigen 4 cell adhesion inhibitory activity measured by binding of fluorescently labeled Ramos cells to immobilized VCAM-1. | Bioorg Med Chem Lett 11: 2955-8 (2001) BindingDB Entry DOI: 10.7270/Q20001CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50106086 ((S)-3-(3,4-Dimethoxy-phenyl)-3-[2-((3-methoxy-prop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro Very late antigen 4 cell adhesion inhibitory activity measured by binding of fluorescently labeled Ramos cells to immobilized VCAM-1. | Bioorg Med Chem Lett 11: 2955-8 (2001) BindingDB Entry DOI: 10.7270/Q20001CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50106085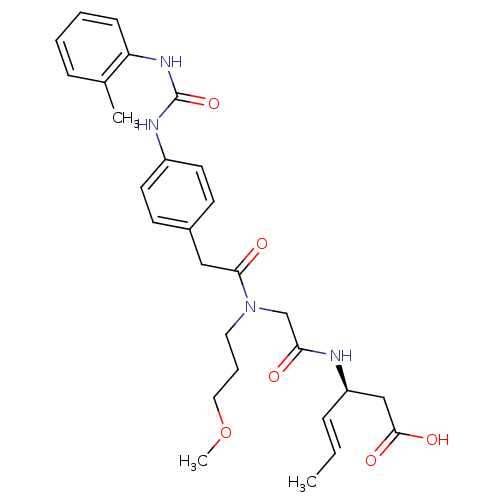 ((E)-(S)-3-[2-((3-Methoxy-propyl)-{2-[4-(3-o-tolyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of fluorescent Ramos cell adhesion to VCAM-1 by mouse Very late antigen 4 (VLA-4) integrin | Bioorg Med Chem Lett 11: 2955-8 (2001) BindingDB Entry DOI: 10.7270/Q20001CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50106085 ((E)-(S)-3-[2-((3-Methoxy-propyl)-{2-[4-(3-o-tolyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of fluorescent Ramos cell adhesion to fibronectin by Very late antigen 4 (VLA-4) | Bioorg Med Chem Lett 11: 2955-8 (2001) BindingDB Entry DOI: 10.7270/Q20001CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50106082 ((S)-3-[2-((3-Methoxy-propyl)-{2-[4-(3-o-tolyl-urei...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro Very late antigen 4 cell adhesion inhibitory activity measured by binding of fluorescently labeled Ramos cells to immobilized VCAM-1. | Bioorg Med Chem Lett 11: 2955-8 (2001) BindingDB Entry DOI: 10.7270/Q20001CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50469560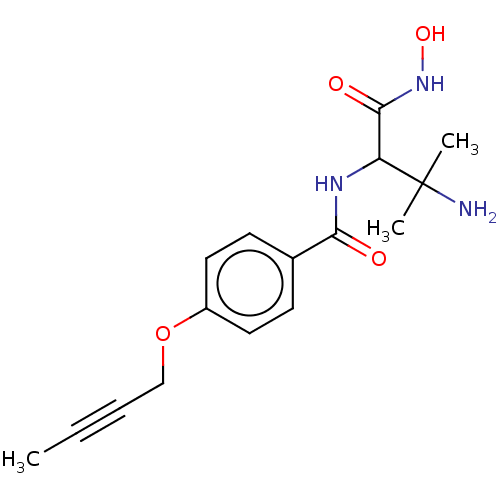 (CHEMBL4083624) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa LpxC using UDP-3-O-(R-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 30 mins followed by sub... | J Med Chem 60: 5002-5014 (2017) Article DOI: 