

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM470573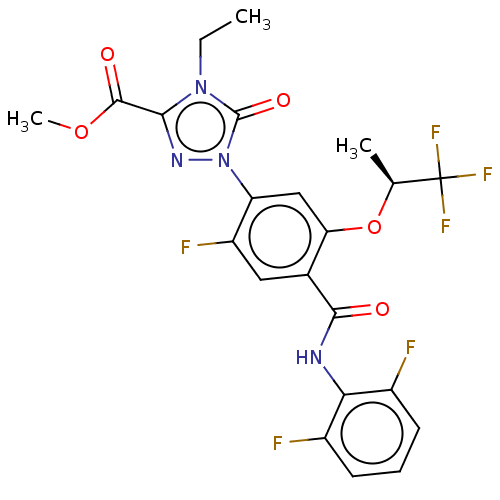 (US10815215, Example 233 | US11130745, Example 233 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The enzymatic assay couples DHODH activity with bleaching of the dye 2,6-dichlorophenolindophenol (DCIP) (Knecht and Loffler, 1998; Miller et al., 19... | Citation and Details BindingDB Entry DOI: 10.7270/Q20C4ZXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50520160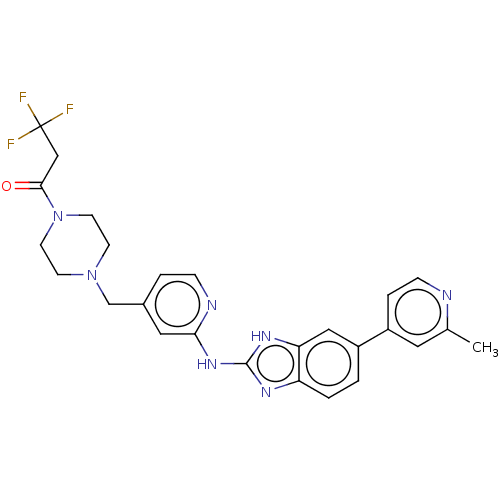 (CHEMBL4515413 | US10894784, Example 01.03) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal GST-tagged FLT3 (564 to end residues) expressed in sf21 cells using biotin labelled Ahx-GGEEEEYFELVKKKK pe... | J Med Chem 63: 601-612 (2020) Article DOI: 10.1021/acs.jmedchem.9b01460 BindingDB Entry DOI: 10.7270/Q2CN7795 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase TBK1 (Homo sapiens (Human)) | BDBM50520150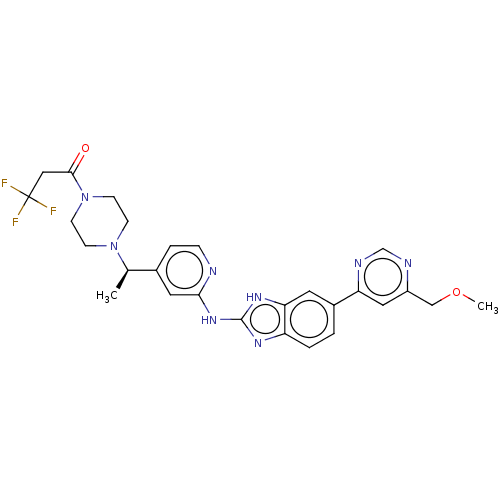 (CHEMBL4566796) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Inhibition of recombinant full length human N-terminal His-tagged TBK1 using biotin-labelled Ahx-GDEDFSSFAEPG peptide as substrate preincubated with ... | J Med Chem 63: 601-612 (2020) Article DOI: 10.1021/acs.jmedchem.9b01460 BindingDB Entry DOI: 10.7270/Q2CN7795 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM490556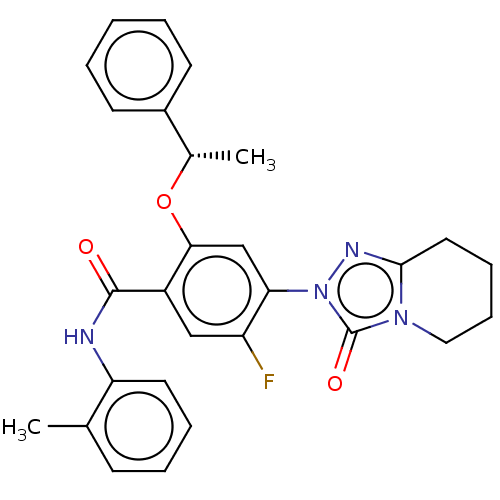 (5-fluoro-N-(2-methylphenyl)-4-(3-oxo-5,6,7,8-tetra...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER AKTIENGESELLSCHAFT; BAYER PHARMA AKTIENGESELLSCHAFT; THE BROAD INSTITUTE, INC.; PRESIDENT AND FELLOWS OF HARVARD COLLEGE; THE GENERAL HOSPITAL CORPORATION US Patent | Assay Description Assay—2: The enzymatic assay couples DHODH activity with bleaching of the dye 2,6-Dichlorophenolindophenol (DCIP) (Knecht and Loffler, 1998; Miller e... | US Patent US10968216 (2021) BindingDB Entry DOI: 10.7270/Q2WM1HJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50520175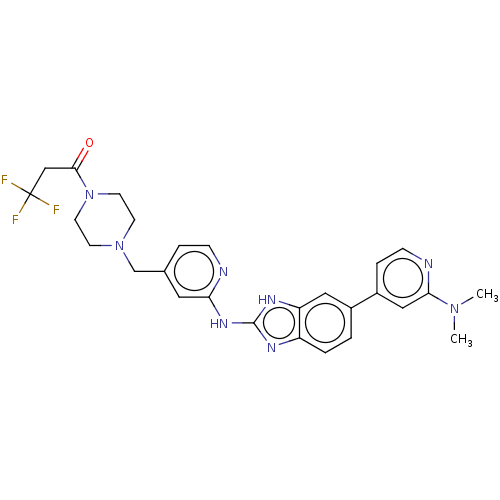 (CHEMBL4451595 | US10894784, Example 135.02) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal GST-tagged FLT3 (564 to end residues) expressed in sf21 cells using biotin labelled Ahx-GGEEEEYFELVKKKK pe... | J Med Chem 63: 601-612 (2020) Article DOI: 10.1021/acs.jmedchem.9b01460 BindingDB Entry DOI: 10.7270/Q2CN7795 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase TBK1 (Homo sapiens (Human)) | BDBM50520142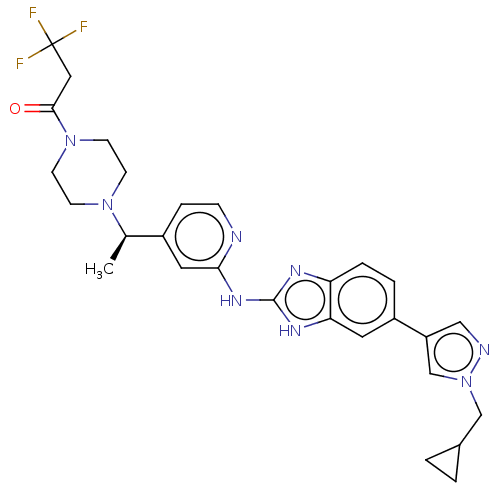 (CHEMBL4435393) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Inhibition of recombinant full length human N-terminal His-tagged TBK1 using biotin-labelled Ahx-GDEDFSSFAEPG peptide as substrate preincubated with ... | J Med Chem 63: 601-612 (2020) Article DOI: 10.1021/acs.jmedchem.9b01460 BindingDB Entry DOI: 10.7270/Q2CN7795 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM470358 (4-(3-ethyl-4-methyl-5-oxo-4,5-dihydro-1H-1,2,4-tri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER AKTIENGESELLSCHAFT; BAYER PHARMA AKTIENGELLSCHAFT; THE BROAD INSTITUTE, INC.; PRESIDENT AND FELLOWS OF HARVARD COLLEGE; THE GENERAL HOSPITAL CORPORATION US Patent | Assay Description Assay 2: The enzymatic assay couples DHODH activity with bleaching of the dye 2,6-Dichlorophenolindophenol (DCIP) (Knecht and Loffler, 1998; Miller e... | US Patent US10815215 (2020) BindingDB Entry DOI: 10.7270/Q2RJ4NJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM470561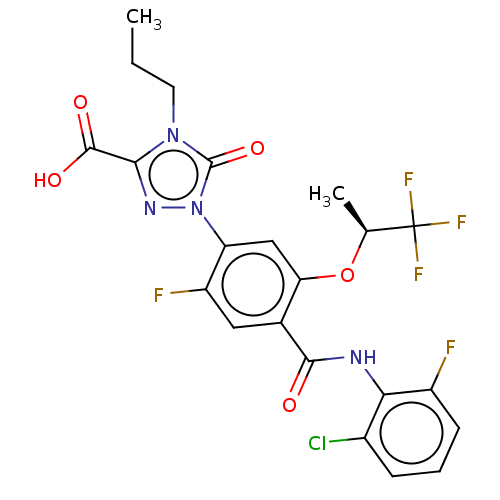 (1-(4-[(2-chloro-6-fluorophenyl)carbamoyl]-2-fluoro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER AKTIENGESELLSCHAFT; BAYER PHARMA AKTIENGELLSCHAFT; THE BROAD INSTITUTE, INC.; PRESIDENT AND FELLOWS OF HARVARD COLLEGE; THE GENERAL HOSPITAL CORPORATION US Patent | Assay Description Assay 1: The enzymatic assay couples DHODH activity with bleaching of the dye 2,6-dichlorophenolindophenol (DCIP) (Knecht and Loffler, 1998; Miller e... | US Patent US10815215 (2020) BindingDB Entry DOI: 10.7270/Q2RJ4NJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM490338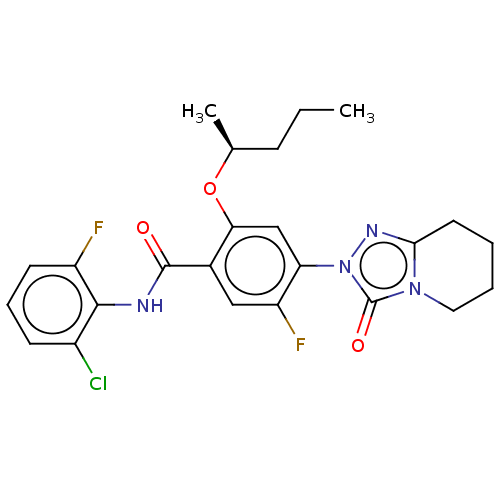 (N-(2-chloro-6-fluorophenyl)-5-fluoro-4-(3-oxo-5,6,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER AKTIENGESELLSCHAFT; BAYER PHARMA AKTIENGESELLSCHAFT; THE BROAD INSTITUTE, INC.; PRESIDENT AND FELLOWS OF HARVARD COLLEGE; THE GENERAL HOSPITAL CORPORATION US Patent | Assay Description Assay—1: The enzymatic assay couples DHODH activity with bleaching of the dye 2,6-dichlorophenolindophenol (DCIP) (Knecht and Loffler, 1998; Miller e... | US Patent US10968216 (2021) BindingDB Entry DOI: 10.7270/Q2WM1HJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50520159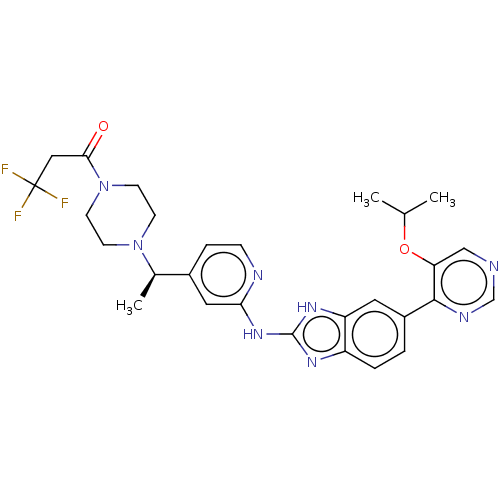 (CHEMBL4543618) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal GST-tagged FLT3 (564 to end residues) expressed in sf21 cells using biotin labelled Ahx-GGEEEEYFELVKKKK pe... | J Med Chem 63: 601-612 (2020) Article DOI: 10.1021/acs.jmedchem.9b01460 BindingDB Entry DOI: 10.7270/Q2CN7795 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM490402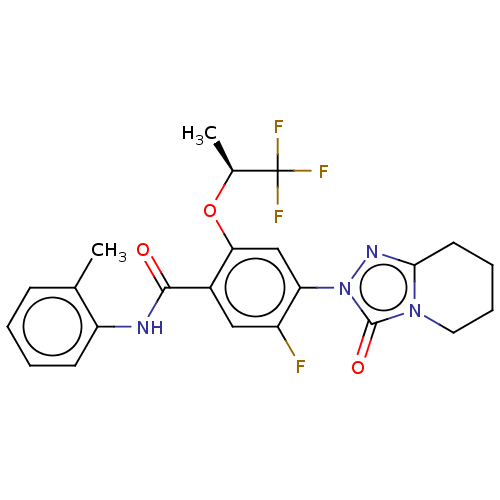 (5-fluoro-N-(2-methylphenyl)-4-(3-oxo-5,6,7,8-tetra...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER AKTIENGESELLSCHAFT; BAYER PHARMA AKTIENGESELLSCHAFT; THE BROAD INSTITUTE, INC.; PRESIDENT AND FELLOWS OF HARVARD COLLEGE; THE GENERAL HOSPITAL CORPORATION US Patent | Assay Description Assay—2: The enzymatic assay couples DHODH activity with bleaching of the dye 2,6-Dichlorophenolindophenol (DCIP) (Knecht and Loffler, 1998; Miller e... | US Patent US10968216 (2021) BindingDB Entry DOI: 10.7270/Q2WM1HJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM470358 (4-(3-ethyl-4-methyl-5-oxo-4,5-dihydro-1H-1,2,4-tri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The enzymatic assay couples DHODH activity with