

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM113220 (US8633206, 139) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.00125 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Pfizer Inc. US Patent | Assay Description A peptide mobility shift assay was used to quantify the phosphorylation of the JAKtide (JAK2 and JAK3) or the IRS-1 peptide (JAK1 and Tyk2). Reaction... | US Patent US8633206 (2014) BindingDB Entry DOI: 10.7270/Q2ZS2V44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM113219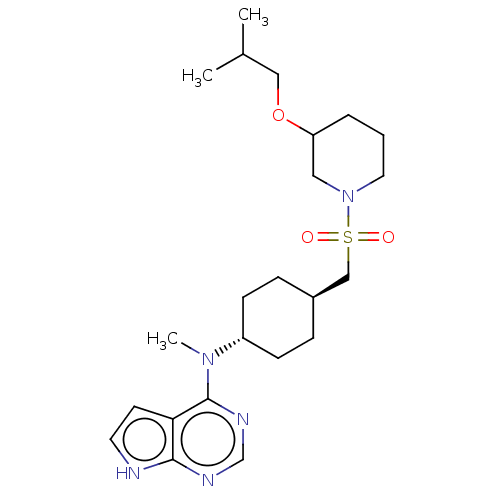 (US8633206, 138) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.00133 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Pfizer Inc. US Patent | Assay Description A peptide mobility shift assay was used to quantify the phosphorylation of the JAKtide (JAK2 and JAK3) or the IRS-1 peptide (JAK1 and Tyk2). Reaction... | US Patent US8633206 (2014) BindingDB Entry DOI: 10.7270/Q2ZS2V44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM113216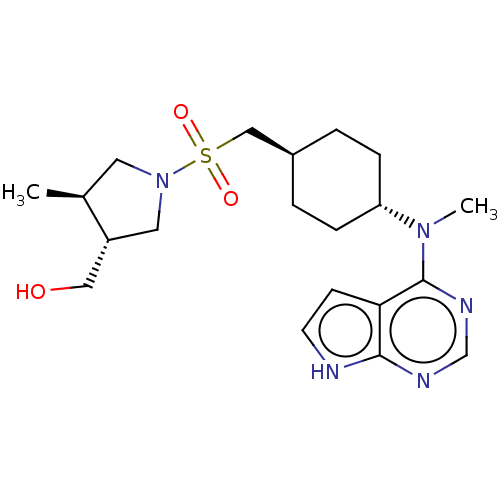 (US8633206, 135) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.00199 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Pfizer Inc. US Patent | Assay Description A peptide mobility shift assay was used to quantify the phosphorylation of the JAKtide (JAK2 and JAK3) or the IRS-1 peptide (JAK1 and Tyk2). Reaction... | US Patent US8633206 (2014) BindingDB Entry DOI: 10.7270/Q2ZS2V44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM113220 (US8633206, 139) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.00405 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Pfizer Inc. US Patent | Assay Description A peptide mobility shift assay was used to quantify the phosphorylation of the JAKtide (JAK2 and JAK3) or the IRS-1 peptide (JAK1 and Tyk2). Reaction... | US Patent US8633206 (2014) BindingDB Entry DOI: 10.7270/Q2ZS2V44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM113214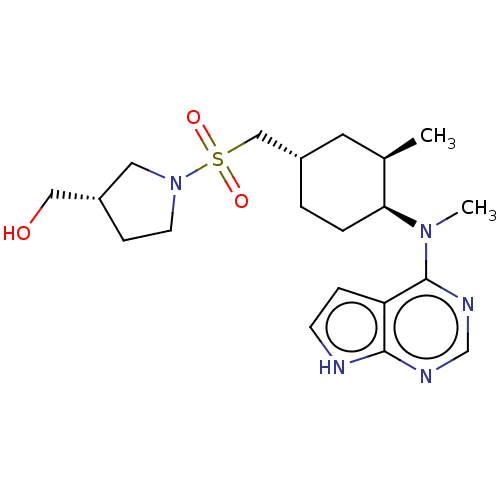 (US8633206, 133) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.00508 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Pfizer Inc. US Patent | Assay Description A peptide mobility shift assay was used to quantify the phosphorylation of the JAKtide (JAK2 and JAK3) or the IRS-1 peptide (JAK1 and Tyk2). Reaction... | US