 Found 15 hits with Last Name = 'srivastava' and Initial = 'sp'
Found 15 hits with Last Name = 'srivastava' and Initial = 'sp' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50320877

(4-(4-Fluorophenyl)-7,7-dimethyl-2-phenyl-4,6,7,8-t...)Show SMILES CC1(C)CC(=O)C2C(C=C(N=C2C1)c1ccccc1)c1ccc(F)cc1 |c:8,10| Show InChI InChI=1S/C23H22FNO/c1-23(2)13-20-22(21(26)14-23)18(15-8-10-17(24)11-9-15)12-19(25-20)16-6-4-3-5-7-16/h3-12,18,22H,13-14H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as p-nitrophenolate ion production pretreated for 10 mins measured after 30 mins |
Bioorg Med Chem 18: 4138-48 (2010)
Article DOI: 10.1016/j.bmc.2009.11.061
BindingDB Entry DOI: 10.7270/Q2DJ5FSF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50320882
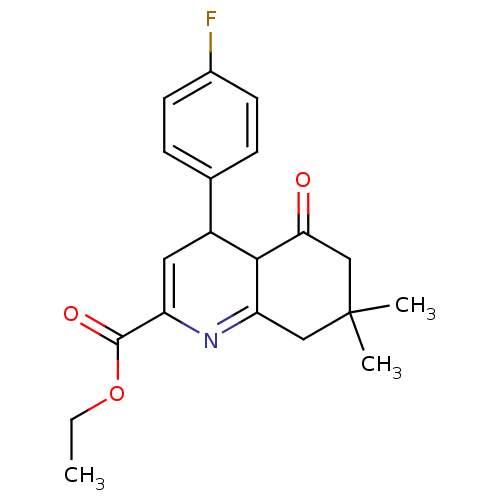
(CHEMBL1165682 | Ethyl-4-(4-fluorophenyl)-7,7-dimet...)Show SMILES CCOC(=O)C1=CC(C2C(=O)CC(C)(C)CC2=N1)c1ccc(F)cc1 |c:17,t:5| Show InChI InChI=1S/C20H22FNO3/c1-4-25-19(24)15-9-14(12-5-7-13(21)8-6-12)18-16(22-15)10-20(2,3)11-17(18)23/h5-9,14,18H,4,10-11H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as p-nitrophenolate ion production pretreated for 10 mins measured after 30 mins |
Bioorg Med Chem 18: 4138-48 (2010)
Article DOI: 10.1016/j.bmc.2009.11.061
BindingDB Entry DOI: 10.7270/Q2DJ5FSF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50320875
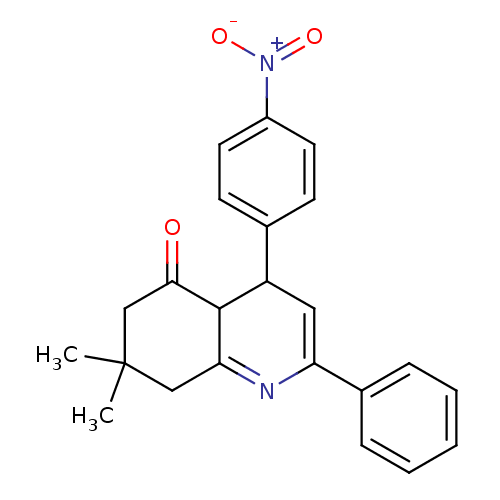
(7,7-Dimethyl-4-(4-nitrophenyl)-2-phenyl-4,6,7,8-te...)Show SMILES CC1(C)CC(=O)C2C(C=C(N=C2C1)c1ccccc1)c1ccc(cc1)[N+]([O-])=O |c:8,10| Show InChI InChI=1S/C23H22N2O3/c1-23(2)13-20-22(21(26)14-23)18(15-8-10-17(11-9-15)25(27)28)12-19(24-20)16-6-4-3-5-7-16/h3-12,18,22H,13-14H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as p-nitrophenolate ion production pretreated for 10 mins measured after 30 mins |
