

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM50502427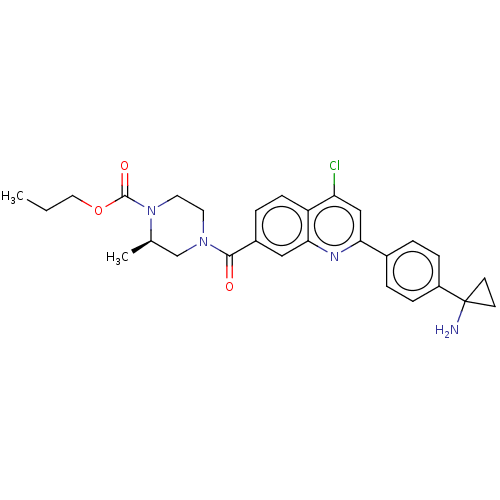 (CHEMBL4542517) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Drug Development Centre Curated by ChEMBL | Assay Description Irreversible inhibition of SMYD3 (unknown origin) using MAP3K2 peptide as substrate pretreated for 5 mins followed by substrate addition and measured... | ACS Med Chem Lett 10: 978-984 (2019) Article DOI: 10.1021/acsmedchemlett.9b00170 BindingDB Entry DOI: 10.7270/Q23B63DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM50502433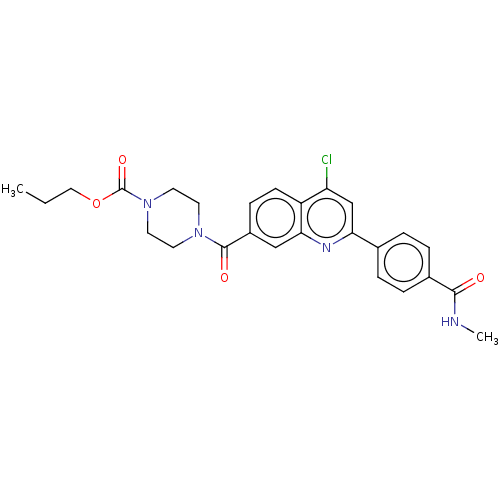 (CHEMBL4575866) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Drug Development Centre Curated by ChEMBL | Assay Description Irreversible inhibition of SMYD3 (unknown origin) using MAP3K2 peptide as substrate pretreated for 5 mins followed by substrate addition and measured... | ACS Med Chem Lett 10: 978-984 (2019) Article DOI: 10.1021/acsmedchemlett.9b00170 BindingDB Entry DOI: 10.7270/Q23B63DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM50502434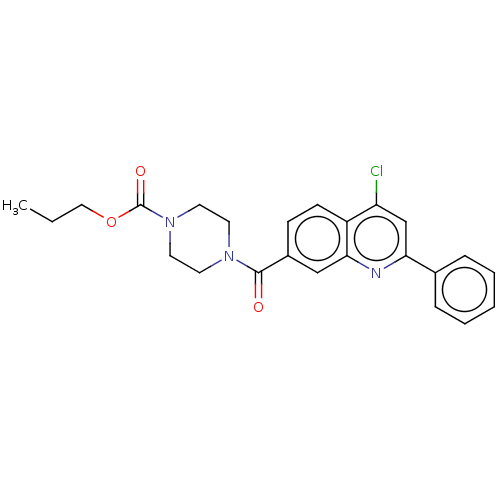 (CHEMBL4551647) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Drug Development Centre Curated by ChEMBL | Assay Description Irreversible inhibition of SMYD3 (unknown origin) using MAP3K2 peptide as substrate pretreated for 5 mins followed by substrate addition and measured... | ACS Med Chem Lett 10: 978-984 (2019) Article DOI: 10.1021/acsmedchemlett.9b00170 BindingDB Entry DOI: 10.7270/Q23B63DW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| DNA gyrase subunit B (Escherichia coli (strain K12)) | BDBM50470399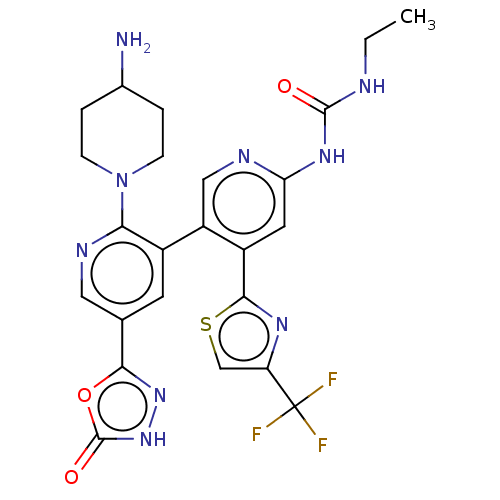 (CHEMBL4286573) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GyrB (1 to 220 residues) expressed in Escherichia coli BL21(DE3) | Eur J Med Chem 157: 610-621 (2018) Article DOI: 10.1016/j.ejmech.2018.08.025 BindingDB Entry DOI: 10.7270/Q28P637N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit B (Escherichia coli (strain K12)) | BDBM50470392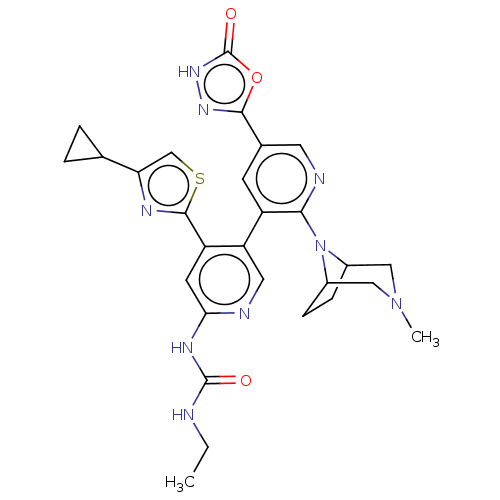 (CHEMBL4283208) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GyrB (1 to 220 residues) expressed in Escherichia coli BL21(DE3) | Eur J Med Chem 157: 610-621 (2018) Article DOI: 10.1016/j.ejmech.2018.08.025 BindingDB Entry DOI: 10.7270/Q28P637N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit B (Escherichia coli (strain K12)) | BDBM50470392 (CHEMBL4283208) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of Escherichia