10.1021/acs.jmedchem.7b00377 BindingDB Entry DOI: 10.7270/Q2FN18MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50106086 ((S)-3-(3,4-Dimethoxy-phenyl)-3-[2-((3-methoxy-prop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of fluorescent Ramos cell adhesion to fibronectin by Very late antigen 5 (VLA-5) | Bioorg Med Chem Lett 11: 2955-8 (2001) BindingDB Entry DOI: 10.7270/Q20001CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50106082 ((S)-3-[2-((3-Methoxy-propyl)-{2-[4-(3-o-tolyl-urei...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of fluorescent Ramos cell adhesion to fibronectin by Very late antigen 4 (VLA-4) | Bioorg Med Chem Lett 11: 2955-8 (2001) BindingDB Entry DOI: 10.7270/Q20001CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50106085 ((E)-(S)-3-[2-((3-Methoxy-propyl)-{2-[4-(3-o-tolyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro Very late antigen 4 cell adhesion inhibitory activity measured by binding of fluorescently labeled Ramos cells to immobilized VCAM-1. | Bioorg Med Chem Lett 11: 2955-8 (2001) BindingDB Entry DOI: 10.7270/Q20001CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50469563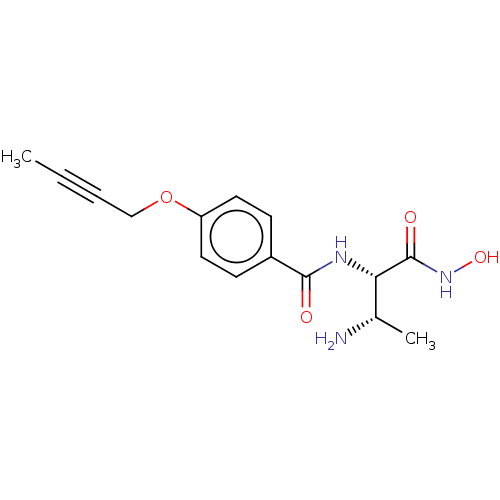 (CHEMBL4079368) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa LpxC using UDP-3-O-(R-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 30 mins followed by sub... | J Med Chem 60: 5002-5014 (2017) Article DOI: 10.1021/acs.jmedchem.7b00377 BindingDB Entry DOI: 10.7270/Q2FN18MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50106098 ((R)-3-((S)-4-Methyl-2-{2-[4-(3-o-tolyl-ureido)-phe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro Very late antigen 4 cell adhesion inhibitory activity measured by binding of fluorescently labeled Ramos cells to immobilized VCAM-1. | Bioorg Med Chem Lett 11: 2955-8 (2001) BindingDB Entry DOI: 10.7270/Q20001CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50469550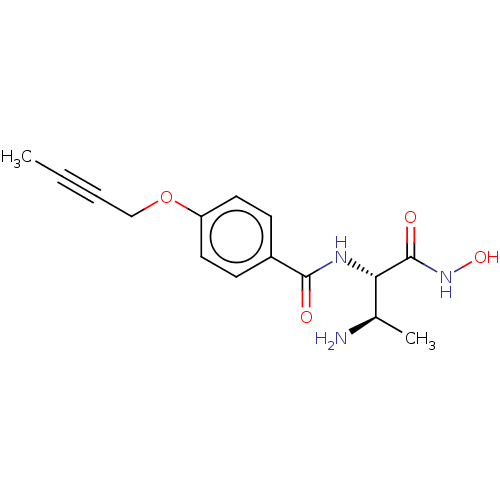 (CHEMBL4070478) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa LpxC using UDP-3-O-(R-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 30 mins followed by sub... | J Med Chem 60: 5002-5014 (2017) Article DOI: 10.1021/acs.jmedchem.7b00377 BindingDB Entry DOI: 10.7270/Q2FN18MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50106093 ((S)-3-(3,4-Dimethoxy-phenyl)-3-[2-((3-methyl-butyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro Very late antigen 4 cell adhesion inhibitory activity measured by binding of fluorescently labeled Ramos cells to immobilized VCAM-1. | Bioorg Med Chem Lett 11: 2955-8 (2001) BindingDB Entry DOI: 10.7270/Q20001CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50106085 ((E)-(S)-3-[2-((3-Methoxy-propyl)-{2-[4-(3-o-tolyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of fluorescent Ramos cell adhesion to VCAM-1 by rat Very late antigen 4 (VLA-4) integrin | Bioorg Med Chem Lett 11: 2955-8 (2001) BindingDB Entry DOI: 10.7270/Q20001CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50106086 ((S)-3-(3,4-Dimethoxy-phenyl)-3-[2-((3-methoxy-prop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of fluorescent Ramos cell adhesion to VCAM-1 by rat Very late antigen 4 (VLA-4) integrin | Bioorg Med Chem Lett 11: 2955-8 (2001) BindingDB Entry DOI: 10.7270/Q20001CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50106082 ((S)-3-[2-((3-Methoxy-propyl)-{2-[4-(3-o-tolyl-urei...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of fluorescent Ramos cell adhesion to VCAM-1 by mouse Very late antigen 4 (VLA-4) integrin | Bioorg Med Chem Lett 11: 2955-8 (2001) BindingDB Entry DOI: 10.7270/Q20001CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-L/Intercellular adhesion molecule 1 (Homo sapiens (Human)) | BDBM50161080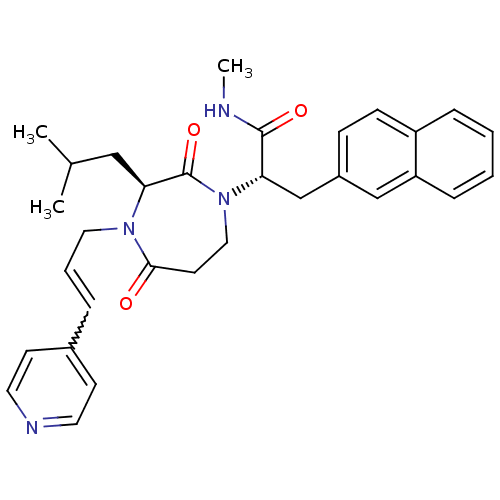 ((S)-2-[(S)-3-Isobutyl-2,5-dioxo-4-((E)-3-pyridin-4...