

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM370555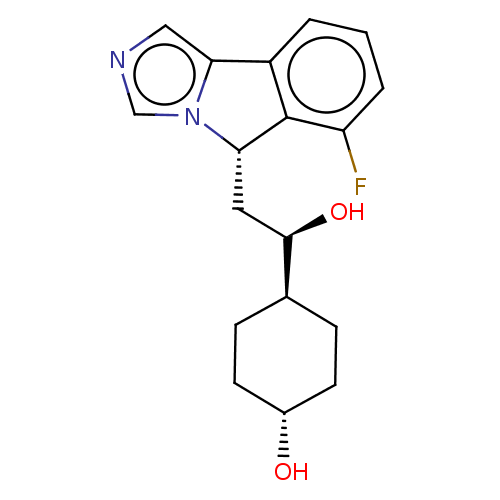 ((1R,4r)-4-((R)-2-((S)-6-fluoro-5H-imidazo[5,1- a]i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NewLink Genetics Corporation Curated by ChEMBL | Assay Description Inhibition of purified human IDO1 using varying levels of L-tryptophan as substrate preincubated for 5 mins followed by substrate addition and measur... | J Med Chem 62: 6705-6733 (2019) Article DOI: 10.1021/acs.jmedchem.9b00662 BindingDB Entry DOI: 10.7270/Q21G0QNZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM370452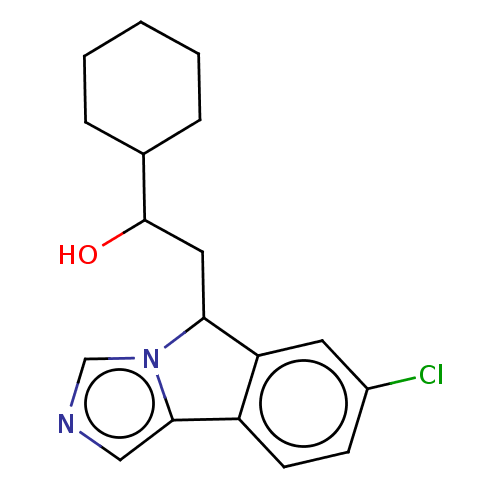 (2-(7-chloro-5H-imidazo[5,1-a]isoindol-5-yl)-1- cyc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
NewLink Genetics Corporation Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) using midazolam as substrate | J Med Chem 62: 6705-6733 (2019) Article DOI: 10.1021/acs.jmedchem.9b00662 BindingDB Entry DOI: 10.7270/Q21G0QNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50138828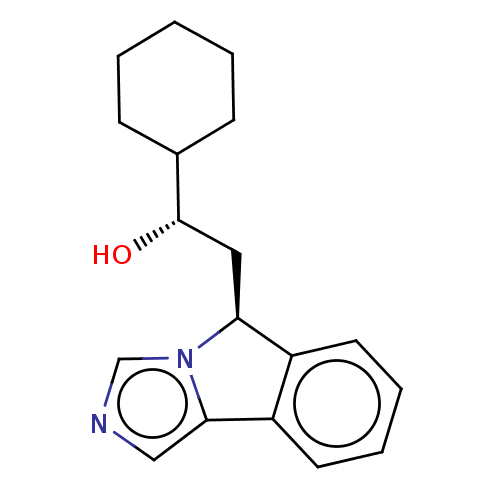 (CHEMBL3752060 | US10233190, Example 1417) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
NewLink Genetics Corporation Curated by ChEMBL | Assay Description Inhibition of purified human IDO1 using L-tryptophan as substrate preincubated for 5 mins followed by substrate addition and measured after 15 mins b... | J Med Chem 62: 6705-6733 (2019) Article DOI: 10.1021/acs.jmedchem.9b00662 BindingDB Entry DOI: 10.7270/Q21G0QNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM370555 ((1R,4r)-4-((R)-2-((S)-6-fluoro-5H-imidazo[5,1- a]i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
NewLink Genetics Corporation Curated by ChEMBL | Assay Description Inhibition of purified human IDO1 using L-tryptophan as substrate preincubated for 5 mins followed by substrate addition and measured after 15 mins b... | J Med Chem 62: 6705-6733 (2019) Article DOI: 10.1021/acs.jmedchem.9b00662 BindingDB Entry DOI: 10.7270/Q21G0QNZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50138819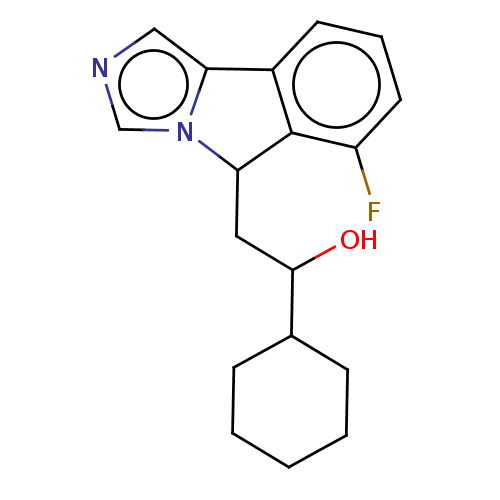 (CHEMBL3753837 | US10233190, Example 1357) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