coli ParE | Eur J Med Chem 157: 610-621 (2018) Article DOI: 10.1016/j.ejmech.2018.08.025 BindingDB Entry DOI: 10.7270/Q28P637N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit B (Escherichia coli (strain K12)) | BDBM50470397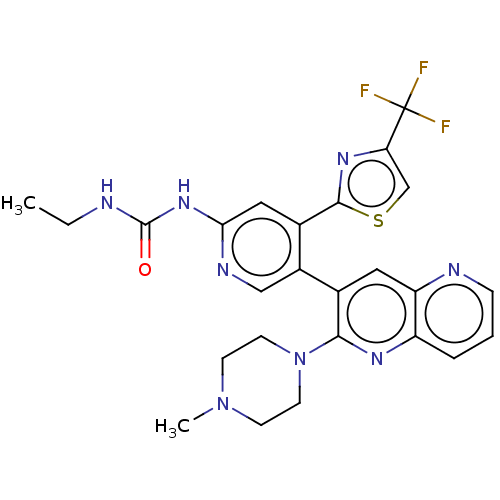 (CHEMBL4294536) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GyrB (1 to 220 residues) expressed in Escherichia coli BL21(DE3) | Eur J Med Chem 157: 610-621 (2018) Article DOI: 10.1016/j.ejmech.2018.08.025 BindingDB Entry DOI: 10.7270/Q28P637N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit B (Escherichia coli (strain K12)) | BDBM50470406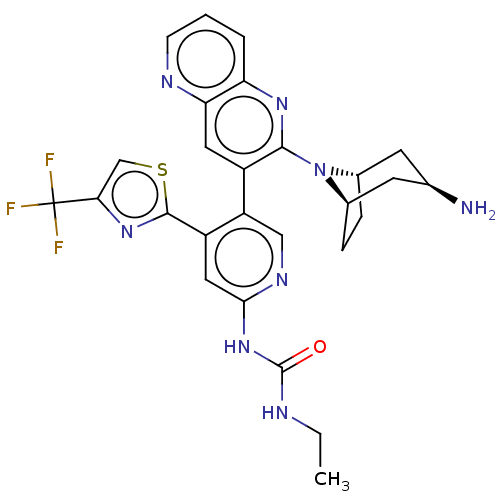 (CHEMBL4295081) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GyrB (1 to 220 residues) expressed in Escherichia coli BL21(DE3) | Eur J Med Chem 157: 610-621 (2018) Article DOI: 10.1016/j.ejmech.2018.08.025 BindingDB Entry DOI: 10.7270/Q28P637N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit B (Escherichia coli (strain K12)) | BDBM50470393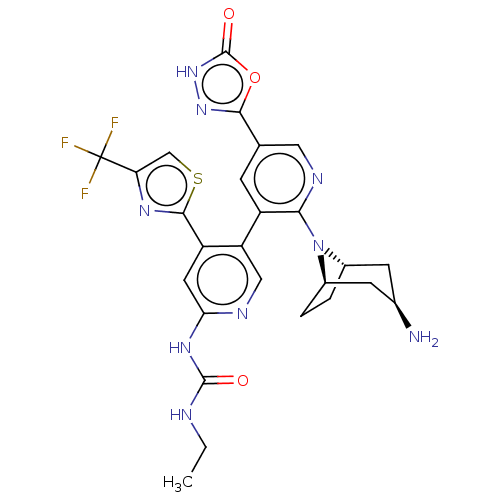 (CHEMBL4291061) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GyrB (1 to 220 residues) expressed in Escherichia coli BL21(DE3) | Eur J Med Chem 157: 610-621 (2018) Article DOI: 10.1016/j.ejmech.2018.08.025 BindingDB Entry DOI: 10.7270/Q28P637N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit B (Escherichia coli (strain K12)) | BDBM50470403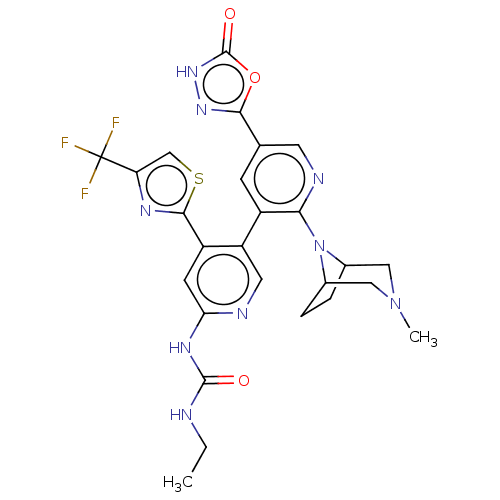 (CHEMBL4294467) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of Escherichia coli ParE | Eur J Med Chem 157: 610-621 (2018) Article DOI: 10.1016/j.ejmech.2018.08.025 BindingDB Entry DOI: 10.7270/Q28P637N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit B (Escherichia coli (strain K12)) | BDBM50470407 (CHEMBL4286625) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GyrB (1 to 220 residues) expressed in Escherichia coli BL21(DE3) | Eur J Med Chem 157: 610-621 (2018) Article DOI: 10.1016/j.ejmech.2018.08.025 BindingDB Entry DOI: 10.7270/Q28P637N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit B (Escherichia coli (strain K12)) | BDBM50470400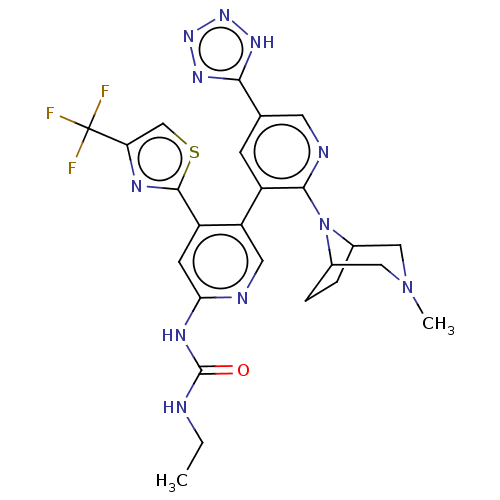 (CHEMBL4289998) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of Escherichia coli ParE | Eur J Med Chem 157: 610-621 (2018) Article DOI: 10.1016/j.ejmech.2018.08.025 BindingDB Entry DOI: 10.7270/Q28P637N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit B (Escherichia coli (strain K12)) | BDBM50470401 (CHEMBL4290062) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GyrB (1 to 220 residues) expressed in Escherichia coli BL21(DE3) | Eur J Med Chem 157: 610-621 (2018) Article DOI: 10.1016/j.ejmech.2018.08.025 BindingDB Entry DOI: 10.7270/Q28P637N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit B (Escherichia coli (strain K12)) | BDBM50470394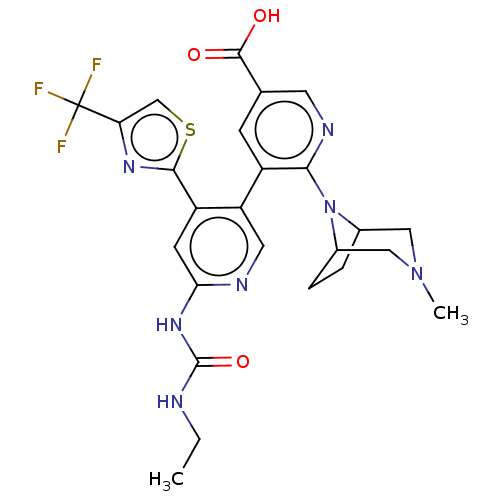 (CHEMBL4281775) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GyrB (1 to 220 residues) expressed in Escherichia coli BL21(DE3) | Eur J Med Chem 157: 610-621 (2018) Article DOI: 10.1016/j.ejmech.2018.08.025 BindingDB Entry DOI: 10.7270/Q28P637N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit B (Escherichia coli (strain K12)) | BDBM50470403 (CHEMBL4294467) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GyrB (1 to 220 residues) expressed in Escherichia coli BL21(DE3) | Eur J Med Chem 157: 610-621 (2018) Article DOI: 10.1016/j.ejmech.2018.08.025 BindingDB Entry DOI: 10.7270/Q28P637N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit B (Escherichia coli (strain K12)) | BDBM50006565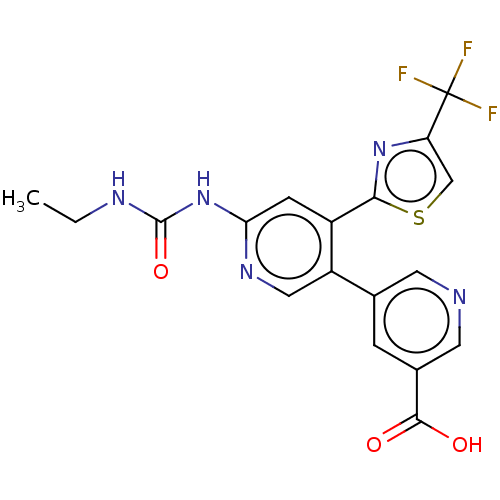 (CHEMBL3235085) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GyrB (1 to 220 residues) expressed in Escherichia coli BL21(DE3) | Eur J Med Chem 157: 610-621 (2018) Article DOI: 10.1016/j.ejmech.2018.08.025 BindingDB Entry DOI: 10.7270/Q28P637N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit B (Escherichia coli (strain K12)) | BDBM50470396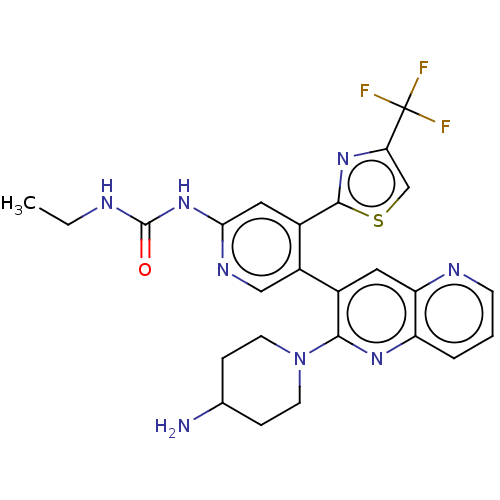 (CHEMBL4293447) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GyrB (1 to 220 residues) expressed in Escherichia coli BL21(DE3) | Eur J Med Chem 157: 610-621 (2018) Article DOI: 10.1016/j.ejmech.2018.08.025 BindingDB Entry DOI: 10.7270/Q28P637N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit B (Escherichia coli (strain K12)) | BDBM50470404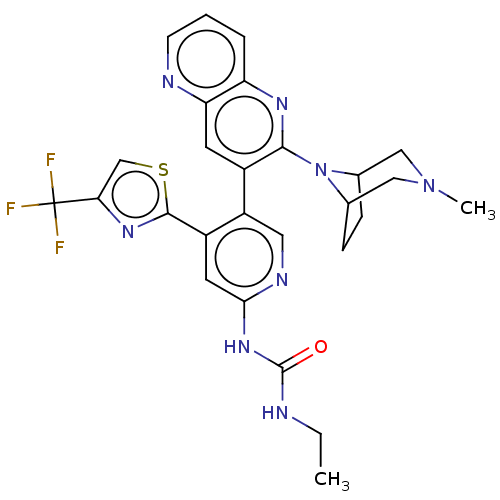 (CHEMBL4279382) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GyrB (1 to 220 residues) expressed in Escherichia coli BL21(DE3) | Eur J Med Chem 157: 610-621 (2018) Article DOI: 10.1016/j.ejmech.2018.08.025 BindingDB Entry DOI: 10.7270/Q28P637N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit B (Escherichia coli (strain K12)) | BDBM50470395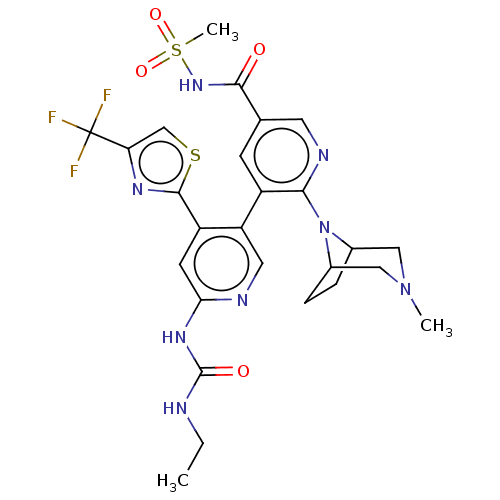 (CHEMBL4279313) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GyrB (1 to 220 residues) expressed in Escherichia coli BL21(DE3) | Eur J Med Chem 157: 610-621 (2018) Article DOI: 10.1016/j.ejmech.2018.08.025 BindingDB