Bioorg Med Chem 18: 4138-48 (2010)
Article DOI: 10.1016/j.bmc.2009.11.061
BindingDB Entry DOI: 10.7270/Q2DJ5FSF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50320876
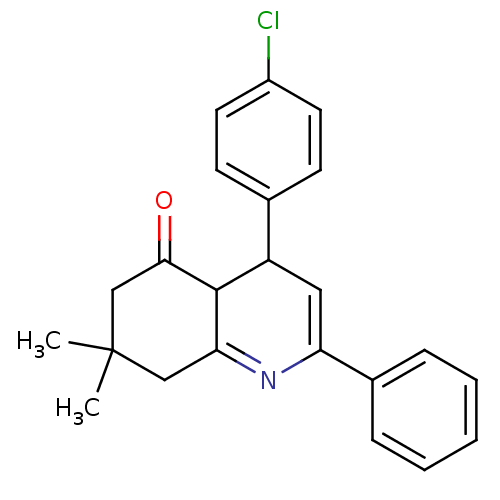
(4-(4-Chlorophenyl)-7,7-dimethyl-2-phenyl-4,6,7,8-t...)Show SMILES CC1(C)CC(=O)C2C(C=C(N=C2C1)c1ccccc1)c1ccc(Cl)cc1 |c:8,10| Show InChI InChI=1S/C23H22ClNO/c1-23(2)13-20-22(21(26)14-23)18(15-8-10-17(24)11-9-15)12-19(25-20)16-6-4-3-5-7-16/h3-12,18,22H,13-14H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as p-nitrophenolate ion production pretreated for 10 mins measured after 30 mins |
Bioorg Med Chem 18: 4138-48 (2010)
Article DOI: 10.1016/j.bmc.2009.11.061
BindingDB Entry DOI: 10.7270/Q2DJ5FSF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50320881
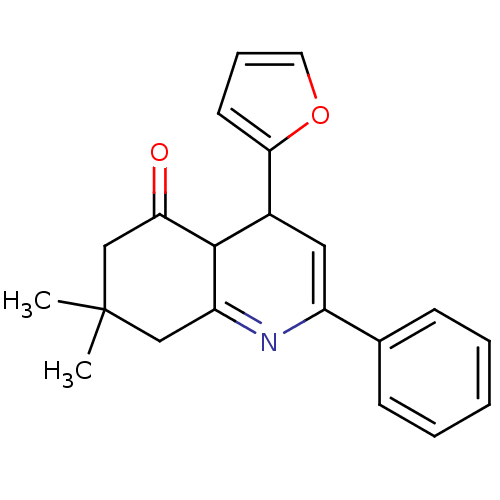
(4-(Furan-2-yl)-7,7-dimethyl-2-phenyl-4,6,7,8-tetra...)Show SMILES CC1(C)CC(=O)C2C(C=C(N=C2C1)c1ccccc1)c1ccco1 |c:8,10| Show InChI InChI=1S/C21H21NO2/c1-21(2)12-17-20(18(23)13-21)15(19-9-6-10-24-19)11-16(22-17)14-7-4-3-5-8-14/h3-11,15,20H,12-13H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as p-nitrophenolate ion production pretreated for 10 mins measured after 30 mins |
Bioorg Med Chem 18: 4138-48 (2010)
Article DOI: 10.1016/j.bmc.2009.11.061
BindingDB Entry DOI: 10.7270/Q2DJ5FSF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50401043
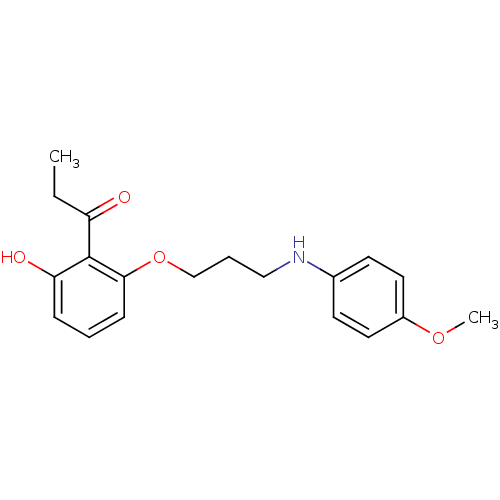
