 Found 40 hits with Last Name = 'cole' and Initial = 'st'
Found 40 hits with Last Name = 'cole' and Initial = 'st' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Enoyl-[acyl-carrier-protein] reductase [NADH]
(Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50443008

(Pyridomycin)Show SMILES CC\C(C)=C1/OC(=O)[C@H](C)[C@H](O)[C@H](Cc2cccnc2)NC(=O)[C@@H](NC(=O)c2ncccc2O)[C@@H](C)OC1=O |r| Show InChI InChI=1S/C27H32N4O8/c1-5-14(2)23-27(37)38-16(4)20(31-25(35)21-19(32)9-7-11-29-21)24(34)30-18(12-17-8-6-10-28-13-17)22(33)15(3)26(36)39-23/h6-11,13,15-16,18,20,22,32-33H,5,12H2,1-4H3,(H,30,34)(H,31,35)/b23-14-/t15-,16-,18+,20+,22+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Federal Institute of Technology (ETH) Zurich
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis InhA S94A mutant assessed as NADH oxidation using 2-trans-dodecenoyl-coA as substrate measured every min for... |
ACS Med Chem Lett 4: 264-8 (2013)
Article DOI: 10.1021/ml300385q
BindingDB Entry DOI: 10.7270/Q27W6G4M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Enoyl-[acyl-carrier-protein] reductase [NADH]
(Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50489465
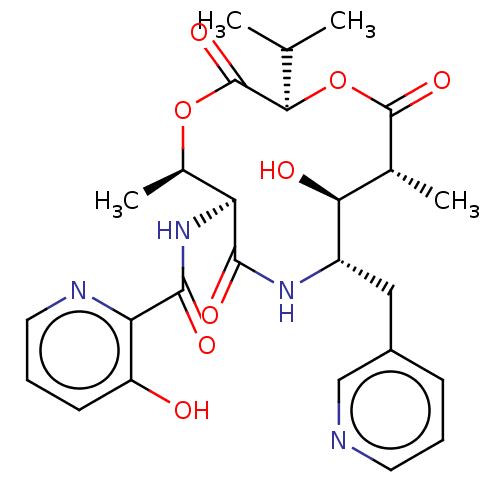
(CHEMBL2323583)Show SMILES CC(C)[C@H]1OC(=O)[C@H](C)[C@H](O)[C@H](Cc2cccnc2)NC(=O)[C@@H](NC(=O)c2ncccc2O)[C@@H](C)OC1=O |r| Show InChI InChI=1S/C26H32N4O8/c1-13(2)22-26(36)37-15(4)19(30-24(34)20-18(31)8-6-10-28-20)23(33)29-17(11-16-7-5-9-27-12-16)21(32)14(3)25(35)38-22/h5-10,12-15,17,19,21-22,31-32H,11H2,1-4H3,(H,29,33)(H,30,34)/t14-,15-,17+,19+,21+,22-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 7.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Federal Institute of Technology (ETH) Zurich
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis InhA S94A mutant assessed as NADH oxidation using 2-trans-dodecenoyl-coA as substrate measured every min for... |
ACS Med Chem Lett 4: 264-8 (2013)
Article DOI: 10.1021/ml300385q
BindingDB Entry DOI: 10.7270/Q27W6G4M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50499991

(CHEMBL3742347)Show SMILES [O-][N+](=O)c1cc(cc2[n+]([O-])c(sc12)C(=O)N1CCCCC1)C(F)(F)F Show InChI InChI=1S/C14H12F3N3O4S/c15-14(16,17)8-6-9-11(10(7-8)20(23)24)25-13(19(9)22)12(21)18-4-2-1-3-5-18/h6-7H,1-5H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2D6 by fluorescence assay |
Bioorg Med Chem 23: 7694-710 (2015)
Article DOI: 10.1016/j.bmc.2015.11.017
BindingDB Entry DOI: 10.7270/Q2PR800F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50499992

(CHEMBL3739863)Show SMILES [O-][N+](=O)c1cc(cc2nc(sc12)C1=N[C@@H](CO1)C(=O)N1CCCC1)C(F)(F)F |r,t:14| Show InChI InChI=1S/C16H13F3N4O4S/c17-16(18,19)8-5-9-12(11(6-8)23(25)26)28-14(21-9)13-20-10(7-27-13)15(24)22-3-1-2-4-22/h5-6,10H,1-4,7H2/t10-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2D6 by fluorescence assay |
Bioorg Med Chem 23: 7694-710 (2015)
Article DOI: 10.1016/j.bmc.2015.11.017
BindingDB Entry DOI: 10.7270/Q2PR800F |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADH]
(Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50443008

(Pyridomycin)Show SMILES CC\C(C)=C1/OC(=O)[C@H](C)[C@H](O)[C@H](Cc2cccnc2)NC(=O)[C@@H](NC(=O)c2ncccc2O)[C@@H](C)OC1=O |r| Show InChI InChI=1S/C27H32N4O8/c1-5-14(2)23-27(37)38-16(4)20(31-25(35)21-19(32)9-7-11-29-21)24(34)30-18(12-17-8-6-10-28-13-17)22(33)15(3)26(36)39-23/h6-11,13,15-16,18,20,22,32-33H,5,12H2,1-4H3,(H,30,34)(H,31,35)/b23-14-/t15-,16-,18+,20+,22+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 3.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ETH Zurich
Curated by ChEMBL
| Assay Description
Inhibition of bacterial InhA harboring S94A mutant using 2-trans-octenoyl-CoA or 2-trans-dodecenoyl-CoA as substrate |
J Med Chem 63: 1105-1131 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01457
BindingDB Entry DOI: 10.7270/Q2BR8WJ8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50499991