Entry DOI: 10.7270/Q28P637N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit B (Escherichia coli (strain K12)) | BDBM50470402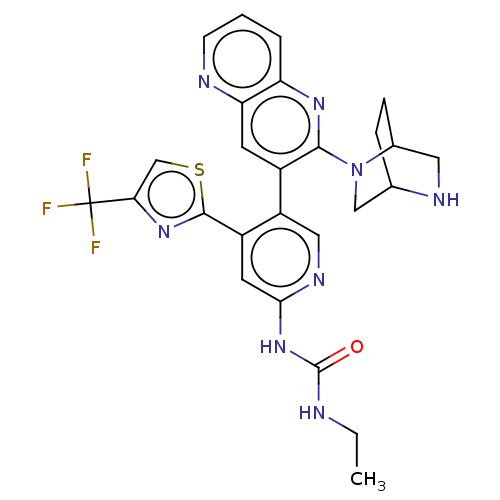 (CHEMBL4278317) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GyrB (1 to 220 residues) expressed in Escherichia coli BL21(DE3) | Eur J Med Chem 157: 610-621 (2018) Article DOI: 10.1016/j.ejmech.2018.08.025 BindingDB Entry DOI: 10.7270/Q28P637N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit B (Escherichia coli (strain K12)) | BDBM50470394 (CHEMBL4281775) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of Escherichia coli ParE | Eur J Med Chem 157: 610-621 (2018) Article DOI: 10.1016/j.ejmech.2018.08.025 BindingDB Entry DOI: 10.7270/Q28P637N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit B (Escherichia coli (strain K12)) | BDBM50470405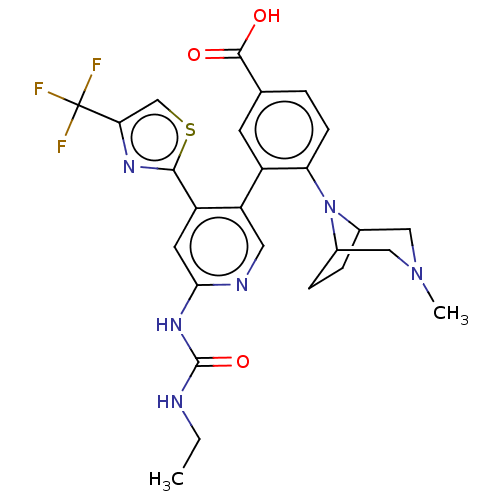 (CHEMBL4289650) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GyrB (1 to 220 residues) expressed in Escherichia coli BL21(DE3) | Eur J Med Chem 157: 610-621 (2018) Article DOI: 10.1016/j.ejmech.2018.08.025 BindingDB Entry DOI: 10.7270/Q28P637N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit B (Escherichia coli (strain K12)) | BDBM50470395 (CHEMBL4279313) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of Escherichia coli ParE | Eur J Med Chem 157: 610-621 (2018) Article DOI: 10.1016/j.ejmech.2018.08.025 BindingDB Entry DOI: 10.7270/Q28P637N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit B (Escherichia coli (strain K12)) | BDBM50470400 (CHEMBL4289998) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GyrB (1 to 220 residues) expressed in Escherichia coli BL21(DE3) | Eur J Med Chem 157: 610-621 (2018) Article DOI: 10.1016/j.ejmech.2018.08.025 BindingDB Entry DOI: 10.7270/Q28P637N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit B (Escherichia coli (strain K12)) | BDBM50470405 (CHEMBL4289650) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of Escherichia coli ParE | Eur J Med Chem 157: 610-621 (2018) Article DOI: 10.1016/j.ejmech.2018.08.025 BindingDB Entry DOI: 10.7270/Q28P637N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit B (Escherichia coli (strain K12)) | BDBM50470393 (CHEMBL4291061) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of Escherichia coli ParE | Eur J Med Chem 157: 610-621 (2018) Article DOI: 10.1016/j.ejmech.2018.08.025 BindingDB Entry DOI: 10.7270/Q28P637N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM50502427 (CHEMBL4542517) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Drug Development Centre Curated by ChEMBL | Assay Description Inhibition of SMYD3 (unknown origin) using MAP3K2 peptide as substrate pretreated for 30 mins followed by substrate addition and measured after 30 mi... | ACS Med Chem Lett 10: 978-984 (2019) Article DOI: 10.1021/acsmedchemlett.9b00170 BindingDB Entry DOI: 10.7270/Q23B63DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit B (Escherichia coli (strain K12)) | BDBM50470404 (CHEMBL4279382) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of Escherichia coli ParE | Eur J Med Chem 157: 610-621 (2018) Article DOI: 10.1016/j.ejmech.2018.08.025 BindingDB Entry DOI: 10.7270/Q28P637N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit B (Escherichia coli (strain K12)) | BDBM50470398 (CHEMBL4286232) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GyrB (1 to 220 residues) expressed in Escherichia coli BL21(DE3) | Eur J Med Chem 157: 610-621 (2018) Article DOI: 10.1016/j.ejmech.2018.08.025 BindingDB Entry DOI: 10.7270/Q28P637N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit B (Escherichia coli (strain K12)) | BDBM50470402 (CHEMBL4278317) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of Escherichia coli ParE | Eur J Med Chem 157: 610-621 (2018) Article DOI: 10.1016/j.ejmech.2018.08.025 BindingDB Entry DOI: 10.7270/Q28P637N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit B (Escherichia coli (strain K12)) | BDBM50470399 (CHEMBL4286573) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of Escherichia coli ParE | Eur J Med Chem 157: 610-621 (2018) Article DOI: 10.1016/j.ejmech.2018.08.025 BindingDB Entry DOI: 10.7270/Q28P637N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit B (Escherichia coli (strain K12)) | BDBM50470406 (CHEMBL4295081) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of Escherichia coli ParE | Eur J Med Chem 157: 610-621 (2018) Article DOI: 10.1016/j.ejmech.2018.08.025 BindingDB Entry DOI: 10.7270/Q28P637N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit B (Escherichia coli (strain K12)) | BDBM50470398 (CHEMBL4286232) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of Escherichia coli ParE | Eur J Med Chem 157: 610-621 (2018) Article DOI: 10.1016/j.ejmech.2018.08.025 BindingDB Entry DOI: 10.7270/Q28P637N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit B (Escherichia coli (strain K12)) | BDBM50470396 (CHEMBL4293447) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of Escherichia coli ParE | Eur J Med