(CHEMBL2206342)Show InChI InChI=1S/C19H23NO4/c1-3-16(21)19-17(22)6-4-7-18(19)24-13-5-12-20-14-8-10-15(23-2)11-9-14/h4,6-11,20,22H,3,5,12-13H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
Bioorg Med Chem 20: 2172-9 (2012)
Article DOI: 10.1016/j.bmc.2011.12.027
BindingDB Entry DOI: 10.7270/Q2T72JKG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50401044
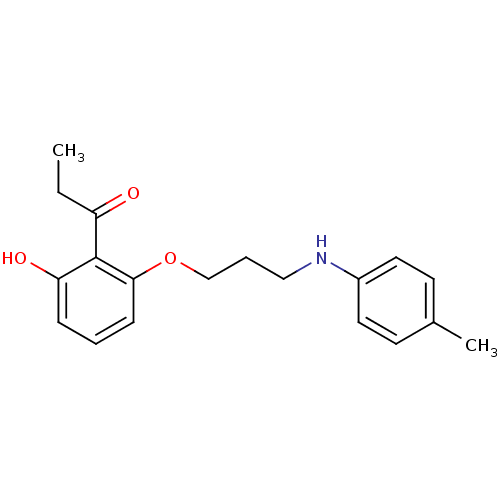
(CHEMBL2206341)Show InChI InChI=1S/C19H23NO3/c1-3-16(21)19-17(22)6-4-7-18(19)23-13-5-12-20-15-10-8-14(2)9-11-15/h4,6-11,20,22H,3,5,12-13H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
Bioorg Med Chem 20: 2172-9 (2012)
Article DOI: 10.1016/j.bmc.2011.12.027
BindingDB Entry DOI: 10.7270/Q2T72JKG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50320879
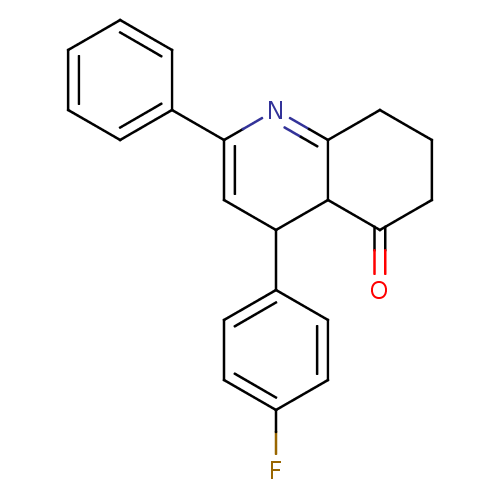
(4-(4-Fluorophenyl)-2-phenyl-4,6,7,8-tetrahydroquin...)Show SMILES Fc1ccc(cc1)C1C=C(N=C2CCCC(=O)C12)c1ccccc1 |c:9,t:11| Show InChI InChI=1S/C21H18FNO/c22-16-11-9-14(10-12-16)17-13-19(15-5-2-1-3-6-15)23-18-7-4-8-20(24)21(17)18/h1-3,5-6,9-13,17,21H,4,7-8H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as p-nitrophenolate ion production pretreated for 10 mins measured after 30 mins |
Bioorg Med Chem 18: 4138-48 (2010)
Article DOI: 10.1016/j.bmc.2009.11.061
BindingDB Entry DOI: 10.7270/Q2DJ5FSF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50320878
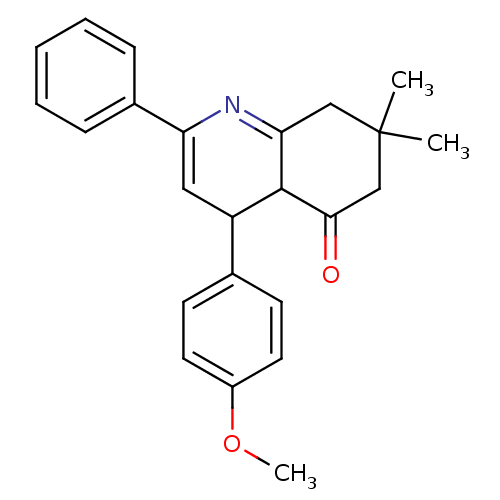
(4-(4-Methoxyphenyl)-7,7-dimethyl-2-phenyl-4,6,7,8-...)Show SMILES COc1ccc(cc1)C1C=C(N=C2CC(C)(C)CC(=O)C12)c1ccccc1 |c:10,t:12| Show InChI InChI=1S/C24H25NO2/c1-24(2)14-21-23(22(26)15-24)19(16-9-11-18(27-3)12-10-16)13-20(25-21)17-7-5-4-6-8-17/h4-13,19,23H,14-15H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as p-nitrophenolate ion production pretreated for 10 mins measured after 30 mins |