bleaching of the dye 2,6-Dichlorophenolindophenol (DCIP) (Knecht and Loffler, 1998; Miller et al., 19... | Citation and Details BindingDB Entry DOI: 10.7270/Q20C4ZXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM470561 (1-(4-[(2-chloro-6-fluorophenyl)carbamoyl]-2-fluoro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The enzymatic assay couples DHODH activity with bleaching of the dye 2,6-dichlorophenolindophenol (DCIP) (Knecht and Loffler, 1998; Miller et al., 19... | Citation and Details BindingDB Entry DOI: 10.7270/Q20C4ZXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM470358 (4-(3-ethyl-4-methyl-5-oxo-4,5-dihydro-1H-1,2,4-tri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2HQ4414 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM470561 (1-(4-[(2-chloro-6-fluorophenyl)carbamoyl]-2-fluoro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2HQ4414 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM490548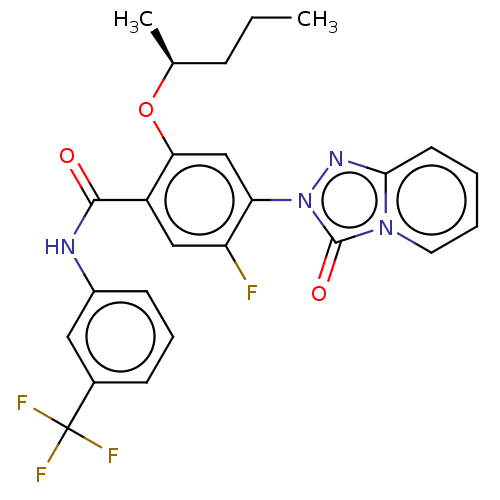 (5-fluoro-4-(3-oxo[1,2,4]triazolo[4,3-a]pyridin-2(3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER AKTIENGESELLSCHAFT; BAYER PHARMA AKTIENGESELLSCHAFT; THE BROAD INSTITUTE, INC.; PRESIDENT AND FELLOWS OF HARVARD COLLEGE; THE GENERAL HOSPITAL CORPORATION US Patent | Assay Description Assay—2: The enzymatic assay couples DHODH activity with bleaching of the dye 2,6-Dichlorophenolindophenol (DCIP) (Knecht and Loffler, 1998; Miller e... | US Patent US10968216 (2021) BindingDB Entry DOI: 10.7270/Q2WM1HJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM470555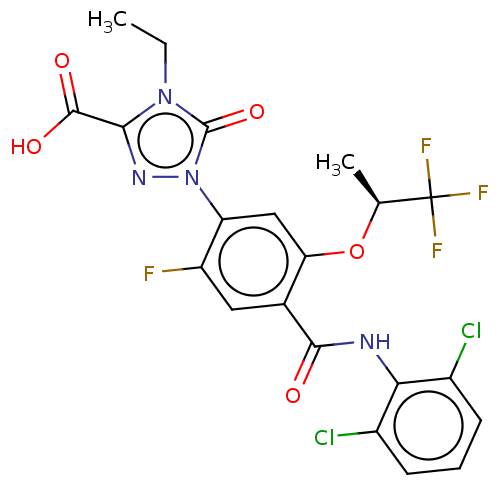 (1-(4-[(2,6-dichlorophenyl)carbamoyl]-2-fluoro-5-{[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER AKTIENGESELLSCHAFT; BAYER PHARMA AKTIENGELLSCHAFT; THE BROAD INSTITUTE, INC.; PRESIDENT AND FELLOWS OF HARVARD COLLEGE; THE GENERAL HOSPITAL CORPORATION US Patent | Assay Description Assay 1: The enzymatic assay couples DHODH activity with bleaching of the dye 2,6-dichlorophenolindophenol (DCIP) (Knecht and Loffler, 1998; Miller e... | US Patent US10815215 (2020) BindingDB Entry DOI: 10.7270/Q2RJ4NJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM470552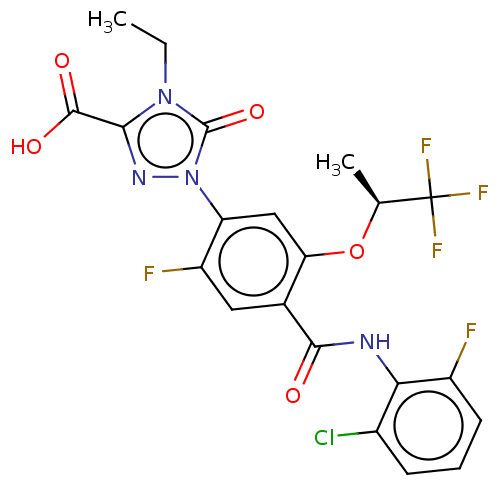 (1-(4-[(2-chloro-6-fluorophenyl)carbamoyl]-2-fluoro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER AKTIENGESELLSCHAFT; BAYER PHARMA AKTIENGELLSCHAFT; THE BROAD INSTITUTE, INC.; PRESIDENT AND FELLOWS OF HARVARD COLLEGE; THE GENERAL HOSPITAL CORPORATION US Patent | Assay Description Assay 1: The enzymatic assay couples DHODH activity with bleaching of the dye 2,6-dichlorophenolindophenol (DCIP) (Knecht and Loffler, 1998; Miller e... | US Patent US10815215 (2020) BindingDB Entry DOI: 10.7270/Q2RJ4NJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM470552 (1-(4-[(2-chloro-6-fluorophenyl)carbamoyl]-2-fluoro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The enzymatic assay couples DHODH activity