NewLink Genetics Corporation Curated by ChEMBL | Assay Description Inhibition of purified human IDO1 using L-tryptophan as substrate preincubated for 5 mins followed by substrate addition and measured after 15 mins b... | J Med Chem 62: 6705-6733 (2019) Article DOI: 10.1021/acs.jmedchem.9b00662 BindingDB Entry DOI: 10.7270/Q21G0QNZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM370487 (1-(4-(1-hydroxy-2-(5H-imidazo[5,1-a]isoindol- 5-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
NewLink Genetics Corporation Curated by ChEMBL | Assay Description Inhibition of purified human IDO1 using L-tryptophan as substrate preincubated for 5 mins followed by substrate addition and measured after 15 mins b... | J Med Chem 62: 6705-6733 (2019) Article DOI: 10.1021/acs.jmedchem.9b00662 BindingDB Entry DOI: 10.7270/Q21G0QNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM370519 (2-(4-fluorophenyl)-1-(4-(1-hydroxy-2-(5H- imidazo[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
NewLink Genetics Corporation Curated by ChEMBL | Assay Description Inhibition of purified human IDO1 using L-tryptophan as substrate preincubated for 5 mins followed by substrate addition and measured after 15 mins b... | J Med Chem 62: 6705-6733 (2019) Article DOI: 10.1021/acs.jmedchem.9b00662 BindingDB Entry DOI: 10.7270/Q21G0QNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM370553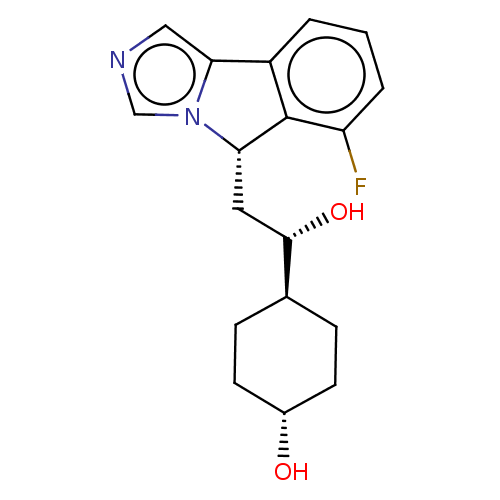 ((1S,4r)-4-((S)-2-((S)-6-fluoro-5H-imidazo[5,1- a]i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
NewLink Genetics Corporation Curated by ChEMBL | Assay Description Inhibition of purified human IDO1 using L-tryptophan as substrate preincubated for 5 mins followed by substrate addition and measured after 15 mins b... | J Med Chem 62: 6705-6733 (2019) Article DOI: 10.1021/acs.jmedchem.9b00662 BindingDB Entry DOI: 10.7270/Q21G0QNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM370526 (4-(1-hydroxy-2-(5H-imidazo[5,1-a]isoindol-5- yl)et...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
NewLink Genetics Corporation Curated by ChEMBL | Assay Description Inhibition of purified human IDO1 using L-tryptophan as substrate preincubated for 5 mins followed by substrate addition and measured after 15 mins b... | J Med Chem 62: 6705-6733 (2019) Article DOI: 10.1021/acs.jmedchem.9b00662 BindingDB Entry DOI: 10.7270/Q21G0QNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM370489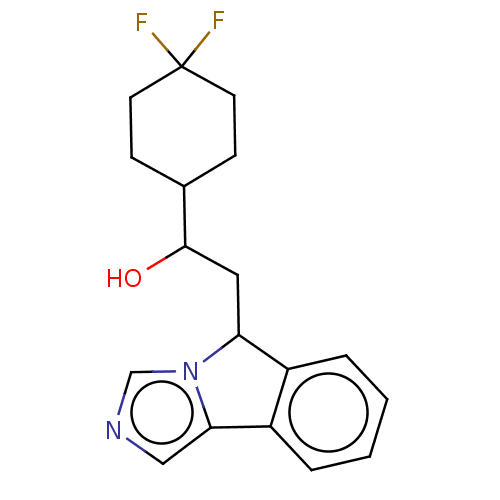 (1-(4,4-difluorocyclohexyl)-2-(5H-imidazo[5,1- a]is...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