Bioorg Med Chem 18: 4138-48 (2010)
Article DOI: 10.1016/j.bmc.2009.11.061
BindingDB Entry DOI: 10.7270/Q2DJ5FSF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50391109

(CHEMBL179166 | Sodium orthovanadate (SOV) | Vanada...) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
Bioorg Med Chem 20: 2172-9 (2012)
Article DOI: 10.1016/j.bmc.2011.12.027
BindingDB Entry DOI: 10.7270/Q2T72JKG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50320883
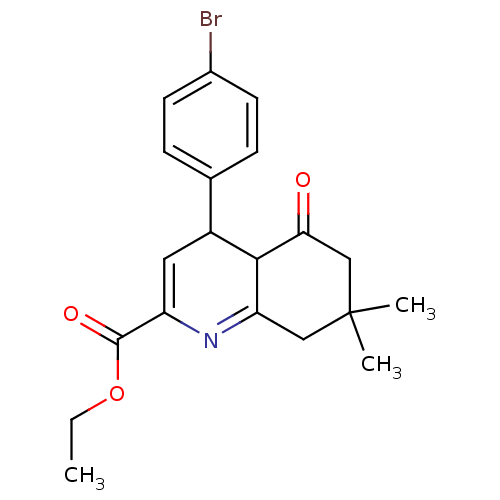
(CHEMBL1164164 | Ethyl-4-(4-bromophenyl)-7,7-dimeth...)Show SMILES CCOC(=O)C1=CC(C2C(=O)CC(C)(C)CC2=N1)c1ccc(Br)cc1 |c:17,t:5| Show InChI InChI=1S/C20H22BrNO3/c1-4-25-19(24)15-9-14(12-5-7-13(21)8-6-12)18-16(22-15)10-20(2,3)11-17(18)23/h5-9,14,18H,4,10-11H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.66E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as p-nitrophenolate ion production pretreated for 10 mins measured after 30 mins |
Bioorg Med Chem 18: 4138-48 (2010)
Article DOI: 10.1016/j.bmc.2009.11.061
BindingDB Entry DOI: 10.7270/Q2DJ5FSF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50401041
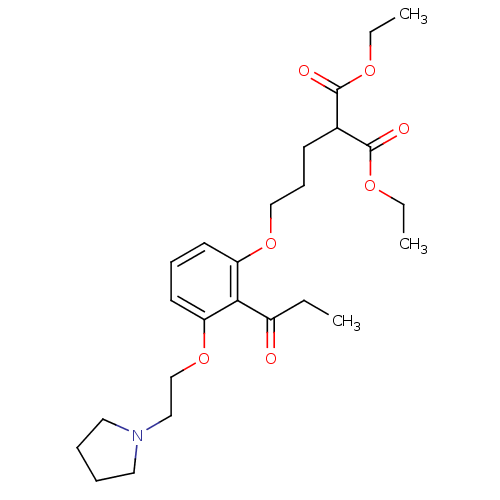
(CHEMBL2206831)Show SMILES CCOC(=O)C(CCCOc1cccc(OCCN2CCCC2)c1C(=O)CC)C(=O)OCC Show InChI InChI=1S/C25H37NO7/c1-4-20(27)23-21(12-9-13-22(23)33-18-16-26-14-7-8-15-26)32-17-10-11-19(24(28)30-5-2)25(29)31-6-3/h9,12-13,19H,4-8,10-11,14-18H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.86E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
Bioorg Med Chem 20: 2172-9 (2012)
Article DOI: 10.1016/j.bmc.2011.12.027