with bleaching of the dye 2,6-dichlorophenolindophenol (DCIP) (Knecht and Loffler, 1998; Miller et al., 19... | Citation and Details BindingDB Entry DOI: 10.7270/Q20C4ZXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM470552 (1-(4-[(2-chloro-6-fluorophenyl)carbamoyl]-2-fluoro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2HQ4414 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM470555 (1-(4-[(2,6-dichlorophenyl)carbamoyl]-2-fluoro-5-{[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2HQ4414 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM490357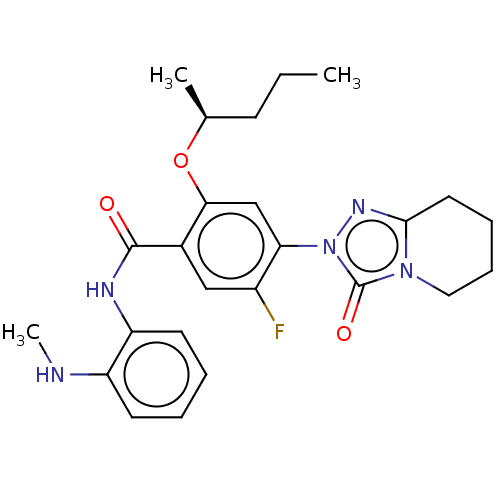 (5-fluoro-N-[2-(methylamino)phenyl]-4-(3-oxo-5,6,7,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER AKTIENGESELLSCHAFT; BAYER PHARMA AKTIENGESELLSCHAFT; THE BROAD INSTITUTE, INC.; PRESIDENT AND FELLOWS OF HARVARD COLLEGE; THE GENERAL HOSPITAL CORPORATION US Patent | Assay Description Assay—2: The enzymatic assay couples DHODH activity with bleaching of the dye 2,6-Dichlorophenolindophenol (DCIP) (Knecht and Loffler, 1998; Miller e... | US Patent US10968216 (2021) BindingDB Entry DOI: 10.7270/Q2WM1HJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM470555 (1-(4-[(2,6-dichlorophenyl)carbamoyl]-2-fluoro-5-{[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The enzymatic assay couples DHODH activity with bleaching of the dye 2,6-dichlorophenolindophenol (DCIP) (Knecht and Loffler, 1998; Miller et al., 19... | Citation and Details BindingDB Entry DOI: 10.7270/Q20C4ZXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM490359 (N-(4-amino-2-methylphenyl)-5-fluoro-4-(3-oxo-5,6,7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER AKTIENGESELLSCHAFT; BAYER PHARMA AKTIENGESELLSCHAFT; THE BROAD INSTITUTE, INC.; PRESIDENT AND FELLOWS OF HARVARD COLLEGE; THE GENERAL HOSPITAL CORPORATION US Patent | Assay Description Assay—1: The enzymatic assay couples DHODH activity with bleaching of the dye 2,6-dichlorophenolindophenol (DCIP) (Knecht and Loffler, 1998; Miller e... | US Patent US10968216 (2021) BindingDB Entry DOI: 10.7270/Q2WM1HJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM470513 (4-(3-ethyl-4-methyl-5-oxo-4,5-dihydro-1H-1,2,4-tri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The enzymatic assay couples DHODH activity with bleaching of the dye 2,6-Dichlorophenolindophenol (DCIP) (Knecht and Loffler, 1998; Miller et al., 19... | Citation and Details BindingDB Entry DOI: 10.7270/Q20C4ZXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM470513 (4-(3-ethyl-4-methyl-5-oxo-4,5-dihydro-1H-1,2,4-tri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2HQ4414 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM470513 (4-(3-ethyl-4-methyl-5-oxo-4,5-dihydro-1H-1,2,4-tri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER AKTIENGESELLSCHAFT; BAYER PHARMA AKTIENGELLSCHAFT; THE BROAD INSTITUTE, INC.; PRESIDENT AND FELLOWS OF HARVARD COLLEGE; THE