Chem 157: 610-621 (2018) Article DOI: 10.1016/j.ejmech.2018.08.025 BindingDB Entry DOI: 10.7270/Q28P637N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM50502413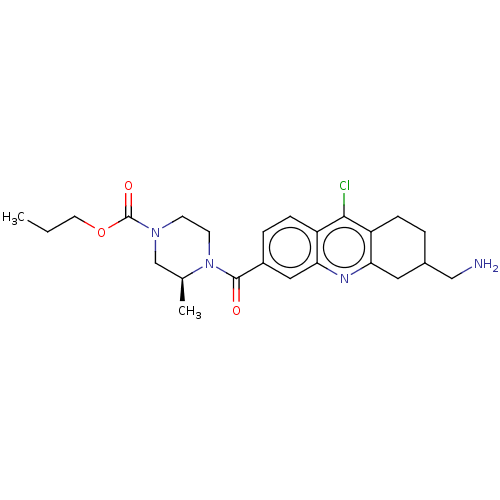 (CHEMBL4521324) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Drug Development Centre Curated by ChEMBL | Assay Description Inhibition of SMYD3 (unknown origin) using MAP3K2 peptide as substrate pretreated for 30 mins followed by substrate addition and measured after 30 mi... | ACS Med Chem Lett 10: 978-984 (2019) Article DOI: 10.1021/acsmedchemlett.9b00170 BindingDB Entry DOI: 10.7270/Q23B63DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM50502429 (CHEMBL4475972) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Drug Development Centre Curated by ChEMBL | Assay Description Inhibition of SMYD3 (unknown origin) using MAP3K2 peptide as substrate pretreated for 30 mins followed by substrate addition and measured after 30 mi... | ACS Med Chem Lett 10: 978-984 (2019) Article DOI: 10.1021/acsmedchemlett.9b00170 BindingDB Entry DOI: 10.7270/Q23B63DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM50502416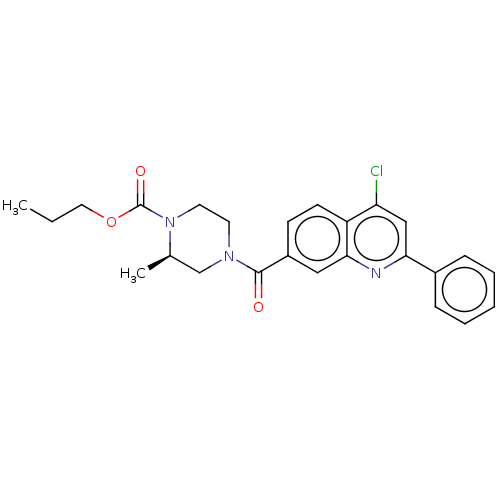 (CHEMBL4472455) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Drug Development Centre Curated by ChEMBL | Assay Description Inhibition of SMYD3 (unknown origin) using MAP3K2 peptide as substrate pretreated for 30 mins followed by substrate addition and measured after 30 mi... | ACS Med Chem Lett 10: 978-984 (2019) Article DOI: 10.1021/acsmedchemlett.9b00170 BindingDB Entry DOI: 10.7270/Q23B63DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit B (Escherichia coli (strain K12)) | BDBM50470401 (CHEMBL4290062) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of Escherichia coli ParE | Eur J Med Chem 157: 610-621 (2018) Article DOI: 10.1016/j.ejmech.2018.08.025 BindingDB Entry DOI: 10.7270/Q28P637N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM50502436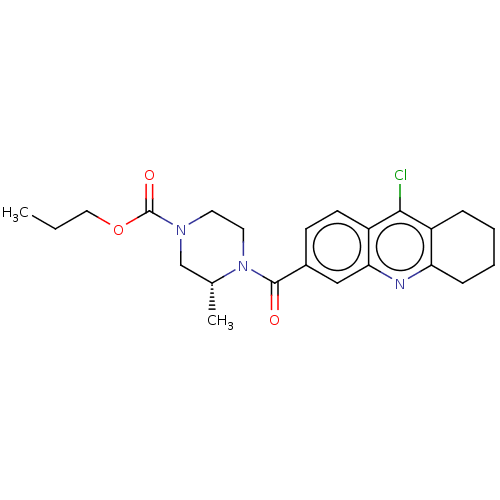 (CHEMBL4450314) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Drug Development Centre Curated by ChEMBL | Assay Description Inhibition of SMYD3 (unknown origin) using MAP3K2 peptide as substrate pretreated for 30 mins followed by substrate addition and measured after 30 mi... | ACS Med Chem Lett 10: 978-984 (2019) Article DOI: 10.1021/acsmedchemlett.9b00170 BindingDB Entry DOI: 10.7270/Q23B63DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM50502433 (CHEMBL4575866) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Drug