Patent US8633206 (2014) BindingDB Entry DOI: 10.7270/Q2ZS2V44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM113219 (US8633206, 138) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.00767 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Pfizer Inc. US Patent | Assay Description A peptide mobility shift assay was used to quantify the phosphorylation of the JAKtide (JAK2 and JAK3) or the IRS-1 peptide (JAK1 and Tyk2). Reaction... | US Patent US8633206 (2014) BindingDB Entry DOI: 10.7270/Q2ZS2V44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM113218 (US8633206, 137) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.00777 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Pfizer Inc. US Patent | Assay Description A peptide mobility shift assay was used to quantify the phosphorylation of the JAKtide (JAK2 and JAK3) or the IRS-1 peptide (JAK1 and Tyk2). Reaction... | US Patent US8633206 (2014) BindingDB Entry DOI: 10.7270/Q2ZS2V44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50523039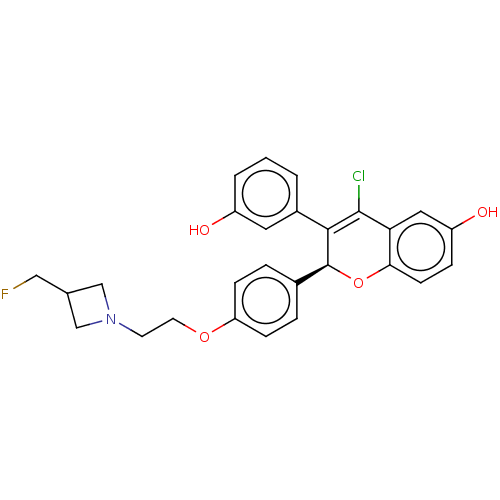 (CHEMBL4591338) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Induction of ERalpha degradation in human MCF7 cells after 4 hrs by FITC/Hoechst 33342 staining based immunofluorescence imaging analysis | Bioorg Med Chem Lett 29: 2090-2093 (2019) Article DOI: 10.1016/j.bmcl.2019.07.013 BindingDB Entry DOI: 10.7270/Q2ZK5M3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM113216 (US8633206, 135) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0165 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Pfizer Inc. US Patent | Assay Description A peptide mobility shift assay was used to quantify the phosphorylation of the JAKtide (JAK2 and JAK3) or the IRS-1 peptide (JAK1 and Tyk2). Reaction... | US Patent US8633206 (2014) BindingDB Entry DOI: 10.7270/Q2ZS2V44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM113214 (US8633206, 133) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0199 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Pfizer Inc. US Patent | Assay Description A peptide mobility shift assay was used to quantify the phosphorylation of the JAKtide (JAK2 and JAK3) or the IRS-1 peptide (JAK1 and Tyk2). Reaction... | US Patent US8633206 (2014) BindingDB Entry DOI: 