(CHEMBL3742347)Show SMILES [O-][N+](=O)c1cc(cc2[n+]([O-])c(sc12)C(=O)N1CCCCC1)C(F)(F)F Show InChI InChI=1S/C14H12F3N3O4S/c15-14(16,17)8-6-9-11(10(7-8)20(23)24)25-13(19(9)22)12(21)18-4-2-1-3-5-18/h6-7H,1-5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4 by fluorescence assay |
Bioorg Med Chem 23: 7694-710 (2015)
Article DOI: 10.1016/j.bmc.2015.11.017
BindingDB Entry DOI: 10.7270/Q2PR800F |
More data for this
Ligand-Target Pair | |
Geranylgeranyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50388398
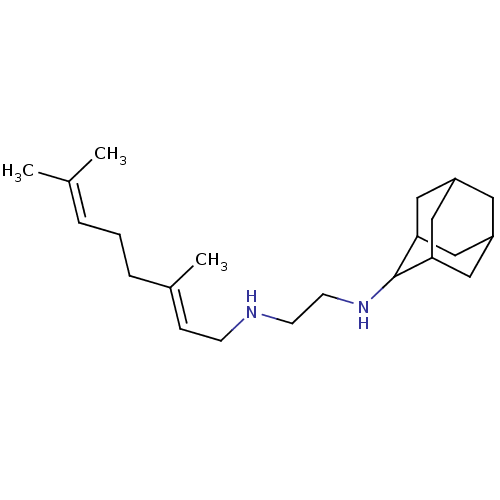
(CHEMBL561057 | SQ-109)Show SMILES CC(C)=CCC\C(C)=C\CNCCNC1C2CC3CC(C2)CC1C3 |TLB:18:19:16.17.23:14,23:22:20:16.17.18,13:14:20:16.17.18,13:14:16.17.23:20.19.21,THB:18:17:14:20.19.21,23:17:20:22.14.21,21:19:16:23.22.14,21:22:16:20.18.19,13:14:16:20.18.19,(-5.14,2.27,;-5.13,1.44,;-5.84,1.02,;-4.41,1.04,;-3.7,1.46,;-2.98,1.06,;-2.28,1.48,;-2.29,2.3,;-1.56,1.08,;-.85,1.5,;-.13,1.09,;.58,1.51,;1.3,1.11,;2.04,1.48,;2.72,1.02,;2.74,.22,;2.11,-.47,;2.91,-.24,;3.67,-.52,;4.2,.17,;3.46,-.03,;4.19,.99,;3.44,1.28,;2.89,.61,)| Show InChI InChI=1S/C22H38N2/c1-16(2)5-4-6-17(3)7-8-23-9-10-24-22-20-12-18-11-19(14-20)15-21(22)13-18/h5,7,18-24H,4,6,8-15H2,1-3H3/b17-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Inhibition of human geranylgeranyl diphosphate synthase |
J Med Chem 57: 3126-39 (2014)
Article DOI: 10.1021/jm500131s
BindingDB Entry DOI: 10.7270/Q27P90ZN |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50499993

(CHEMBL3742122)Show SMILES COCCN1CCN(CC1)C(=O)c1nc2cc(cc([N+]([O-])=O)c2s1)C(F)(F)F Show InChI InChI=1S/C16H17F3N4O4S/c1-27-7-6-21-2-4-22(5-3-21)15(24)14-20-11-8-10(16(17,18)19)9-12(23(25)26)13(11)28-14/h8-9H,2-7H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2D6 by fluorescence assay |
Bioorg Med Chem 23: 7694-710 (2015)
Article DOI: 10.1016/j.bmc.2015.11.017
BindingDB Entry DOI: 10.7270/Q2PR800F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50499994
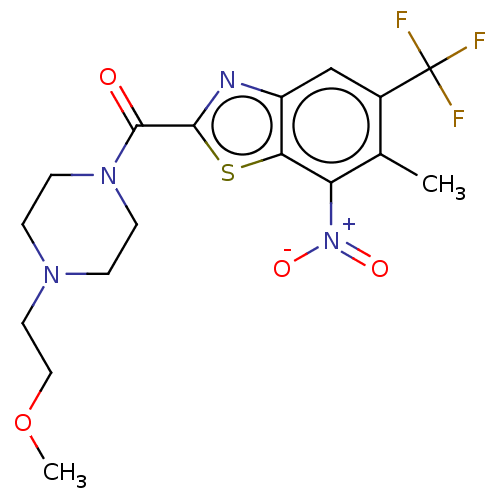
(CHEMBL3741545)Show SMILES COCCN1CCN(CC1)C(=O)c1nc2cc(c(C)c([N+]([O-])=O)c2s1)C(F)(F)F Show InChI InChI=1S/C17H19F3N4O4S/c1-10-11(17(18,19)20)9-12-14(13(10)24(26)27)29-15(21-12)16(25)23-5-3-22(4-6-23)7-8-28-2/h9H,3-8H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 9.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP1A2 by fluorescence assay |
Bioorg Med Chem 23: 7694-710 (2015)
Article DOI: 10.1016/j.bmc.2015.11.017
BindingDB Entry DOI: 10.7270/Q2PR800F |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADH]
(Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50520246
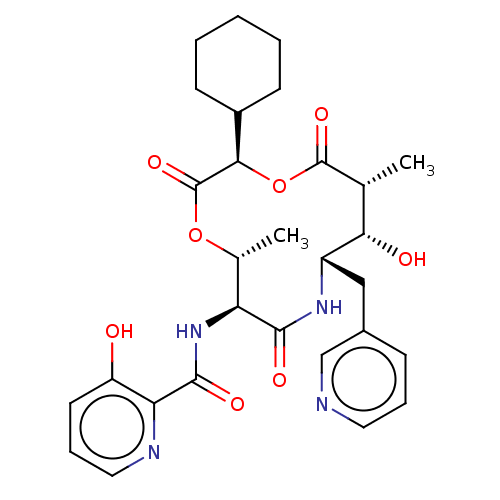
(CHEMBL4564729)Show SMILES C[C@H]1OC(=O)[C@H](OC(=O)[C@H](C)[C@H](O)[C@H](Cc2cccnc2)NC(=O)[C@H]1NC(=O)c1ncccc1O)C1CCCCC1 |r| Show InChI InChI=1S/C29H36N4O8/c1-16-24(35)20(14-18-8-6-12-30-15-18)32-26(36)22(33-27(37)23-21(34)11-7-13-31-23)17(2)40-29(39)25(41-28(16)38)19-9-4-3-5-10-19/h6-8,11-13,15-17,19-20,22,24-25,34-35H,3-5,9-10,14H2,1-2H3,(H,32,36)(H,33,37)/t16-,17-,20+,22+,24+,25-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ETH Zurich
Curated by ChEMBL
| Assay Description
Inhibition of bacterial InhA harboring S94A mutant using 2-trans-octenoyl-CoA or 2-trans-dodecenoyl-CoA as substrate |
J Med Chem 63: 1105-1131 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01457
BindingDB Entry DOI: 10.7270/Q2BR8WJ8 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50499992