GENERAL HOSPITAL CORPORATION US Patent | Assay Description Assay 2: The enzymatic assay couples DHODH activity with bleaching of the dye 2,6-Dichlorophenolindophenol (DCIP) (Knecht and Loffler, 1998; Miller e... | US Patent US10815215 (2020) BindingDB Entry DOI: 10.7270/Q2RJ4NJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM490359 (N-(4-amino-2-methylphenyl)-5-fluoro-4-(3-oxo-5,6,7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER AKTIENGESELLSCHAFT; BAYER PHARMA AKTIENGESELLSCHAFT; THE BROAD INSTITUTE, INC.; PRESIDENT AND FELLOWS OF HARVARD COLLEGE; THE GENERAL HOSPITAL CORPORATION US Patent | Assay Description Assay—2: The enzymatic assay couples DHODH activity with bleaching of the dye 2,6-Dichlorophenolindophenol (DCIP) (Knecht and Loffler, 1998; Miller e... | US Patent US10968216 (2021) BindingDB Entry DOI: 10.7270/Q2WM1HJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM490481 (2-[(1S)-1-cyclohexylethoxy]-5-fluoro-N-(2-fluoroph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER AKTIENGESELLSCHAFT; BAYER PHARMA AKTIENGESELLSCHAFT; THE BROAD INSTITUTE, INC.; PRESIDENT AND FELLOWS OF HARVARD COLLEGE; THE GENERAL HOSPITAL CORPORATION US Patent | Assay Description Assay—2: The enzymatic assay couples DHODH activity with bleaching of the dye 2,6-Dichlorophenolindophenol (DCIP) (Knecht and Loffler, 1998; Miller e... | US Patent US10968216 (2021) BindingDB Entry DOI: 10.7270/Q2WM1HJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase TBK1 (Homo sapiens (Human)) | BDBM50520157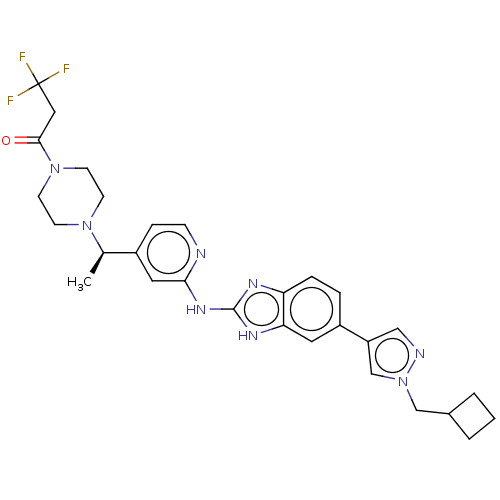 (CHEMBL4436188) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Inhibition of recombinant full length human N-terminal His-tagged TBK1 using biotin-labelled Ahx-GDEDFSSFAEPG peptide as substrate preincubated with ... | J Med Chem 63: 601-612 (2020) Article DOI: 10.1021/acs.jmedchem.9b01460 BindingDB Entry DOI: 10.7270/Q2CN7795 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase TBK1 (Homo sapiens (Human)) | BDBM50520165 (CHEMBL4454902) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Inhibition of recombinant full length human N-terminal His-tagged TBK1 using biotin-labelled Ahx-GDEDFSSFAEPG peptide as substrate preincubated with ... | J Med Chem 63: 601-612 (2020) Article DOI: 10.1021/acs.jmedchem.9b01460 BindingDB Entry DOI: 10.7270/Q2CN7795 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit epsilon (Homo sapiens (Human)) | BDBM50520151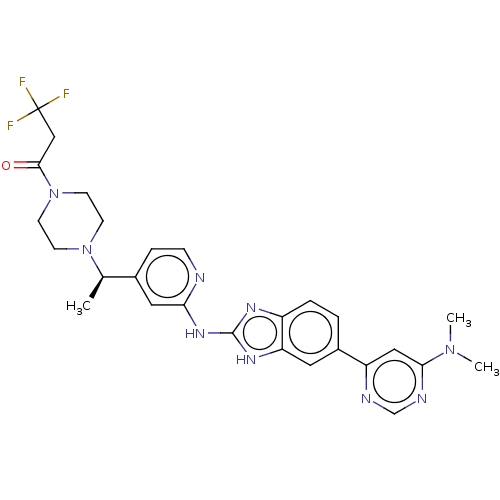 (CHEMBL4537673) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Inhibition of recombinant full length human C-terminal GST-tagged IKKepsilon expressed in baculovirus expression system using biotin-labelled Ahx-GDE... | J Med Chem 63: 601-612 (2020) Article DOI: 10.1021/acs.jmedchem.9b01460 BindingDB Entry DOI: 10.7270/Q2CN7795 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit epsilon (Homo sapiens (Human)) | BDBM50520150 (CHEMBL4566796) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Inhibition of recombinant full length human C-terminal GST-tagged IKKepsilon expressed in baculovirus expression system using biotin-labelled Ahx-GDE... | J Med Chem 63: 601-612 (2020) Article DOI: 10.1021/acs.jmedchem.9b01460 BindingDB Entry DOI: 10.7270/Q2CN7795 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase TBK1 (Homo sapiens (Human)) | BDBM50520159 (CHEMBL4543618) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Inhibition of recombinant full length human N-terminal His-tagged TBK1 using biotin-labelled Ahx-GDEDFSSFAEPG peptide as substrate preincubated with ... | J Med Chem 63: 601-612 (2020) Article DOI: 10.1021/acs.jmedchem.9b01460 BindingDB Entry DOI: 10.7270/Q2CN7795 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase TBK1 (Homo sapiens (Human)) | BDBM50520151 (CHEMBL4537673) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Inhibition of recombinant full length human N-terminal His-tagged TBK1 using biotin-labelled Ahx-GDEDFSSFAEPG peptide as substrate preincubated with ... | J Med Chem 63: 601-612 (2020) Article DOI: 10.1021/acs.jmedchem.9b01460 BindingDB Entry DOI: 10.7270/Q2CN7795 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM470565 (1-(4-[(2-chloro-6-fluorophenyl)carbamoyl]-2-fluoro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER AKTIENGESELLSCHAFT; BAYER PHARMA AKTIENGELLSCHAFT; THE BROAD INSTITUTE, INC.; PRESIDENT AND FELLOWS OF HARVARD COLLEGE; THE GENERAL HOSPITAL CORPORATION US Patent | Assay Description Assay 1: The enzymatic assay couples DHODH activity with bleaching of the dye 2,6-dichlorophenolindophenol (DCIP) (Knecht and Loffler, 1998; Miller e... | US Patent US10815215 (2020) BindingDB Entry DOI: 10.7270/Q2RJ4NJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM490360 (5-fluoro-N-(2-fluoro-6-methylphenyl)-4-(3-oxo-5,6,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER AKTIENGESELLSCHAFT; BAYER PHARMA AKTIENGESELLSCHAFT; THE BROAD INSTITUTE, INC.; PRESIDENT AND FELLOWS OF HARVARD COLLEGE; THE GENERAL HOSPITAL CORPORATION US Patent | Assay Description Assay—2: The enzymatic assay couples DHODH activity with bleaching of the dye 2,6-Dichlorophenolindophenol (DCIP) (Knecht and Loffler, 1998; Miller e... | US Patent US10968216 (2021) BindingDB Entry DOI: 10.7270/Q2WM1HJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM490333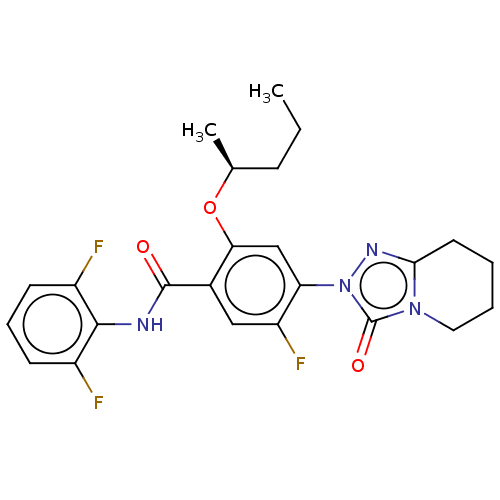 (N-(2,6-difluorophenyl)-5-fluoro-4-(3-oxo-5,6,7,8-t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER AKTIENGESELLSCHAFT; BAYER PHARMA AKTIENGESELLSCHAFT; THE BROAD INSTITUTE, INC.; PRESIDENT AND FELLOWS OF HARVARD COLLEGE; THE GENERAL HOSPITAL CORPORATION US Patent | Assay Description Assay—2: The enzymatic assay couples DHODH activity with bleaching of the dye 2,6-Dichlorophenolindophenol (DCIP) (Knecht and Loffler, 1998; Miller e... | US Patent US10968216 (2021) BindingDB Entry DOI: 