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description In vitro inhibitory concentrationfor Lymphocyte function associated antigen 1/Intercellular adhesion molecule 1 (LFA-1/ICAM-1) | Bioorg Med Chem Lett 15: 1217-20 (2005) Article DOI: 10.1016/j.bmcl.2004.11.072 BindingDB Entry DOI: 10.7270/Q20Z72S2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50106082 ((S)-3-[2-((3-Methoxy-propyl)-{2-[4-(3-o-tolyl-urei...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of fluorescent Ramos cell adhesion to VCAM-1 by rat Very late antigen 4 (VLA-4) integrin | Bioorg Med Chem Lett 11: 2955-8 (2001) BindingDB Entry DOI: 10.7270/Q20001CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50106083 ((S)-3-(4-Methoxy-phenyl)-3-[2-((3-methoxy-propyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of fluorescent Ramos cell adhesion to VCAM-1 by rat Very late antigen 4 (VLA-4) integrin | Bioorg Med Chem Lett 11: 2955-8 (2001) BindingDB Entry DOI: 10.7270/Q20001CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50106086 ((S)-3-(3,4-Dimethoxy-phenyl)-3-[2-((3-methoxy-prop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of fluorescent Ramos cell adhesion to VCAM-1 by mouse Very late antigen 4 (VLA-4) integrin | Bioorg Med Chem Lett 11: 2955-8 (2001) BindingDB Entry DOI: 10.7270/Q20001CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50106099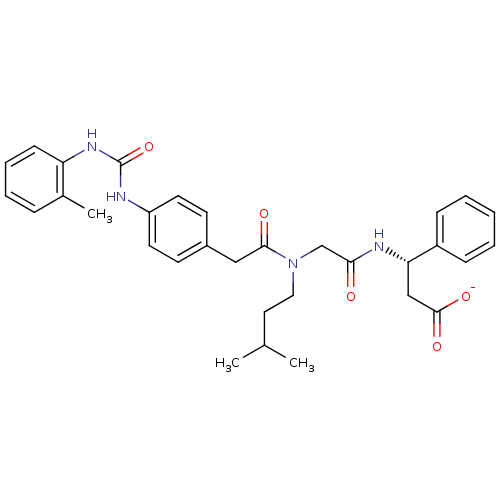 (CHEMBL321739 | Lithium; (S)-3-[2-((3-methyl-butyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro Very late antigen 4 cell adhesion inhibitory activity measured by binding of fluorescently labeled Ramos cells to immobilized VCAM-1. | Bioorg Med Chem Lett 11: 2955-8 (2001) BindingDB Entry DOI: 10.7270/Q20001CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50106083 ((S)-3-(4-Methoxy-phenyl)-3-[2-((3-methoxy-propyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of fluorescent Ramos cell adhesion to fibronectin by Very late antigen 5 (VLA-5) | Bioorg Med Chem Lett 11: 2955-8 (2001) BindingDB Entry DOI: 10.7270/Q20001CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50469557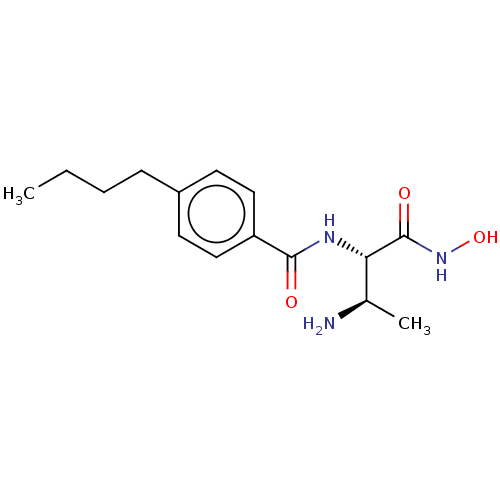 (CHEMBL4091408) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 37.