(CHEMBL3739863)Show SMILES [O-][N+](=O)c1cc(cc2nc(sc12)C1=N[C@@H](CO1)C(=O)N1CCCC1)C(F)(F)F |r,t:14| Show InChI InChI=1S/C16H13F3N4O4S/c17-16(18,19)8-5-9-12(11(6-8)23(25)26)28-14(21-9)13-20-10(7-27-13)15(24)22-3-1-2-4-22/h5-6,10H,1-4,7H2/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C9 by fluorescence assay |
Bioorg Med Chem 23: 7694-710 (2015)
Article DOI: 10.1016/j.bmc.2015.11.017
BindingDB Entry DOI: 10.7270/Q2PR800F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50499991

(CHEMBL3742347)Show SMILES [O-][N+](=O)c1cc(cc2[n+]([O-])c(sc12)C(=O)N1CCCCC1)C(F)(F)F Show InChI InChI=1S/C14H12F3N3O4S/c15-14(16,17)8-6-9-11(10(7-8)20(23)24)25-13(19(9)22)12(21)18-4-2-1-3-5-18/h6-7H,1-5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP1A2 by fluorescence assay |
Bioorg Med Chem 23: 7694-710 (2015)
Article DOI: 10.1016/j.bmc.2015.11.017
BindingDB Entry DOI: 10.7270/Q2PR800F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50499991

(CHEMBL3742347)Show SMILES [O-][N+](=O)c1cc(cc2[n+]([O-])c(sc12)C(=O)N1CCCCC1)C(F)(F)F Show InChI InChI=1S/C14H12F3N3O4S/c15-14(16,17)8-6-9-11(10(7-8)20(23)24)25-13(19(9)22)12(21)18-4-2-1-3-5-18/h6-7H,1-5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.77E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C19 by fluorescence assay |
Bioorg Med Chem 23: 7694-710 (2015)
Article DOI: 10.1016/j.bmc.2015.11.017
BindingDB Entry DOI: 10.7270/Q2PR800F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50499993

(CHEMBL3742122)Show SMILES COCCN1CCN(CC1)C(=O)c1nc2cc(cc([N+]([O-])=O)c2s1)C(F)(F)F Show InChI InChI=1S/C16H17F3N4O4S/c1-27-7-6-21-2-4-22(5-3-21)15(24)14-20-11-8-10(16(17,18)19)9-12(23(25)26)13(11)28-14/h8-9H,2-7H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.79E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP1A2 by fluorescence assay |
Bioorg Med Chem 23: 7694-710 (2015)
Article DOI: 10.1016/j.bmc.2015.11.017
BindingDB Entry DOI: 10.7270/Q2PR800F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50499992

(CHEMBL3739863)Show SMILES [O-][N+](=O)c1cc(cc2nc(sc12)C1=N[C@@H](CO1)C(=O)N1CCCC1)C(F)(F)F |r,t:14| Show InChI InChI=1S/C16H13F3N4O4S/c17-16(18,19)8-5-9-12(11(6-8)23(25)26)28-14(21-9)13-20-10(7-27-13)15(24)22-3-1-2-4-22/h5-6,10H,1-4,7H2/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.86E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4 by fluorescence assay |
Bioorg Med Chem 23: 7694-710 (2015)
Article DOI: 10.1016/j.bmc.2015.11.017
BindingDB Entry DOI: 10.7270/Q2PR800F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50499993