10.7270/Q2WM1HJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase TBK1 (Homo sapiens (Human)) | BDBM50520148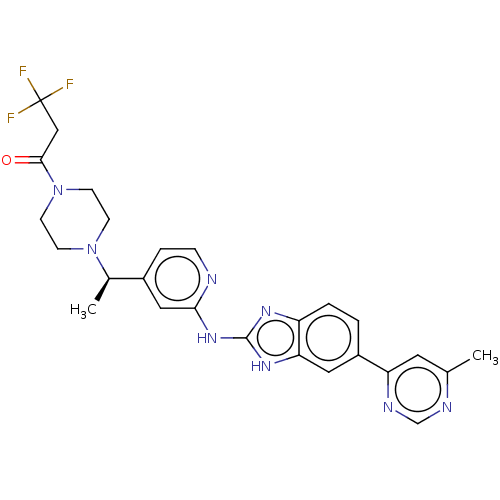 (CHEMBL4538751 | US10894784, Example 30.03.01.A) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Inhibition of recombinant full length human N-terminal His-tagged TBK1 using biotin-labelled Ahx-GDEDFSSFAEPG peptide as substrate preincubated with ... | J Med Chem 63: 601-612 (2020) Article DOI: 10.1021/acs.jmedchem.9b01460 BindingDB Entry DOI: 10.7270/Q2CN7795 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM490477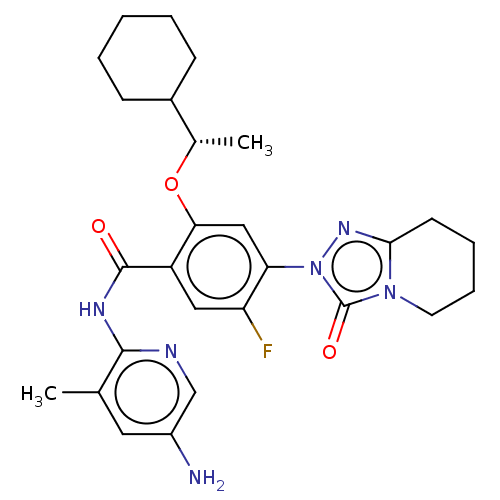 (N-(5-amino-3-methylpyridin-2-yl)-2-[(1S)-1-cyclohe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER AKTIENGESELLSCHAFT; BAYER PHARMA AKTIENGESELLSCHAFT; THE BROAD INSTITUTE, INC.; PRESIDENT AND FELLOWS OF HARVARD COLLEGE; THE GENERAL HOSPITAL CORPORATION US Patent | Assay Description Assay—2: The enzymatic assay couples DHODH activity with bleaching of the dye 2,6-Dichlorophenolindophenol (DCIP) (Knecht and Loffler, 1998; Miller e... | US Patent US10968216 (2021) BindingDB Entry DOI: 10.7270/Q2WM1HJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM490403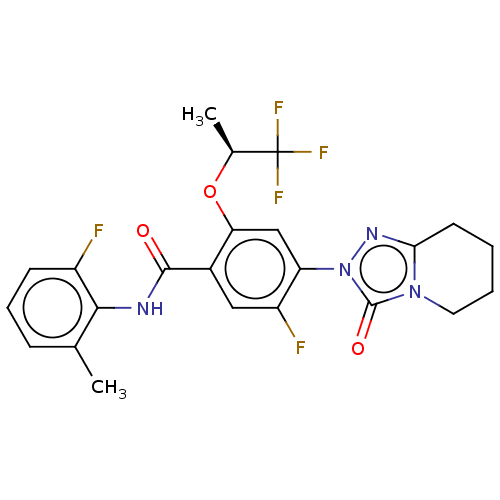 (5-fluoro-N-(2-fluoro-6-methylphenyl)-4-(3-oxo-5,6,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER AKTIENGESELLSCHAFT; BAYER PHARMA AKTIENGESELLSCHAFT; THE BROAD INSTITUTE, INC.; PRESIDENT AND FELLOWS OF HARVARD COLLEGE; THE GENERAL HOSPITAL CORPORATION US Patent | Assay Description Assay—2: The enzymatic assay couples DHODH activity with bleaching of the dye 2,6-Dichlorophenolindophenol (DCIP) (Knecht and Loffler, 1998; Miller e... | US Patent US10968216 (2021) BindingDB Entry DOI: 10.7270/Q2WM1HJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM490393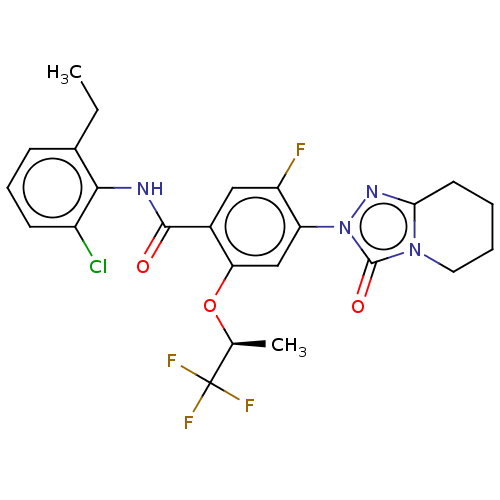 (N-(2-chloro-6-methylphenyl)-5-fluoro-4-(3-oxo-5,6,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER AKTIENGESELLSCHAFT; BAYER PHARMA AKTIENGESELLSCHAFT; THE BROAD INSTITUTE, INC.; PRESIDENT AND FELLOWS OF HARVARD COLLEGE; THE GENERAL HOSPITAL CORPORATION US Patent | Assay Description Assay—1: The enzymatic assay couples DHODH activity with bleaching of the dye 2,6-dichlorophenolindophenol (DCIP) (Knecht and Loffler, 1998; Miller e... | US Patent US10968216 (2021) BindingDB Entry DOI: 10.7270/Q2WM1HJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM470565 (1-(4-[(2-chloro-6-fluorophenyl)carbamoyl]-2-fluoro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The enzymatic assay couples DHODH activity with bleaching of the dye 2,6-dichlorophenolindophenol (DCIP) (Knecht and Loffler, 1998; Miller et al., 19... | Citation and Details BindingDB Entry DOI: 10.7270/Q20C4ZXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM470565 (1-(4-[(2-chloro-6-fluorophenyl)carbamoyl]-2-fluoro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2HQ4414 