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa LpxC using UDP-3-O-(R-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 30 mins followed by sub... | J Med Chem 60: 5002-5014 (2017) Article DOI: 10.1021/acs.jmedchem.7b00377 BindingDB Entry DOI: 10.7270/Q2FN18MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50469554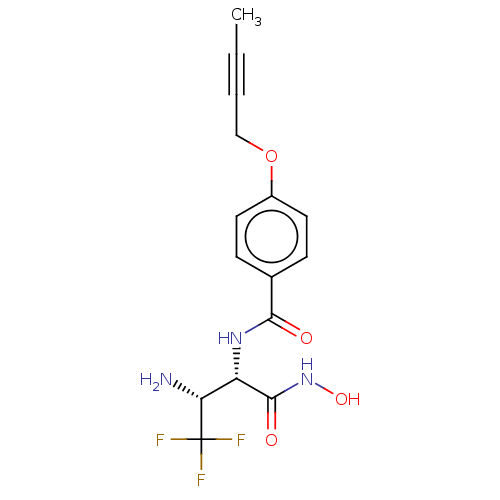 (CHEMBL4061854) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa LpxC using UDP-3-O-(R-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 30 mins followed by sub... | J Med Chem 60: 5002-5014 (2017) Article DOI: 10.1021/acs.jmedchem.7b00377 BindingDB Entry DOI: 10.7270/Q2FN18MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoprenyl transferase (Streptococcus pneumoniae) | BDBM50372752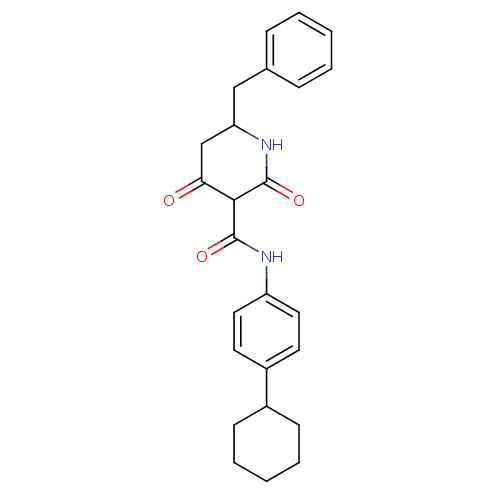 (CHEMBL272547) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae UPPS | Bioorg Med Chem Lett 18: 1840-4 (2008) Article DOI: 10.1016/j.bmcl.2008.02.009 BindingDB Entry DOI: 10.7270/Q2CF9QZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50106102 ((E)-(S)-3-[2-((3-Methyl-butyl)-{2-[4-(3-o-tolyl-ur...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro Very late antigen 4 cell adhesion inhibitory activity measured by binding of fluorescently labeled Ramos cells to immobilized VCAM-1. | Bioorg Med Chem Lett 11: 2955-8 (2001) BindingDB Entry DOI: 10.7270/Q20001CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50106083 ((S)-3-(4-Methoxy-phenyl)-3-[2-((3-methoxy-propyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of fluorescent Ramos cell adhesion to VCAM-1 by mouse Very late antigen 4 (VLA-4) integrin | Bioorg Med Chem Lett 11: 2955-8 (2001) BindingDB Entry DOI: 10.7270/Q20001CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoprenyl transferase (Streptococcus pneumoniae) | BDBM50372771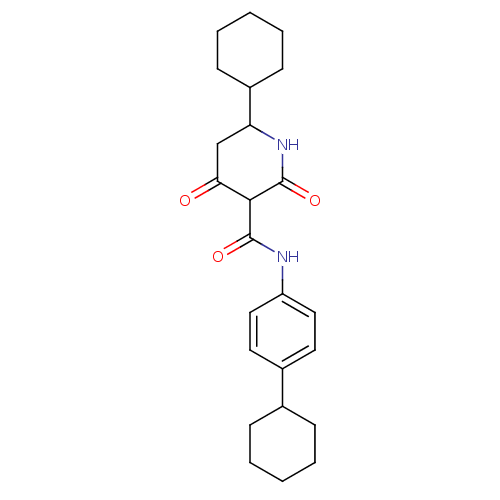 (CHEMBL404127) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae UPPS | Bioorg Med Chem Lett 18: 1840-4 (2008) Article DOI: 10.1016/j.bmcl.2008.02.009 BindingDB Entry DOI: 10.7270/Q2CF9QZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-L/Intercellular adhesion molecule 1 (Homo sapiens (Human)) | BDBM50161067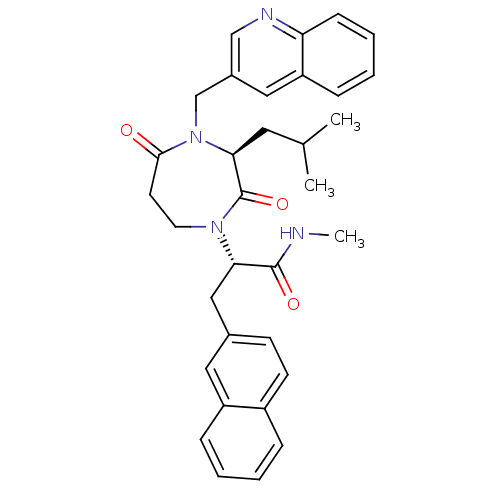 ((2R)-2-[3-ISOBUTYL-2,5-DIOXO-4-(QUINOLIN-3-YLMETHY...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description In vitro inhibitory concentrationfor Lymphocyte function associated antigen 1/Intercellular adhesion molecule 1 (LFA-1/ICAM-1) | Bioorg Med Chem Lett 15: 1217-20 (2005) Article DOI: 10.1016/j.bmcl.2004.11.072 BindingDB Entry DOI: 10.7270/Q20Z72S2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Isoprenyl transferase (Streptococcus pneumoniae) | BDBM50372754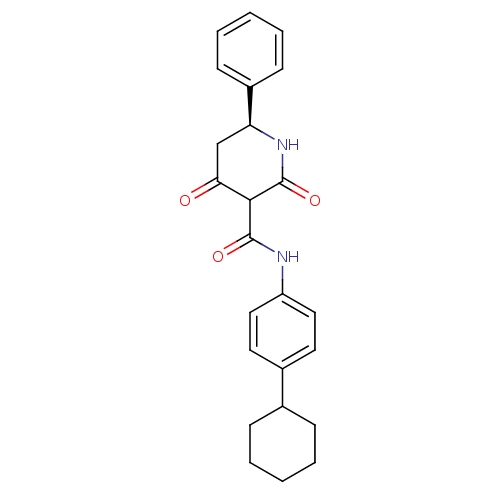 (CHEMBL272054) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae UPPS | Bioorg Med Chem Lett 18: 1840-4 (2008) Article DOI: 10.1016/j.bmcl.2008.02.009 BindingDB Entry DOI: 10.7270/Q2CF9QZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-L/Intercellular adhesion molecule 1 (Homo sapiens (Human)) | BDBM50123241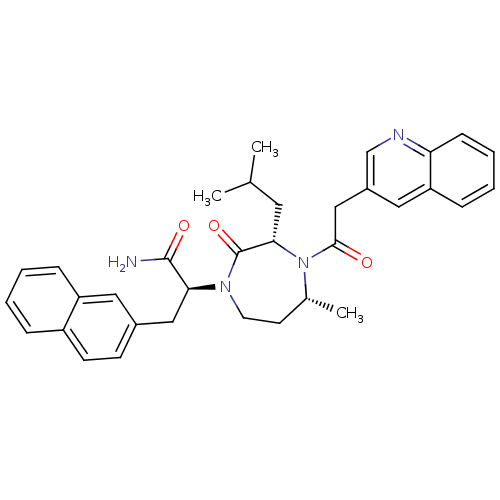 ((S)-2-[(3S,5R)-3-Isobutyl-5-methyl-2-oxo-4-(2-quin...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description In vitro inhibitory concentrationfor Lymphocyte function associated antigen 1/Intercellular adhesion molecule 1 (LFA-1/ICAM-1) | Bioorg Med Chem Lett 15: 1217-20 (2005) Article DOI: 10.1016/j.bmcl.2004.11.072 BindingDB Entry DOI: 10.7270/Q20Z72S2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-L/Integrin beta-2/Intercellular adhesion molecule 1 (Homo sapiens (Human)) | BDBM50123241 ((S)-2-[(3S,5R)-3-Isobutyl-5-methyl-2-oxo-4-(2-quin...