(CHEMBL3742122)Show SMILES COCCN1CCN(CC1)C(=O)c1nc2cc(cc([N+]([O-])=O)c2s1)C(F)(F)F Show InChI InChI=1S/C16H17F3N4O4S/c1-27-7-6-21-2-4-22(5-3-21)15(24)14-20-11-8-10(16(17,18)19)9-12(23(25)26)13(11)28-14/h8-9H,2-7H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4 by fluorescence assay |
Bioorg Med Chem 23: 7694-710 (2015)
Article DOI: 10.1016/j.bmc.2015.11.017
BindingDB Entry DOI: 10.7270/Q2PR800F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50499995
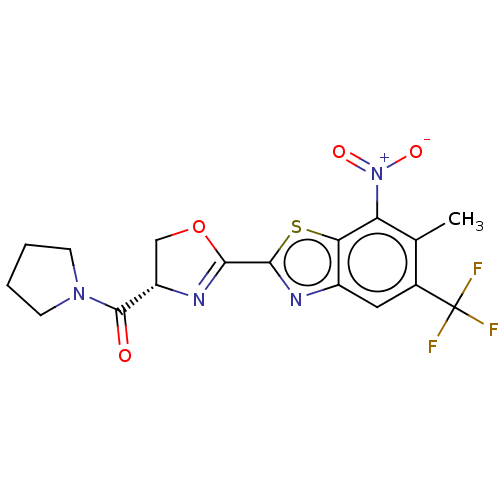
(CHEMBL3739831)Show SMILES Cc1c(cc2nc(sc2c1[N+]([O-])=O)C1=N[C@@H](CO1)C(=O)N1CCCC1)C(F)(F)F |r,t:15| Show InChI InChI=1S/C17H15F3N4O4S/c1-8-9(17(18,19)20)6-10-13(12(8)24(26)27)29-15(22-10)14-21-11(7-28-14)16(25)23-4-2-3-5-23/h6,11H,2-5,7H2,1H3/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C9 by fluorescence assay |
Bioorg Med Chem 23: 7694-710 (2015)
Article DOI: 10.1016/j.bmc.2015.11.017
BindingDB Entry DOI: 10.7270/Q2PR800F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50499992

(CHEMBL3739863)Show SMILES [O-][N+](=O)c1cc(cc2nc(sc12)C1=N[C@@H](CO1)C(=O)N1CCCC1)C(F)(F)F |r,t:14| Show InChI InChI=1S/C16H13F3N4O4S/c17-16(18,19)8-5-9-12(11(6-8)23(25)26)28-14(21-9)13-20-10(7-27-13)15(24)22-3-1-2-4-22/h5-6,10H,1-4,7H2/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C19 by fluorescence assay |
Bioorg Med Chem 23: 7694-710 (2015)
Article DOI: 10.1016/j.bmc.2015.11.017
BindingDB Entry DOI: 10.7270/Q2PR800F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50499991

(CHEMBL3742347)Show SMILES [O-][N+](=O)c1cc(cc2[n+]([O-])c(sc12)C(=O)N1CCCCC1)C(F)(F)F Show InChI InChI=1S/C14H12F3N3O4S/c15-14(16,17)8-6-9-11(10(7-8)20(23)24)25-13(19(9)22)12(21)18-4-2-1-3-5-18/h6-7H,1-5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.66E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C9 by fluorescence assay |
Bioorg Med Chem 23: 7694-710 (2015)
Article DOI: 10.1016/j.bmc.2015.11.017
BindingDB Entry DOI: 10.7270/Q2PR800F |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADH]
(Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50520247
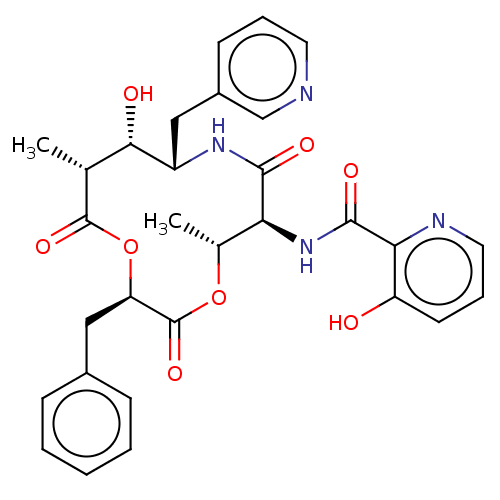
(CHEMBL4565976)Show SMILES C[C@H]1OC(=O)[C@@H](Cc2ccccc2)OC(=O)[C@H](C)[C@H](O)[C@H](Cc2cccnc2)NC(=O)[C@H]1NC(=O)c1ncccc1O |r| Show InChI InChI=1S/C30H32N4O8/c1-17-26(36)21(14-20-10-6-12-31-16-20)33-27(37)24(34-28(38)25-22(35)11-7-13-32-25)18(2)41-30(40)23(42-29(17)39)15-19-8-4-3-5-9-19/h3-13,16-18,21,23-24,26,35-36H,14-15H2,1-2H3,(H,33,37)(H,34,38)/t17-,18-,21+,23-,24+,26+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.91E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ETH Zurich
Curated by ChEMBL
| Assay Description
Inhibition of bacterial InhA harboring S94A mutant using 2-trans-octenoyl-CoA or 2-trans-dodecenoyl-CoA as substrate |
J Med Chem 63: 1105-1131 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01457
BindingDB Entry DOI: 10.7270/Q2BR8WJ8 |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADH]
(Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50520249