10.7270/Q2ZS2V44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM113215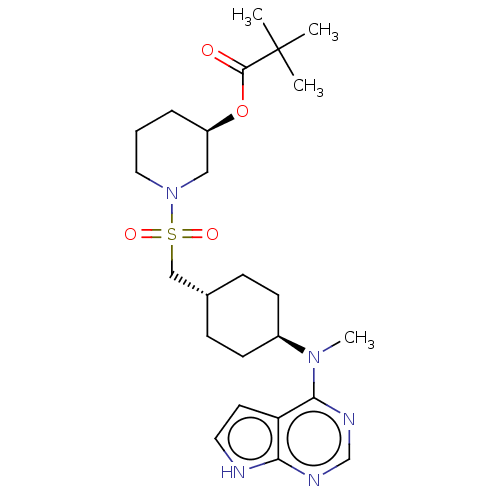 (US8633206, 134) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0227 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Pfizer Inc. US Patent | Assay Description A peptide mobility shift assay was used to quantify the phosphorylation of the JAKtide (JAK2 and JAK3) or the IRS-1 peptide (JAK1 and Tyk2). Reaction... | US Patent US8633206 (2014) BindingDB Entry DOI: 10.7270/Q2ZS2V44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM113221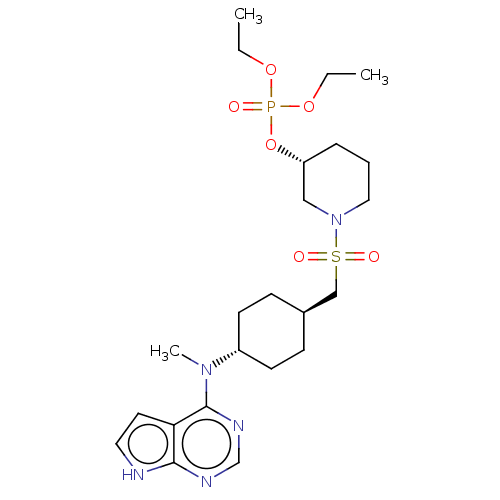 (US8633206, 140) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0235 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Pfizer Inc. US Patent | Assay Description A peptide mobility shift assay was used to quantify the phosphorylation of the JAKtide (JAK2 and JAK3) or the IRS-1 peptide (JAK1 and Tyk2). Reaction... | US Patent US8633206 (2014) BindingDB Entry DOI: 10.7270/Q2ZS2V44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM113217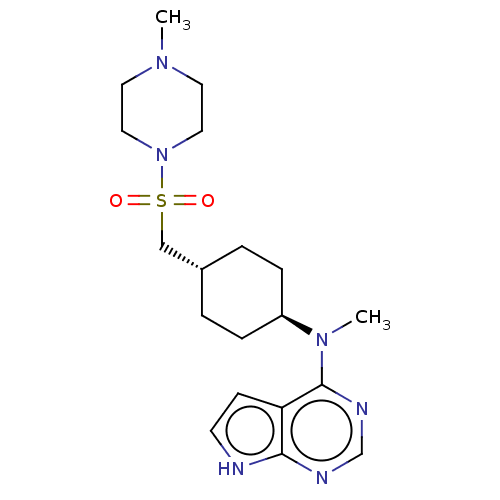 (US8633206, 136) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0245 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Pfizer Inc. US Patent | Assay Description A peptide mobility shift assay was used to quantify the phosphorylation of the JAKtide (JAK2 and JAK3) or the IRS-1 peptide (JAK1 and Tyk2). Reaction... | US Patent US8633206 (2014) BindingDB Entry DOI: 10.7270/Q2ZS2V44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM113222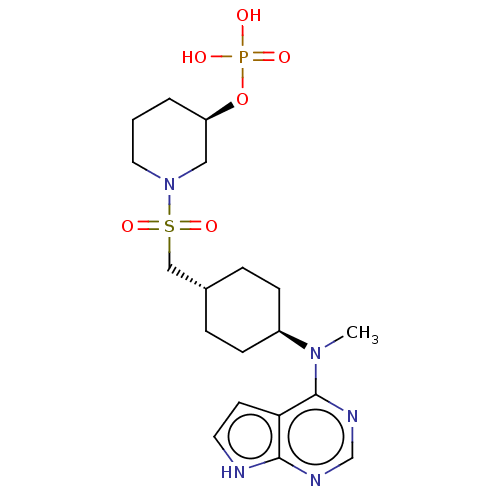 (US8633206, 141) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0263 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Pfizer Inc. US Patent | Assay Description A peptide mobility shift assay was used to quantify the phosphorylation of the JAKtide (JAK2 and JAK3) or the IRS-1 peptide (JAK1 and Tyk2). Reaction... | US Patent US8633206 (2014) BindingDB Entry DOI: 10.7270/Q2ZS2V44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM368199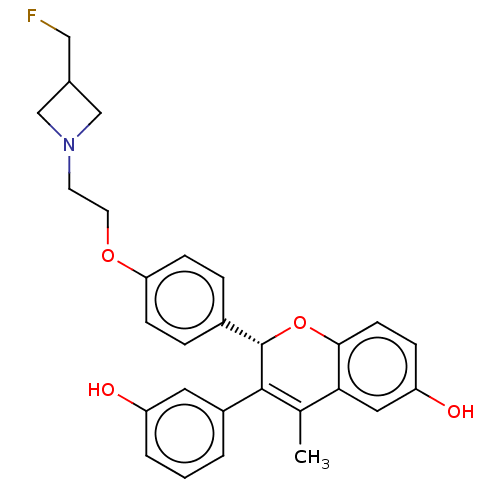 ((S)-2-(4-(2-(3-(fluoromethyl)azetidin-1-yl)ethoxy)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Induction of ERalpha degradation in human MCF7 cells after 4 hrs by FITC/Hoechst 33342 staining based immunofluorescence imaging analysis | Bioorg Med