Development Centre Curated by ChEMBL | Assay Description Inhibition of SMYD3 (unknown origin) using MAP3K2 peptide as substrate pretreated for 30 mins followed by substrate addition and measured after 30 mi... | ACS Med Chem Lett 10: 978-984 (2019) Article DOI: 10.1021/acsmedchemlett.9b00170 BindingDB Entry DOI: 10.7270/Q23B63DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM50502414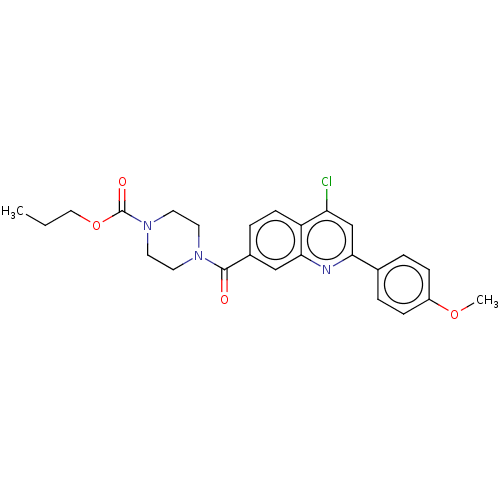 (CHEMBL4440399) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Drug Development Centre Curated by ChEMBL | Assay Description Inhibition of SMYD3 (unknown origin) using MAP3K2 peptide as substrate pretreated for 30 mins followed by substrate addition and measured after 30 mi... | ACS Med Chem Lett 10: 978-984 (2019) Article DOI: 10.1021/acsmedchemlett.9b00170 BindingDB Entry DOI: 10.7270/Q23B63DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM50502434 (CHEMBL4551647) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Drug Development Centre Curated by ChEMBL | Assay Description Inhibition of SMYD3 (unknown origin) using MAP3K2 peptide as substrate pretreated for 30 mins followed by substrate addition and measured after 30 mi... | ACS Med Chem Lett 10: 978-984 (2019) Article DOI: 10.1021/acsmedchemlett.9b00170 BindingDB Entry DOI: 10.7270/Q23B63DW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM50502426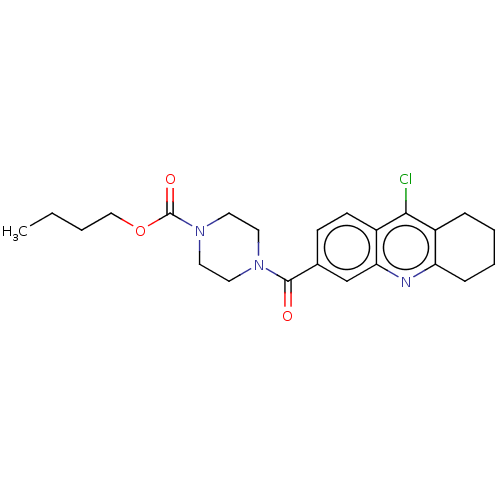 (CHEMBL4448008) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Drug Development Centre Curated by ChEMBL | Assay Description Inhibition of SMYD3 (unknown origin) using MAP3K2 peptide as substrate pretreated for 30 mins followed by substrate addition and measured after 30 mi... | ACS Med Chem Lett 10: 978-984 (2019) Article DOI: 10.1021/acsmedchemlett.9b00170 BindingDB Entry DOI: 10.7270/Q23B63DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM50502438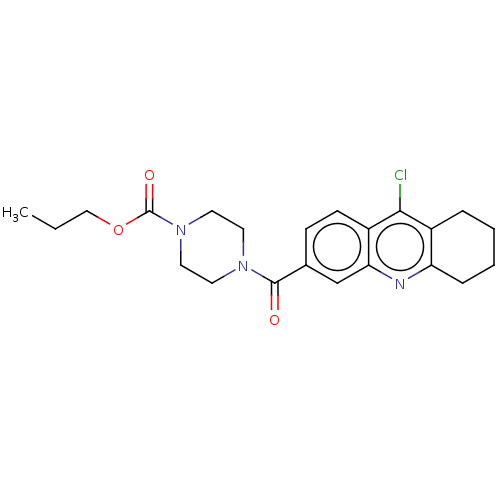 (CHEMBL4530816) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Drug Development Centre Curated by ChEMBL | Assay Description Inhibition of SMYD3 (unknown origin) using MAP3K2 peptide as substrate pretreated for 30 mins followed by substrate addition and measured after 30 mi... | ACS Med Chem Lett 10: 978-984 (2019) Article DOI: 10.1021/acsmedchemlett.9b00170 BindingDB Entry DOI: 10.7270/Q23B63DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM50502415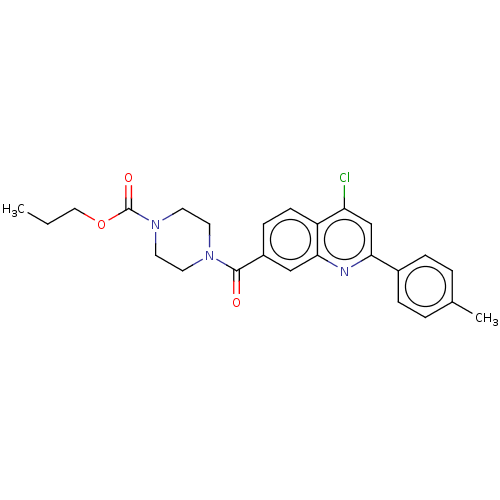 (CHEMBL4579512) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Drug