(CHEMBL4548115)Show SMILES [H][C@@]1(OC(=O)[C@H](C)[C@H](O)[C@H](Cc2cccnc2)NC(=O)[C@@H](NC(=O)c2ncccc2O)[C@@H](C)OC1=O)[C@H](C)CC |r| Show InChI InChI=1S/C27H34N4O8/c1-5-14(2)23-27(37)38-16(4)20(31-25(35)21-19(32)9-7-11-29-21)24(34)30-18(12-17-8-6-10-28-13-17)22(33)15(3)26(36)39-23/h6-11,13-16,18,20,22-23,32-33H,5,12H2,1-4H3,(H,30,34)(H,31,35)/t14-,15-,16-,18+,20+,22+,23-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ETH Zurich
Curated by ChEMBL
| Assay Description
Inhibition of bacterial InhA harboring S94A mutant using 2-trans-octenoyl-CoA or 2-trans-dodecenoyl-CoA as substrate |
J Med Chem 63: 1105-1131 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01457
BindingDB Entry DOI: 10.7270/Q2BR8WJ8 |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADH]
(Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50489465

(CHEMBL2323583)Show SMILES CC(C)[C@H]1OC(=O)[C@H](C)[C@H](O)[C@H](Cc2cccnc2)NC(=O)[C@@H](NC(=O)c2ncccc2O)[C@@H](C)OC1=O |r| Show InChI InChI=1S/C26H32N4O8/c1-13(2)22-26(36)37-15(4)19(30-24(34)20-18(31)8-6-10-28-20)23(33)29-17(11-16-7-5-9-27-12-16)21(32)14(3)25(35)38-22/h5-10,12-15,17,19,21-22,31-32H,11H2,1-4H3,(H,29,33)(H,30,34)/t14-,15-,17+,19+,21+,22-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 3.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ETH Zurich
Curated by ChEMBL
| Assay Description
Inhibition of bacterial InhA harboring S94A mutant using 2-trans-octenoyl-CoA or 2-trans-dodecenoyl-CoA as substrate |
J Med Chem 63: 1105-1131 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01457
BindingDB Entry DOI: 10.7270/Q2BR8WJ8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50499992

(CHEMBL3739863)Show SMILES [O-][N+](=O)c1cc(cc2nc(sc12)C1=N[C@@H](CO1)C(=O)N1CCCC1)C(F)(F)F |r,t:14| Show InChI InChI=1S/C16H13F3N4O4S/c17-16(18,19)8-5-9-12(11(6-8)23(25)26)28-14(21-9)13-20-10(7-27-13)15(24)22-3-1-2-4-22/h5-6,10H,1-4,7H2/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP1A2 by fluorescence assay |
Bioorg Med Chem 23: 7694-710 (2015)
Article DOI: 10.1016/j.bmc.2015.11.017
BindingDB Entry DOI: 10.7270/Q2PR800F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50499993

(CHEMBL3742122)Show SMILES COCCN1CCN(CC1)C(=O)c1nc2cc(cc([N+]([O-])=O)c2s1)C(F)(F)F Show InChI InChI=1S/C16H17F3N4O4S/c1-27-7-6-21-2-4-22(5-3-21)15(24)14-20-11-8-10(16(17,18)19)9-12(23(25)26)13(11)28-14/h8-9H,2-7H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.59E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C19 by fluorescence assay |
Bioorg Med Chem 23: 7694-710 (2015)
Article DOI: 10.1016/j.bmc.2015.11.017
BindingDB Entry DOI: 10.7270/Q2PR800F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50499994

(CHEMBL3741545)Show SMILES COCCN1CCN(CC1)C(=O)c1nc2cc(c(C)c([N+]([O-])=O)c2s1)C(F)(F)F Show InChI InChI=1S/C17H19F3N4O4S/c1-10-11(17(18,19)20)9-12-14(13(10)24(26)27)29-15(21-12)16(25)23-5-3-22(4-6-23)7-8-28-2/h9H,3-8H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 5.86E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2D6 by fluorescence assay |
Bioorg Med Chem 23: 7694-710 (2015)
Article DOI: 10.1016/j.bmc.2015.11.017
BindingDB Entry DOI: 10.7270/Q2PR800F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50499994

(CHEMBL3741545)Show SMILES COCCN1CCN(CC1)C(=O)c1nc2cc(c(C)c([N+]([O-])=O)c2s1)C(F)(F)F Show InChI InChI=1S/C17H19F3N4O4S/c1-10-11(17(18,19)20)9-12-14(13(10)24(26)27)29-15(21-12)16(25)23-5-3-22(4-6-23)7-8-28-2/h9H,3-8H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 6.49E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C19 by fluorescence assay |
Bioorg Med Chem 23: 7694-710 (2015)
Article DOI: 10.1016/j.bmc.2015.11.017
BindingDB Entry DOI: 10.7270/Q2PR800F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50499995