Chem Lett 29: 2090-2093 (2019) Article DOI: 10.1016/j.bmcl.2019.07.013 BindingDB Entry DOI: 10.7270/Q2ZK5M3V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50523049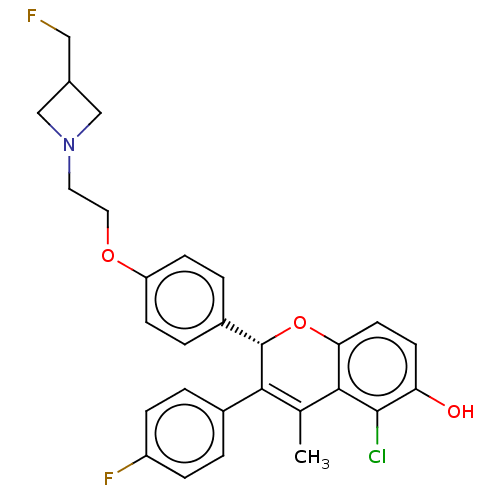 (CHEMBL4441471) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Induction of ERalpha degradation in human MCF7 cells after 4 hrs by FITC/Hoechst 33342 staining based immunofluorescence imaging analysis | Bioorg Med Chem Lett 29: 2090-2093 (2019) Article DOI: 10.1016/j.bmcl.2019.07.013 BindingDB Entry DOI: 10.7270/Q2ZK5M3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM113218 (US8633206, 137) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0366 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Pfizer Inc. US Patent | Assay Description A peptide mobility shift assay was used to quantify the phosphorylation of the JAKtide (JAK2 and JAK3) or the IRS-1 peptide (JAK1 and Tyk2). Reaction... | US Patent US8633206 (2014) BindingDB Entry DOI: 10.7270/Q2ZS2V44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM113220 (US8633206, 139) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0368 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Pfizer Inc. US Patent | Assay Description A peptide mobility shift assay was used to quantify the phosphorylation of the JAKtide (JAK2 and JAK3) or the IRS-1 peptide (JAK1 and Tyk2). Reaction... | US Patent US8633206 (2014) BindingDB Entry DOI: 10.7270/Q2ZS2V44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM113214 (US8633206, 133) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0421 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Pfizer Inc. US Patent | Assay Description A peptide mobility shift assay was used to quantify the phosphorylation of the JAKtide (JAK2 and JAK3) or the IRS-1 peptide (JAK1 and Tyk2). Reaction... | US Patent US8633206 (2014) BindingDB Entry DOI: 10.7270/Q2ZS2V44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50572808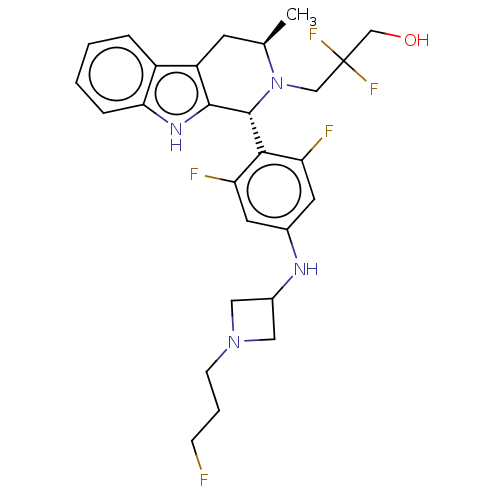 (Gdc-9545 | Giredestrant | RO-7197597 | RO7197597) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00847 BindingDB Entry DOI: 10.7270/Q20R9T67 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50523050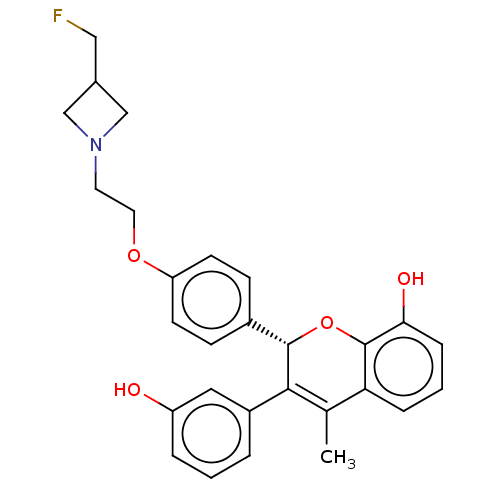 (CHEMBL4455953) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Induction