Development Centre Curated by ChEMBL | Assay Description Inhibition of SMYD3 (unknown origin) using MAP3K2 peptide as substrate pretreated for 30 mins followed by substrate addition and measured after 30 mi... | ACS Med Chem Lett 10: 978-984 (2019) Article DOI: 10.1021/acsmedchemlett.9b00170 BindingDB Entry DOI: 10.7270/Q23B63DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM50502428 (CHEMBL4584168) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Drug Development Centre Curated by ChEMBL | Assay Description Inhibition of SMYD3 (unknown origin) using MAP3K2 peptide as substrate pretreated for 30 mins followed by substrate addition and measured after 30 mi... | ACS Med Chem Lett 10: 978-984 (2019) Article DOI: 10.1021/acsmedchemlett.9b00170 BindingDB Entry DOI: 10.7270/Q23B63DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit B (Escherichia coli (strain K12)) | BDBM50470397 (CHEMBL4294536) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of Escherichia coli ParE | Eur J Med Chem 157: 610-621 (2018) Article DOI: 10.1016/j.ejmech.2018.08.025 BindingDB Entry DOI: 10.7270/Q28P637N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit B (Escherichia coli (strain K12)) | BDBM50006565 (CHEMBL3235085) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of Escherichia coli ParE | Eur J Med Chem 157: 610-621 (2018) Article DOI: 10.1016/j.ejmech.2018.08.025 BindingDB Entry DOI: 10.7270/Q28P637N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM50502418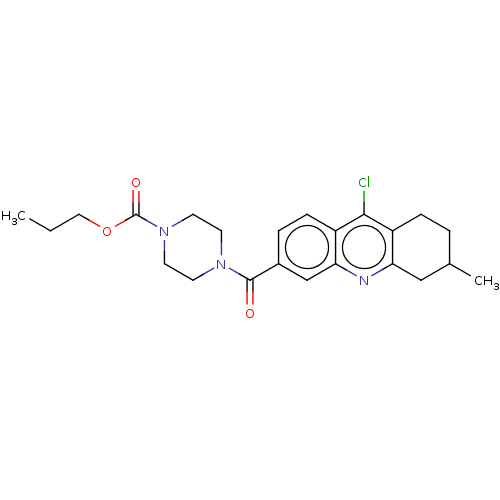 (CHEMBL4532072) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Drug Development Centre Curated by ChEMBL | Assay Description Inhibition of SMYD3 (unknown origin) using MAP3K2 peptide as substrate pretreated for 30 mins followed by substrate addition and measured after 30 mi... | ACS Med Chem Lett 10: 978-984 (2019) Article DOI: 10.1021/acsmedchemlett.9b00170 BindingDB Entry DOI: 10.7270/Q23B63DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM50502419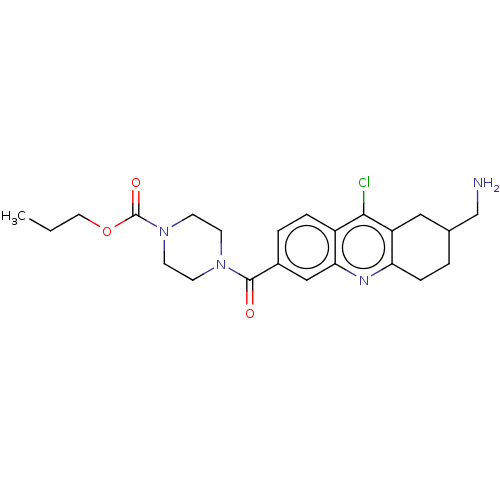 (CHEMBL4462956) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Drug Development Centre Curated by ChEMBL | Assay Description Inhibition of SMYD3 (unknown origin) using MAP3K2 peptide as substrate pretreated for 30 mins followed by substrate addition and measured after 30 mi... | ACS Med Chem Lett 10: 978-984 (2019) Article DOI: 10.1021/acsmedchemlett.9b00170 BindingDB Entry DOI: 10.7270/Q23B63DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 64 total ) | Next | Last >> |