(CHEMBL3739831)Show SMILES Cc1c(cc2nc(sc2c1[N+]([O-])=O)C1=N[C@@H](CO1)C(=O)N1CCCC1)C(F)(F)F |r,t:15| Show InChI InChI=1S/C17H15F3N4O4S/c1-8-9(17(18,19)20)6-10-13(12(8)24(26)27)29-15(22-10)14-21-11(7-28-14)16(25)23-4-2-3-5-23/h6,11H,2-5,7H2,1H3/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C19 by fluorescence assay |
Bioorg Med Chem 23: 7694-710 (2015)
Article DOI: 10.1016/j.bmc.2015.11.017
BindingDB Entry DOI: 10.7270/Q2PR800F |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADH]
(Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50520250
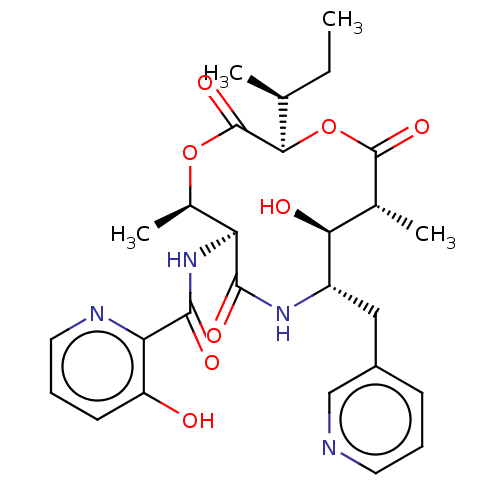
(CHEMBL4575301)Show SMILES [H][C@@]1(OC(=O)[C@H](C)[C@H](O)[C@H](Cc2cccnc2)NC(=O)[C@@H](NC(=O)c2ncccc2O)[C@@H](C)OC1=O)[C@@H](C)CC |r| Show InChI InChI=1S/C27H34N4O8/c1-5-14(2)23-27(37)38-16(4)20(31-25(35)21-19(32)9-7-11-29-21)24(34)30-18(12-17-8-6-10-28-13-17)22(33)15(3)26(36)39-23/h6-11,13-16,18,20,22-23,32-33H,5,12H2,1-4H3,(H,30,34)(H,31,35)/t14-,15+,16+,18-,20-,22-,23+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.72E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ETH Zurich
Curated by ChEMBL
| Assay Description
Inhibition of bacterial InhA harboring S94A mutant using 2-trans-octenoyl-CoA or 2-trans-dodecenoyl-CoA as substrate |
J Med Chem 63: 1105-1131 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01457
BindingDB Entry DOI: 10.7270/Q2BR8WJ8 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50499994

(CHEMBL3741545)Show SMILES COCCN1CCN(CC1)C(=O)c1nc2cc(c(C)c([N+]([O-])=O)c2s1)C(F)(F)F Show InChI InChI=1S/C17H19F3N4O4S/c1-10-11(17(18,19)20)9-12-14(13(10)24(26)27)29-15(21-12)16(25)23-5-3-22(4-6-23)7-8-28-2/h9H,3-8H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 9.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4 by fluorescence assay |
Bioorg Med Chem 23: 7694-710 (2015)
Article DOI: 10.1016/j.bmc.2015.11.017
BindingDB Entry DOI: 10.7270/Q2PR800F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50499995

(CHEMBL3739831)Show SMILES Cc1c(cc2nc(sc2c1[N+]([O-])=O)C1=N[C@@H](CO1)C(=O)N1CCCC1)C(F)(F)F |r,t:15| Show InChI InChI=1S/C17H15F3N4O4S/c1-8-9(17(18,19)20)6-10-13(12(8)24(26)27)29-15(22-10)14-21-11(7-28-14)16(25)23-4-2-3-5-23/h6,11H,2-5,7H2,1H3/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4 by fluorescence assay |
Bioorg Med Chem 23: 7694-710 (2015)
Article DOI: 10.1016/j.bmc.2015.11.017
BindingDB Entry DOI: 10.7270/Q2PR800F |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Homo sapiens (Human)) | BDBM50011480

(CHEMBL3261881)Show SMILES CC(C)=CCC\C(C)=C\COCCNC1C2CC3CC(C2)CC1C3 |TLB:20:19:23:16.15.14,20:15:18.19.21:23,THB:14:15:18:21.22.23,14:22:18:16.20.15,13:14:18.19.21:23,(14.35,-9.79,;12.97,-9.1,;12.88,-7.56,;11.69,-9.95,;10.31,-9.26,;9.02,-10.11,;7.65,-9.42,;7.55,-7.88,;6.36,-10.27,;4.98,-9.58,;3.7,-10.43,;2.32,-9.74,;1.04,-10.59,;-.36,-9.9,;-1.64,-10.75,;-1.65,-12.28,;-3.06,-12.62,;-4.36,-12.13,;-5.56,-13.41,;-4.07,-12.99,;-2.67,-13.55,;-4.08,-11.41,;-3.04,-10.17,;-4.37,-10.65,)| Show InChI InChI=1S/C22H37NO/c1-16(2)5-4-6-17(3)7-9-24-10-8-23-22-20-12-18-11-19(14-20)15-21(22)13-18/h5,7,18-23H,4,6,8-15H2,1-3H3/b17-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Inhibition of human SQS |
J Med Chem 57: 3126-39 (2014)
Article DOI: 10.1021/jm500131s
BindingDB Entry DOI: 10.7270/Q27P90ZN |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50499995