of ERalpha degradation in human MCF7 cells after 4 hrs by FITC/Hoechst 33342 staining based immunofluorescence imaging analysis | Bioorg Med Chem Lett 29: 2090-2093 (2019) Article DOI: 10.1016/j.bmcl.2019.07.013 BindingDB Entry DOI: 10.7270/Q2ZK5M3V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50572809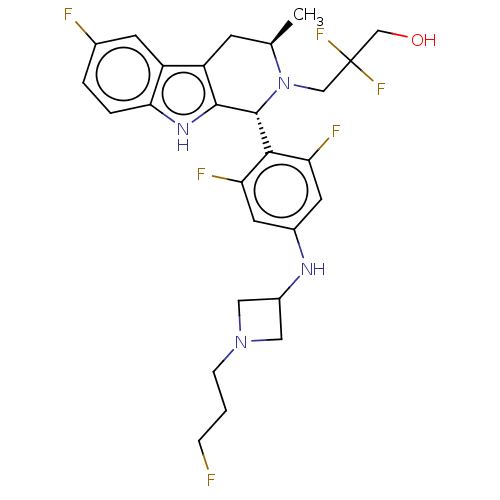 (CHEMBL4866043) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00847 BindingDB Entry DOI: 10.7270/Q20R9T67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50572829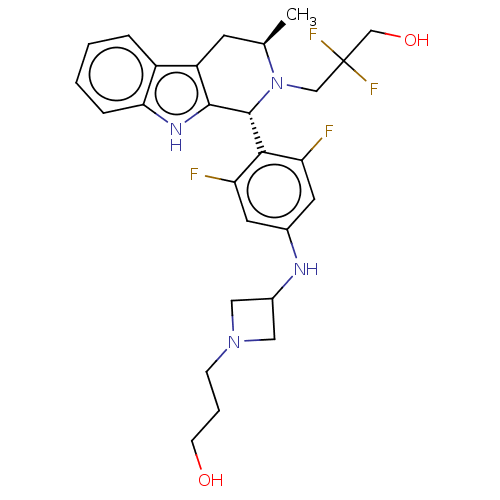 (CHEMBL4856969) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00847 BindingDB Entry DOI: 10.7270/Q20R9T67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50523040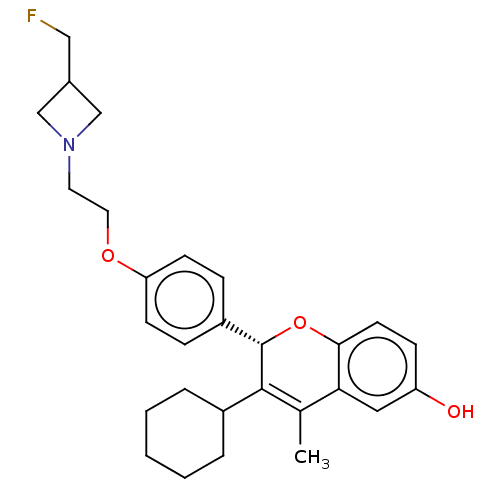 (CHEMBL4463541) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Induction of ERalpha degradation in human MCF7 cells after 4 hrs by FITC/Hoechst 33342 staining based immunofluorescence imaging analysis | Bioorg Med Chem Lett 29: 2090-2093 (2019) Article DOI: 10.1016/j.bmcl.2019.07.013 BindingDB Entry DOI: 10.7270/Q2ZK5M3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50572825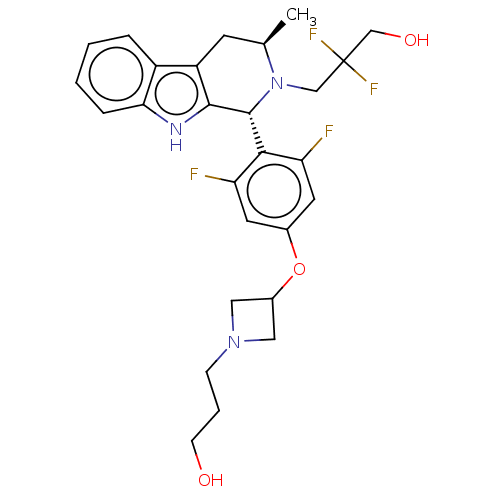 (CHEMBL4856892) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00847 BindingDB Entry DOI: 10.7270/Q20R9T67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50523035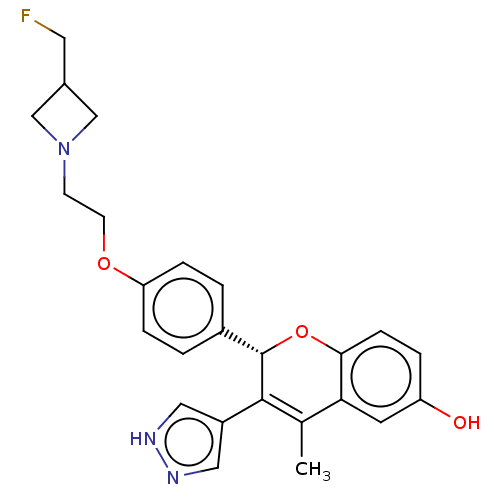 (CHEMBL4594150) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Induction of ERalpha degradation in human MCF7 cells after 4 hrs by FITC/Hoechst 33342 staining based immunofluorescence imaging analysis | Bioorg Med Chem Lett 29: 2090-2093 (2019) Article DOI: 10.1016/j.bmcl.2019.07.013 BindingDB Entry DOI: 10.7270/Q2ZK5M3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50572828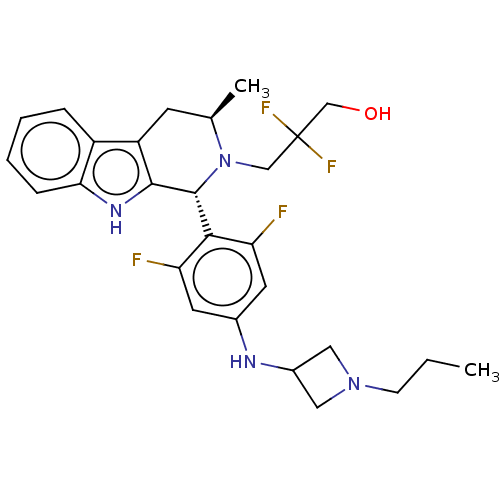 (CHEMBL4864829) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00847 BindingDB Entry DOI: 10.7270/Q20R9T67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50572826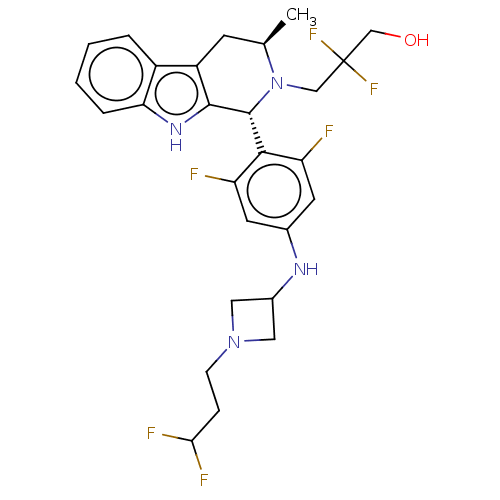 (CHEMBL4869698) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00847 BindingDB Entry DOI: 10.7270/Q20R9T67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50572832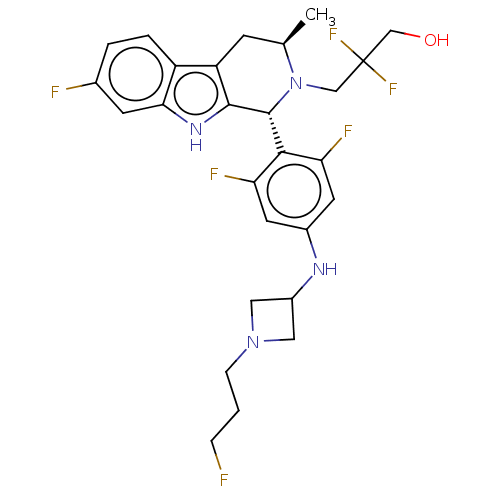 (CHEMBL4845726) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00847 BindingDB Entry DOI: 10.7270/Q20R9T67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM368199 ((S)-2-(4-(2-(3-(fluoromethyl)azetidin-1-yl)ethoxy)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00847 BindingDB Entry DOI: 10.7270/Q20R9T67 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50572821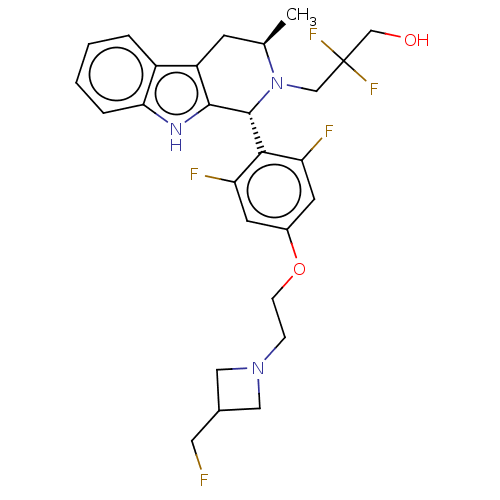 (CHEMBL4871161) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00847 BindingDB Entry DOI: 10.7270/Q20R9T67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM113222 (US8633206, 141) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.106 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Pfizer Inc. US Patent | Assay Description A peptide mobility shift assay was used to quantify the phosphorylation of the JAKtide (JAK2 and JAK3) or the IRS-1 peptide (JAK1 and Tyk2). Reaction... | US Patent US8633206 (2014) BindingDB Entry DOI: 10.7270/Q2ZS2V44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM113217 (US8633206, 136) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.109 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Pfizer Inc. US Patent | Assay Description A peptide mobility shift assay was used to quantify the phosphorylation of the JAKtide (JAK2 and JAK3) or the IRS-1 peptide (JAK1 and Tyk2). Reaction... | US Patent US8633206 (2014) BindingDB Entry DOI: 10.7270/Q2ZS2V44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM113221 (US8633206, 140) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.116 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Pfizer Inc. US Patent | Assay Description A peptide mobility shift assay was used to quantify the phosphorylation of the JAKtide (JAK2 and JAK3) or the IRS-1 peptide (JAK1 and Tyk2). Reaction... | US Patent US8633206 (2014) BindingDB Entry DOI: 10.7270/Q2ZS2V44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM113215 (US8633206, 134) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.129 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Pfizer Inc. US Patent | Assay Description A peptide mobility shift assay was used to quantify the phosphorylation of the JAKtide (JAK2 and JAK3) or the IRS-1 peptide (JAK1 and Tyk2). Reaction... | US Patent US8633206 (2014) BindingDB Entry DOI: 