BindingDB Entry DOI: 10.7270/Q2T72JKG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50401045
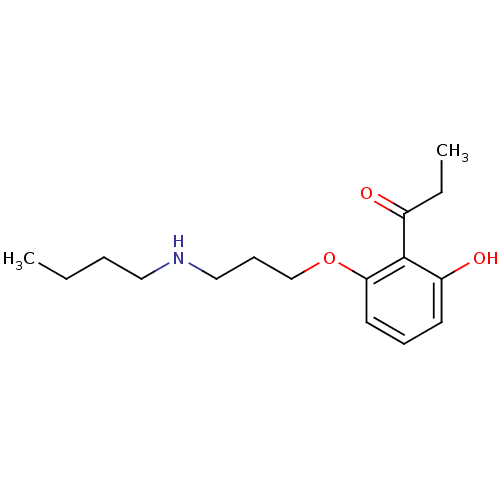
(CHEMBL2206340)Show InChI InChI=1S/C16H25NO3/c1-3-5-10-17-11-7-12-20-15-9-6-8-14(19)16(15)13(18)4-2/h6,8-9,17,19H,3-5,7,10-12H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
Bioorg Med Chem 20: 2172-9 (2012)
Article DOI: 10.1016/j.bmc.2011.12.027
BindingDB Entry DOI: 10.7270/Q2T72JKG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50320880
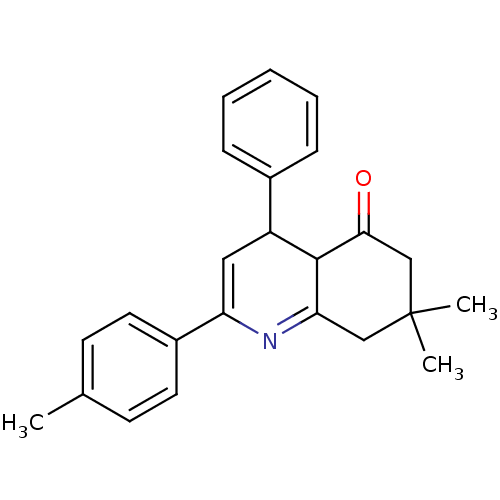
(7,7-Dimethyl-2-phenyl-4-p-tolyl-4,6,7,8-tetrahydro...)Show SMILES Cc1ccc(cc1)C1=CC(C2C(=O)CC(C)(C)CC2=N1)c1ccccc1 |c:20,t:8| Show InChI InChI=1S/C24H25NO/c1-16-9-11-18(12-10-16)20-13-19(17-7-5-4-6-8-17)23-21(25-20)14-24(2,3)15-22(23)26/h4-13,19,23H,14-15H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.96E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as p-nitrophenolate ion production pretreated for 10 mins measured after 30 mins |
Bioorg Med Chem 18: 4138-48 (2010)
Article DOI: 10.1016/j.bmc.2009.11.061
BindingDB Entry DOI: 10.7270/Q2DJ5FSF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50401042
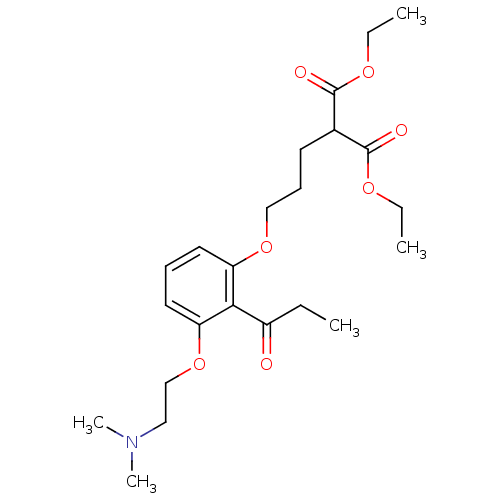
(CHEMBL2206347)Show SMILES CCOC(=O)C(CCCOc1cccc(OCCN(C)C)c1C(=O)CC)C(=O)OCC Show InChI InChI=1S/C23H35NO7/c1-6-18(25)21-19(12-9-13-20(21)31-16-14-24(4)5)30-15-10-11-17(22(26)28-7-2)23(27)29-8-3/h9,12-13,17H,6-8,10-11,14-16H2,1-5H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
Bioorg Med Chem 20: 2172-9 (2012)
Article DOI: 10.1016/j.bmc.2011.12.027
BindingDB Entry DOI: 10.7270/Q2T72JKG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data