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of LFA-1/ICAM-1 interaction in ELISA | Bioorg Med Chem Lett 13: 499-502 (2003) BindingDB Entry DOI: 10.7270/Q29K49K4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoprenyl transferase (Streptococcus pneumoniae) | BDBM50372766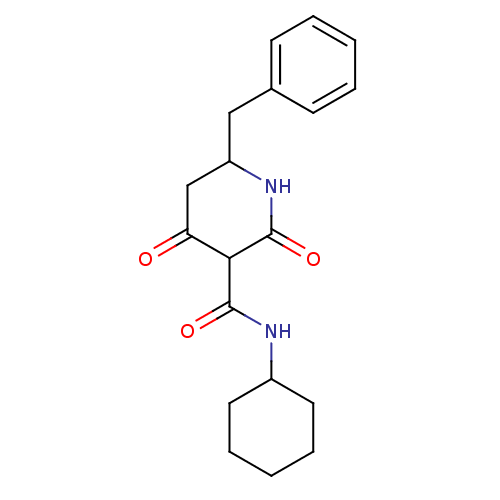 (CHEMBL271482) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae UPPS | Bioorg Med Chem Lett 18: 1840-4 (2008) Article DOI: 10.1016/j.bmcl.2008.02.009 BindingDB Entry DOI: 10.7270/Q2CF9QZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50469553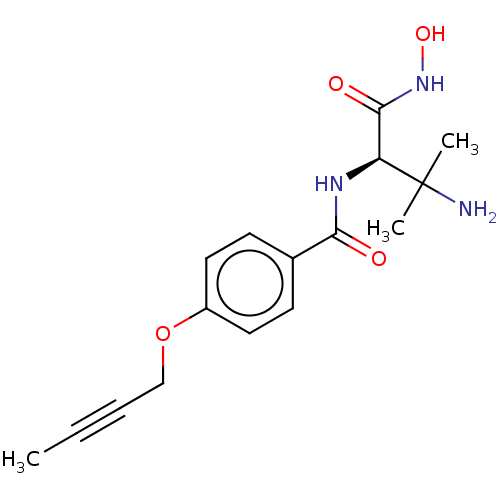 (CHEMBL4102769) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 81.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa LpxC using UDP-3-O-(R-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 30 mins followed by sub... | J Med Chem 60: 5002-5014 (2017) Article DOI: 10.1021/acs.jmedchem.7b00377 BindingDB Entry DOI: 10.7270/Q2FN18MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50106097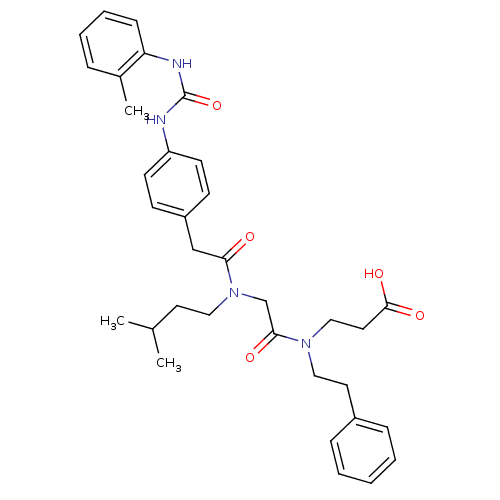 (3-{[2-((3-Methyl-butyl)-{2-[4-(3-o-tolyl-ureido)-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro Very late antigen 4 cell adhesion inhibitory activity measured by binding of fluorescently labeled Ramos cells to immobilized VCAM-1. | Bioorg Med Chem Lett 11: 2955-8 (2001) BindingDB Entry DOI: 10.7270/Q20001CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50469552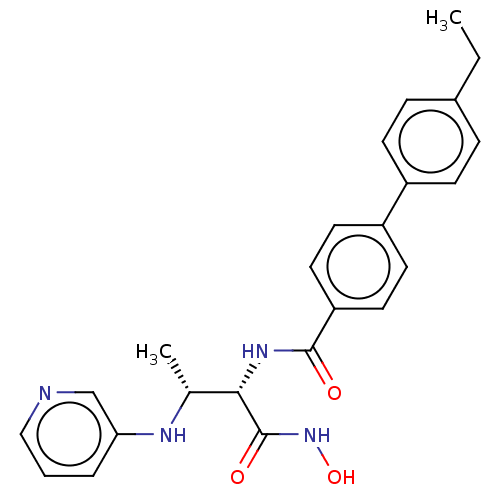 (CHEMBL4072428) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa LpxC using UDP-3-O-(R-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 30 mins followed by sub... | J Med Chem 60: 5002-5014 (2017) Article DOI: 10.1021/acs.jmedchem.7b00377 BindingDB Entry DOI: 10.7270/Q2FN18MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoprenyl transferase (Streptococcus pneumoniae) | BDBM50372750 (CHEMBL272914) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae UPPS | Bioorg Med Chem Lett 18: 1840-4 (2008) Article DOI: 10.1016/j.bmcl.2008.02.009 BindingDB Entry DOI: 10.7270/Q2CF9QZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-L/Integrin beta-2/Intercellular adhesion molecule 1 (Homo sapiens (Human)) | BDBM50123239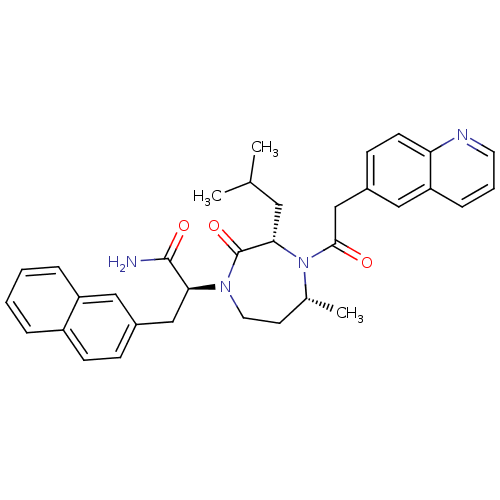 ((S)-2-[(3S,5R)-3-Isobutyl-5-methyl-2-oxo-4-(2-quin...