10.7270/Q2ZS2V44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50572824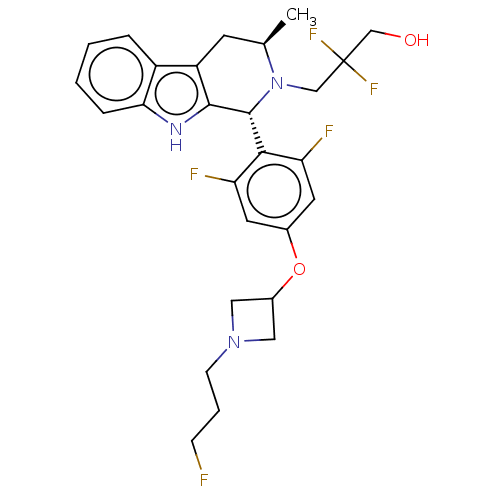 (CHEMBL4857736) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00847 BindingDB Entry DOI: 10.7270/Q20R9T67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM113219 (US8633206, 138) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.132 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Pfizer Inc. US Patent | Assay Description A peptide mobility shift assay was used to quantify the phosphorylation of the JAKtide (JAK2 and JAK3) or the IRS-1 peptide (JAK1 and Tyk2). Reaction... | US Patent US8633206 (2014) BindingDB Entry DOI: 10.7270/Q2ZS2V44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50542086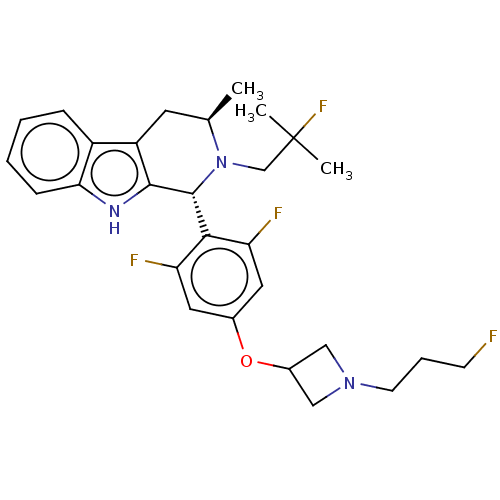 (CHEMBL4649161) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00847 BindingDB Entry DOI: 10.7270/Q20R9T67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50572831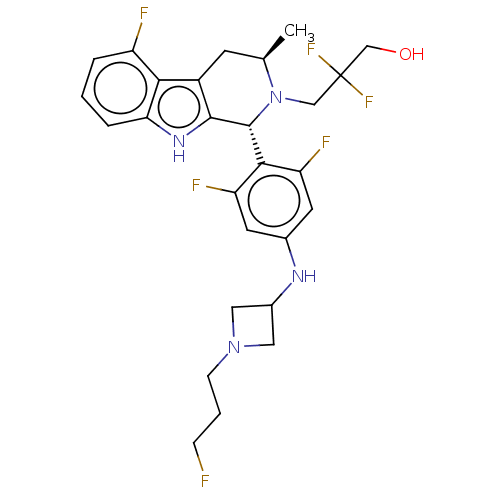 (CHEMBL4877338) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00847 BindingDB Entry DOI: 10.7270/Q20R9T67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50572823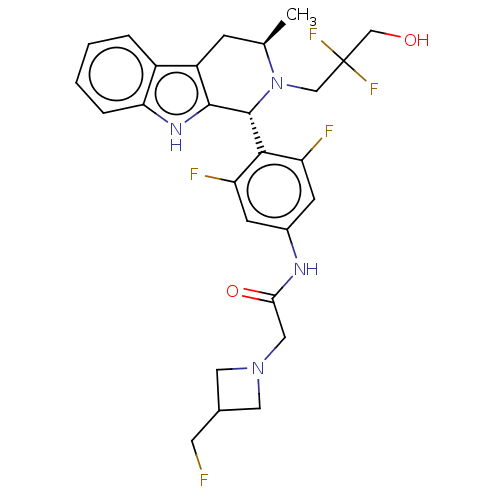 (CHEMBL4860671) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00847 BindingDB Entry DOI: 10.7270/Q20R9T67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50572834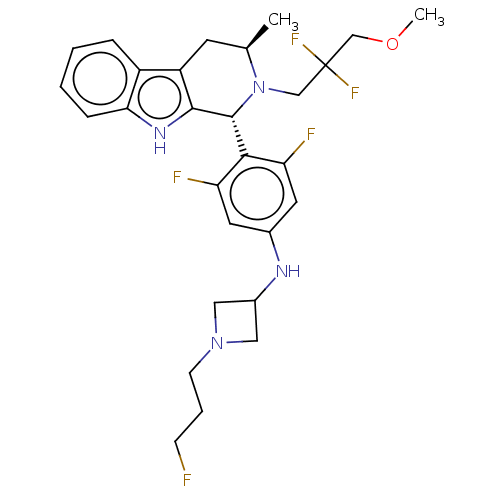 (CHEMBL4853118) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00847 BindingDB Entry DOI: 10.7270/Q20R9T67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50523057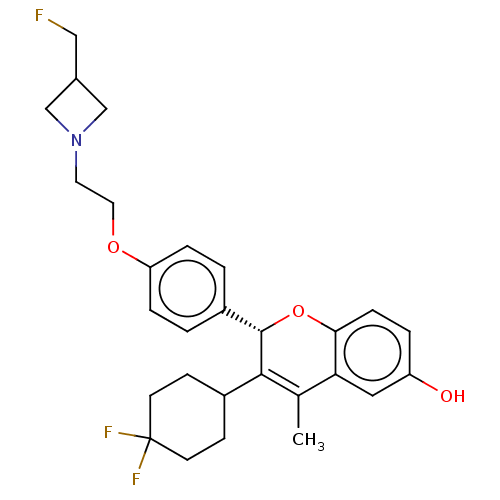 (CHEMBL4447143) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Induction of ERalpha degradation in human MCF7 cells after 4 hrs by FITC/Hoechst 33342 staining based immunofluorescence imaging analysis | Bioorg Med Chem Lett 29: 2090-2093 (2019) Article DOI: 10.1016/j.bmcl.2019.07.013 BindingDB Entry DOI: 10.7270/Q2ZK5M3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM113216 (US8633206, 135) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.198 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Pfizer Inc. US Patent | Assay Description A peptide mobility shift assay was used to quantify the phosphorylation of the JAKtide (JAK2 and JAK3) or the IRS-1 peptide (JAK1 and