(CHEMBL3739831)Show SMILES Cc1c(cc2nc(sc2c1[N+]([O-])=O)C1=N[C@@H](CO1)C(=O)N1CCCC1)C(F)(F)F |r,t:15| Show InChI InChI=1S/C17H15F3N4O4S/c1-8-9(17(18,19)20)6-10-13(12(8)24(26)27)29-15(22-10)14-21-11(7-28-14)16(25)23-4-2-3-5-23/h6,11H,2-5,7H2,1H3/t11-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2D6 by fluorescence assay |
Bioorg Med Chem 23: 7694-710 (2015)
Article DOI: 10.1016/j.bmc.2015.11.017
BindingDB Entry DOI: 10.7270/Q2PR800F |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Homo sapiens (Human)) | BDBM50011479
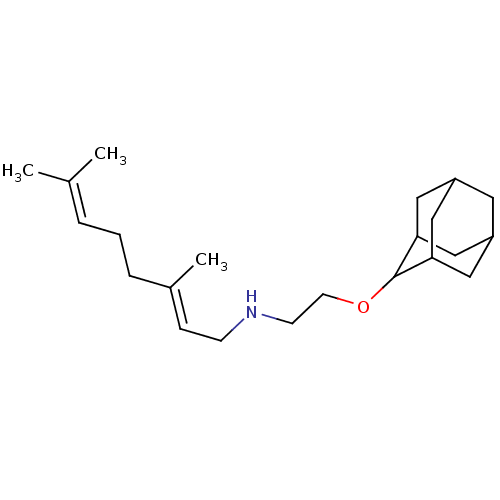
(CHEMBL3261880)Show SMILES CC(C)=CCC\C(C)=C\CNCCOC1C2CC3CC(C2)CC1C3 |TLB:20:19:23:16.15.14,20:15:18.19.21:23,THB:14:15:18:21.22.23,14:22:18:16.20.15,13:14:18.19.21:23,(49.64,-.96,;48.27,-.27,;48.17,1.28,;46.98,-1.12,;45.6,-.43,;44.32,-1.28,;42.94,-.59,;42.85,.95,;41.66,-1.44,;40.28,-.75,;38.99,-1.6,;37.62,-.91,;36.33,-1.76,;34.95,-1.07,;33.67,-1.92,;33.65,-3.45,;32.25,-3.8,;30.93,-3.3,;29.74,-4.58,;31.23,-4.16,;32.64,-4.73,;31.23,-2.58,;32.26,-1.34,;30.93,-1.82,)| Show InChI InChI=1S/C22H37NO/c1-16(2)5-4-6-17(3)7-8-23-9-10-24-22-20-12-18-11-19(14-20)15-21(22)13-18/h5,7,18-23H,4,6,8-15H2,1-3H3/b17-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Inhibition of human SQS |
J Med Chem 57: 3126-39 (2014)
Article DOI: 10.1021/jm500131s
BindingDB Entry DOI: 10.7270/Q27P90ZN |
More data for this
Ligand-Target Pair | |
4,4'-diapophytoene synthase
(Staphylococcus aureus) | BDBM50388398

(CHEMBL561057 | SQ-109)Show SMILES CC(C)=CCC\C(C)=C\CNCCNC1C2CC3CC(C2)CC1C3 |TLB:18:19:16.17.23:14,23:22:20:16.17.18,13:14:20:16.17.18,13:14:16.17.23:20.19.21,THB:18:17:14:20.19.21,23:17:20:22.14.21,21:19:16:23.22.14,21:22:16:20.18.19,13:14:16:20.18.19,(-5.14,2.27,;-5.13,1.44,;-5.84,1.02,;-4.41,1.04,;-3.7,1.46,;-2.98,1.06,;-2.28,1.48,;-2.29,2.3,;-1.56,1.08,;-.85,1.5,;-.13,1.09,;.58,1.51,;1.3,1.11,;2.04,1.48,;2.72,1.02,;2.74,.22,;2.11,-.47,;2.91,-.24,;3.67,-.52,;4.2,.17,;3.46,-.03,;4.19,.99,;3.44,1.28,;2.89,.61,)| Show InChI InChI=1S/C22H38N2/c1-16(2)5-4-6-17(3)7-8-23-9-10-24-22-20-12-18-11-19(14-20)15-21(22)13-18/h5,7,18-24H,4,6,8-15H2,1-3H3/b17-7+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus CrtM |
J Med Chem 57: 3126-39 (2014)
Article DOI: 10.1021/jm500131s
BindingDB Entry DOI: 10.7270/Q27P90ZN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50499995

(CHEMBL3739831)Show SMILES Cc1c(cc2nc(sc2c1[N+]([O-])=O)C1=N[C@@H](CO1)C(=O)N1CCCC1)C(F)(F)F |r,t:15| Show InChI InChI=1S/C17H15F3N4O4S/c1-8-9(17(18,19)20)6-10-13(12(8)24(26)27)29-15(22-10)14-21-11(7-28-14)16(25)23-4-2-3-5-23/h6,11H,2-5,7H2,1H3/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP1A2 by fluorescence assay |
Bioorg Med Chem 23: 7694-710 (2015)
Article DOI: 10.1016/j.bmc.2015.11.017
BindingDB Entry DOI: 10.7270/Q2PR800F |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Homo sapiens (Human)) | BDBM50388398

(CHEMBL561057 | SQ-109)Show SMILES CC(C)=CCC\C(C)=C\CNCCNC1C2CC3CC(C2)CC1C3 |TLB:18:19:16.17.23:14,23:22:20:16.17.18,13:14:20:16.17.18,13:14:16.17.23:20.19.21,THB:18:17:14:20.19.21,23:17:20:22.14.21,21:19:16:23.22.14,21:22:16:20.18.19,13:14:16:20.18.19,(-5.14,2.27,;-5.13,1.44,;-5.84,1.02,;-4.41,1.04,;-3.7,1.46,;-2.98,1.06,;-2.28,1.48,;-2.29,2.3,;-1.56,1.08,;-.85,1.5,;-.13,1.09,;.58,1.51,;1.3,1.11,;2.04,1.48,;2.72,1.02,;2.74,.22,;2.11,-.47,;2.91,-.24,;3.67,-.52,;4.2,.17,;3.46,-.03,;4.19,.99,;3.44,1.28,;2.89,.61,)| Show InChI InChI=1S/C22H38N2/c1-16(2)5-4-6-17(3)7-8-23-9-10-24-22-20-12-18-11-19(14-20)15-21(22)13-18/h5,7,18-24H,4,6,8-15H2,1-3H3/b17-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Inhibition of human SQS |
J Med Chem 57: 3126-39 (2014)
Article DOI: 10.1021/jm500131s
BindingDB Entry DOI: 10.7270/Q27P90ZN |
More data for this
Ligand-Target Pair | |
4,4'-diapophytoene synthase
(Staphylococcus aureus) | BDBM50011480