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of LFA-1/ICAM-1 interaction in ELISA | Bioorg Med Chem Lett 13: 499-502 (2003) BindingDB Entry DOI: 10.7270/Q29K49K4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoprenyl transferase (Streptococcus pneumoniae) | BDBM50372751 (CHEMBL256222) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae UPPS | Bioorg Med Chem Lett 18: 1840-4 (2008) Article DOI: 10.1016/j.bmcl.2008.02.009 BindingDB Entry DOI: 10.7270/Q2CF9QZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50469548 (CHEMBL4073216) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 116 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa LpxC using UDP-3-O-(R-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 30 mins followed by sub... | J Med Chem 60: 5002-5014 (2017) Article DOI: 10.1021/acs.jmedchem.7b00377 BindingDB Entry DOI: 10.7270/Q2FN18MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoprenyl transferase (Streptococcus pneumoniae) | BDBM50372760 (CHEMBL257948) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae UPPS | Bioorg Med Chem Lett 18: 1840-4 (2008) Article DOI: 10.1016/j.bmcl.2008.02.009 BindingDB Entry DOI: 10.7270/Q2CF9QZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-L/Intercellular adhesion molecule 1 (Homo sapiens (Human)) | BDBM50161072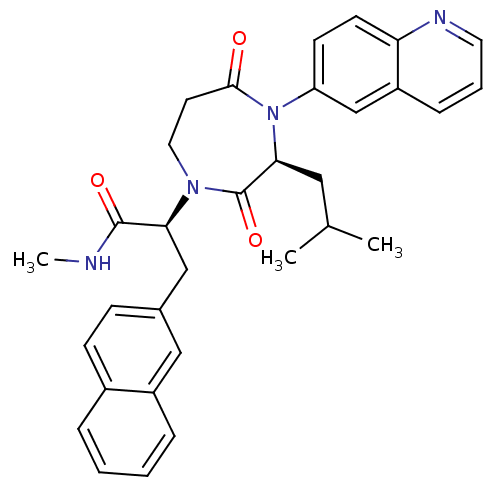 ((S)-2-((S)-3-Isobutyl-2,5-dioxo-4-quinolin-6-yl-[1...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description In vitro inhibitory concentrationfor Lymphocyte function associated antigen 1/Intercellular adhesion molecule 1 (LFA-1/ICAM-1) | Bioorg Med Chem Lett 15: 1217-20 (2005) Article DOI: 10.1016/j.bmcl.2004.11.072 BindingDB Entry DOI: 10.7270/Q20Z72S2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 172 total ) | Next | Last >> |