Tyk2). Reaction... | US Patent US8633206 (2014) BindingDB Entry DOI: 10.7270/Q2ZS2V44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50523053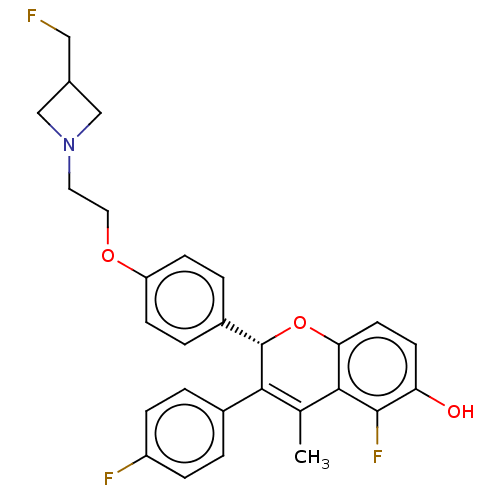 (CHEMBL4567402) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Induction of ERalpha degradation in human MCF7 cells after 4 hrs by FITC/Hoechst 33342 staining based immunofluorescence imaging analysis | Bioorg Med Chem Lett 29: 2090-2093 (2019) Article DOI: 10.1016/j.bmcl.2019.07.013 BindingDB Entry DOI: 10.7270/Q2ZK5M3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM299111 (US10125135, Example 1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00847 BindingDB Entry DOI: 10.7270/Q20R9T67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50523054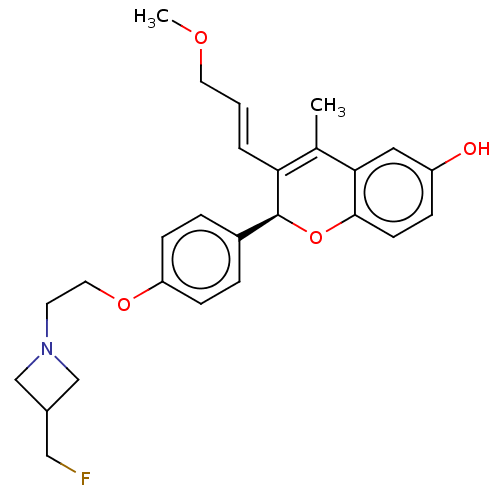 (CHEMBL4476031) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Induction of ERalpha degradation in human MCF7 cells after 4 hrs by FITC/Hoechst 33342 staining based immunofluorescence imaging analysis | Bioorg Med Chem Lett 29: 2090-2093 (2019) Article DOI: 10.1016/j.bmcl.2019.07.013 BindingDB Entry DOI: 10.7270/Q2ZK5M3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50572822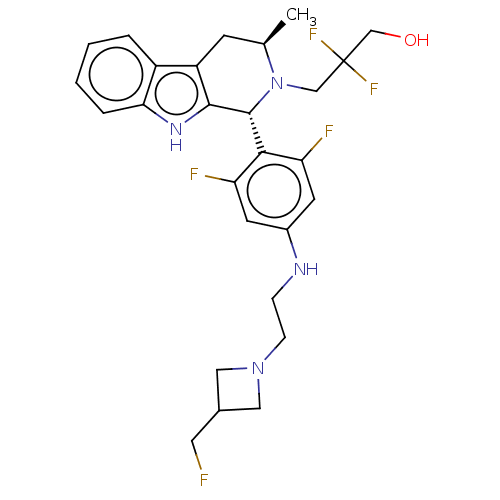 (CHEMBL4866232) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00847 BindingDB Entry DOI: 10.7270/Q20R9T67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50238741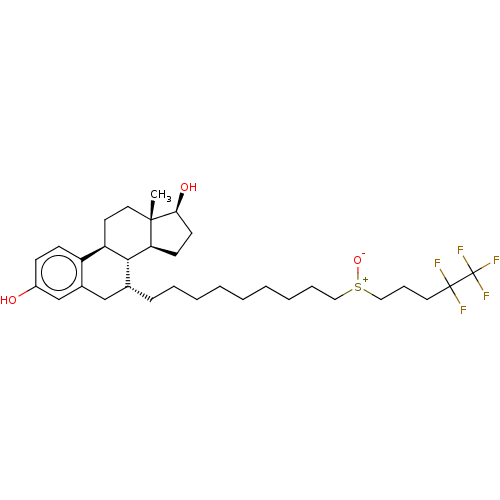 (CHEBI:31638 | Faslodex | Fulvestrant | ICI-182780 ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00847 BindingDB Entry DOI: 10.7270/Q20R9T67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50572833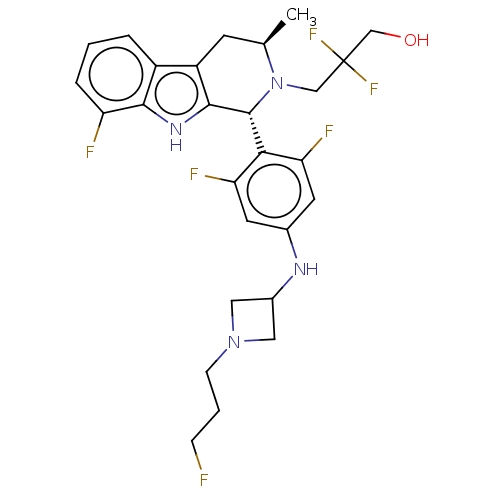 (CHEMBL4873860) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00847 BindingDB Entry DOI: 10.7270/Q20R9T67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50523062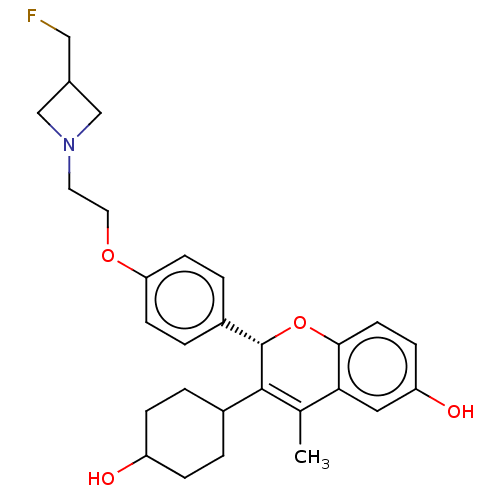 (CHEMBL4450730) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Induction of ERalpha degradation in human MCF7 cells after 4 hrs by FITC/Hoechst 33342 staining based immunofluorescence imaging analysis | Bioorg Med Chem Lett 29: 2090-2093 (2019) Article DOI: 10.1016/j.bmcl.2019.07.013 BindingDB Entry DOI: 10.7270/Q2ZK5M3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 595 total ) | Next | Last >> |