(CHEMBL3261881)Show SMILES CC(C)=CCC\C(C)=C\COCCNC1C2CC3CC(C2)CC1C3 |TLB:20:19:23:16.15.14,20:15:18.19.21:23,THB:14:15:18:21.22.23,14:22:18:16.20.15,13:14:18.19.21:23,(14.35,-9.79,;12.97,-9.1,;12.88,-7.56,;11.69,-9.95,;10.31,-9.26,;9.02,-10.11,;7.65,-9.42,;7.55,-7.88,;6.36,-10.27,;4.98,-9.58,;3.7,-10.43,;2.32,-9.74,;1.04,-10.59,;-.36,-9.9,;-1.64,-10.75,;-1.65,-12.28,;-3.06,-12.62,;-4.36,-12.13,;-5.56,-13.41,;-4.07,-12.99,;-2.67,-13.55,;-4.08,-11.41,;-3.04,-10.17,;-4.37,-10.65,)| Show InChI InChI=1S/C22H37NO/c1-16(2)5-4-6-17(3)7-9-24-10-8-23-22-20-12-18-11-19(14-20)15-21(22)13-18/h5,7,18-23H,4,6,8-15H2,1-3H3/b17-7+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus CrtM |
J Med Chem 57: 3126-39 (2014)
Article DOI: 10.1021/jm500131s
BindingDB Entry DOI: 10.7270/Q27P90ZN |
More data for this
Ligand-Target Pair | |
4,4'-diapophytoene synthase
(Staphylococcus aureus) | BDBM50011479

(CHEMBL3261880)Show SMILES CC(C)=CCC\C(C)=C\CNCCOC1C2CC3CC(C2)CC1C3 |TLB:20:19:23:16.15.14,20:15:18.19.21:23,THB:14:15:18:21.22.23,14:22:18:16.20.15,13:14:18.19.21:23,(49.64,-.96,;48.27,-.27,;48.17,1.28,;46.98,-1.12,;45.6,-.43,;44.32,-1.28,;42.94,-.59,;42.85,.95,;41.66,-1.44,;40.28,-.75,;38.99,-1.6,;37.62,-.91,;36.33,-1.76,;34.95,-1.07,;33.67,-1.92,;33.65,-3.45,;32.25,-3.8,;30.93,-3.3,;29.74,-4.58,;31.23,-4.16,;32.64,-4.73,;31.23,-2.58,;32.26,-1.34,;30.93,-1.82,)| Show InChI InChI=1S/C22H37NO/c1-16(2)5-4-6-17(3)7-8-23-9-10-24-22-20-12-18-11-19(14-20)15-21(22)13-18/h5,7,18-23H,4,6,8-15H2,1-3H3/b17-7+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus CrtM |
J Med Chem 57: 3126-39 (2014)
Article DOI: 10.1021/jm500131s
BindingDB Entry DOI: 10.7270/Q27P90ZN |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADH]
(Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50520245

(CHEMBL4454539)Show SMILES CC[C@H]1OC(=O)[C@H](C)[C@H](O)[C@H](Cc2cccnc2)NC(=O)[C@@H](NC(=O)c2ncccc2O)[C@@H](C)OC1=O |r| Show InChI InChI=1S/C25H30N4O8/c1-4-18-25(35)36-14(3)19(29-23(33)20-17(30)8-6-10-27-20)22(32)28-16(11-15-7-5-9-26-12-15)21(31)13(2)24(34)37-18/h5-10,12-14,16,18-19,21,30-31H,4,11H2,1-3H3,(H,28,32)(H,29,33)/t13-,14-,16+,18-,19+,21+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.27E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
ETH Zurich
Curated by ChEMBL
| Assay Description
Inhibition of bacterial InhA harboring S94A mutant using 2-trans-octenoyl-CoA or 2-trans-dodecenoyl-CoA as substrate |
J Med Chem 63: 1105-1131 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01457
BindingDB Entry DOI: 10.7270/Q2BR8WJ8 |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADH]
(Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50520248

(CHEMBL4557187)Show SMILES C[C@H]1OC(=O)[C@H](NC(=O)[C@@H](C)[C@@H](O)[C@H](Cc2cccnc2)NC(=O)[C@H]1NC(=O)c1ncccc1O)C1CCCCC1 |r| Show InChI InChI=1S/C29H37N5O7/c1-16-25(36)20(14-18-8-6-12-30-15-18)32-27(38)22(33-28(39)24-21(35)11-7-13-31-24)17(2)41-29(40)23(34-26(16)37)19-9-4-3-5-10-19/h6-8,11-13,15-17,19-20,22-23,25,35-36H,3-5,9-10,14H2,1-2H3,(H,32,38)(H,33,39)(H,34,37)/t16-,17+,20-,22-,23+,25+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.91E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
ETH Zurich
Curated by ChEMBL
| Assay Description
Inhibition of bacterial InhA harboring S94A mutant using 2-trans-octenoyl-CoA or 2-trans-dodecenoyl-CoA as substrate |
J Med Chem 63: 1105-1131 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01457
BindingDB Entry DOI: 10.7270/Q2BR8WJ8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data