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM490471 (N-(3-amino-2-methylphenyl)-2-[(1S)-1-cyclohexyleth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER AKTIENGESELLSCHAFT; BAYER PHARMA AKTIENGESELLSCHAFT; THE BROAD INSTITUTE, INC.; PRESIDENT AND FELLOWS OF HARVARD COLLEGE; THE GENERAL HOSPITAL CORPORATION US Patent | Assay Description Assay—2: The enzymatic assay couples DHODH activity with bleaching of the dye 2,6-Dichlorophenolindophenol (DCIP) (Knecht and Loffler, 1998; Miller e... | US Patent US10968216 (2021) BindingDB Entry DOI: 10.7270/Q2WM1HJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM470524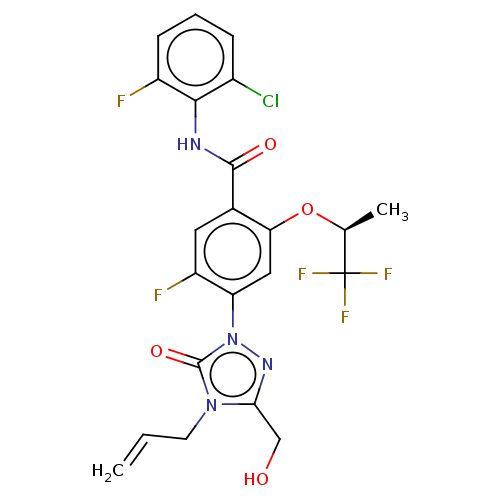 (N-(2-chloro-6-fluorophenyl)-5-fluoro-4-[3-(hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The enzymatic assay couples DHODH activity with bleaching of the dye 2,6-dichlorophenolindophenol (DCIP) (Knecht and Loffler, 1998; Miller et al., 19... | Citation and Details BindingDB Entry DOI: 10.7270/Q20C4ZXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM470524 (N-(2-chloro-6-fluorophenyl)-5-fluoro-4-[3-(hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2HQ4414 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM470524 (N-(2-chloro-6-fluorophenyl)-5-fluoro-4-[3-(hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER AKTIENGESELLSCHAFT; BAYER PHARMA AKTIENGELLSCHAFT; THE BROAD INSTITUTE, INC.; PRESIDENT AND FELLOWS OF HARVARD COLLEGE; THE GENERAL HOSPITAL CORPORATION US Patent | Assay Description Assay 1: The enzymatic assay couples DHODH activity with bleaching of the dye 2,6-dichlorophenolindophenol (DCIP) (Knecht and Loffler, 1998; Miller e... | US Patent US10815215 (2020) BindingDB Entry DOI: 10.7270/Q2RJ4NJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM490450 (2-[(1S)-1-cyclohexylethoxy]-5-fluoro-N-(2-methoxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER AKTIENGESELLSCHAFT; BAYER PHARMA AKTIENGESELLSCHAFT; THE BROAD INSTITUTE, INC.; PRESIDENT AND FELLOWS OF HARVARD COLLEGE; THE GENERAL HOSPITAL CORPORATION US Patent | Assay Description Assay—1: The enzymatic assay couples DHODH activity with bleaching of the dye 2,6-dichlorophenolindophenol (DCIP) (Knecht and Loffler, 1998; Miller e... | US Patent US10968216 (2021) BindingDB Entry DOI: 10.7270/Q2WM1HJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM490478 (2-({2-[(1S)-1-cyclohexylethoxy]-5-fluoro-4-(3-oxo-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER AKTIENGESELLSCHAFT; BAYER PHARMA AKTIENGESELLSCHAFT; THE BROAD INSTITUTE, INC.; PRESIDENT AND FELLOWS OF HARVARD COLLEGE; THE GENERAL HOSPITAL CORPORATION US Patent | Assay Description Assay—2: The enzymatic assay couples DHODH activity with bleaching of the dye 2,6-Dichlorophenolindophenol (DCIP) (Knecht and Loffler, 1998; Miller e... | US Patent US10968216 (2021) BindingDB Entry DOI: 10.7270/Q2WM1HJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1984 total ) | Next | Last >> |