NewLink Genetics Corporation Curated by ChEMBL | Assay Description Inhibition of purified human IDO1 using L-tryptophan as substrate preincubated for 5 mins followed by substrate addition and measured after 15 mins b... | J Med Chem 62: 6705-6733 (2019) Article DOI: 10.1021/acs.jmedchem.9b00662 BindingDB Entry DOI: 10.7270/Q21G0QNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM370572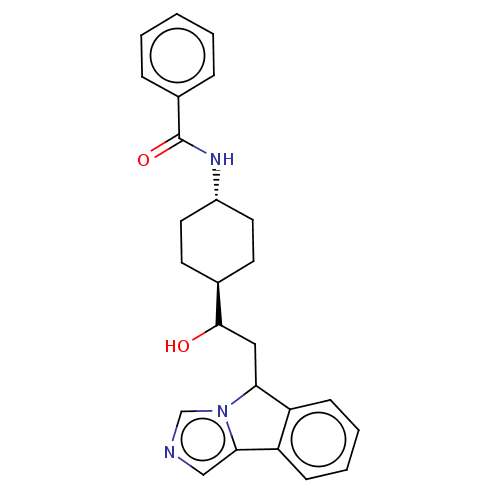 (N-((1r,4r)-4-(1-hydroxy-2-(5H-imidazo[5,1- a]isoin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
NewLink Genetics Corporation Curated by ChEMBL | Assay Description Inhibition of purified human IDO1 using L-tryptophan as substrate preincubated for 5 mins followed by substrate addition and measured after 15 mins b... | J Med Chem 62: 6705-6733 (2019) Article DOI: 10.1021/acs.jmedchem.9b00662 BindingDB Entry DOI: 10.7270/Q21G0QNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM370519 (2-(4-fluorophenyl)-1-(4-(1-hydroxy-2-(5H- imidazo[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
NewLink Genetics Corporation Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) using midazolam as substrate | J Med Chem 62: 6705-6733 (2019) Article DOI: 10.1021/acs.jmedchem.9b00662 BindingDB Entry DOI: 10.7270/Q21G0QNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50126144 (CHEMBL3629569 | US10155972, Compound NewLink 1 | U...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
NewLink Genetics Corporation Curated by ChEMBL | Assay Description Inhibition of purified human IDO1 using L-tryptophan as substrate preincubated for 5 mins followed by substrate addition and measured after 15 mins b... | J Med Chem 62: 6705-6733 (2019) Article DOI: 10.1021/acs.jmedchem.9b00662 BindingDB Entry DOI: 10.7270/Q21G0QNZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM370559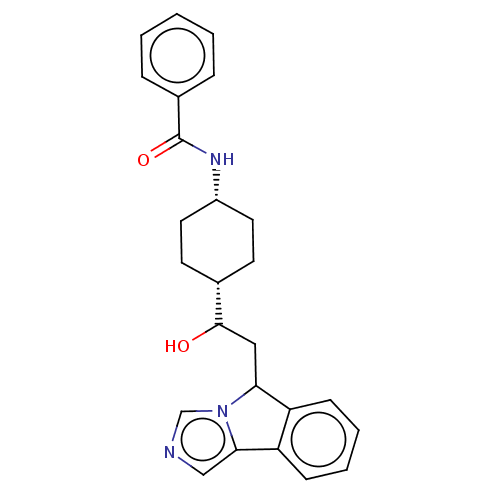 (N-((1s,4s)-4-(1-hydroxy-2-(5H-imidazo[5,1- a]isoin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
NewLink Genetics Corporation Curated by ChEMBL | Assay Description Inhibition of purified human IDO1 using L-tryptophan as substrate preincubated for 5 mins followed by substrate addition and measured after 15 mins b... | J Med Chem 62: 6705-6733 (2019) Article DOI: 10.1021/acs.jmedchem.9b00662 BindingDB Entry DOI: 10.7270/Q21G0QNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM370551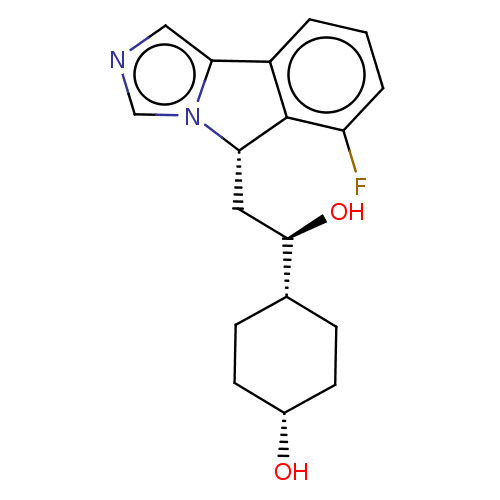 ((1S,4s)-4-((R)-2-((S)-6-fluoro-5H-imidazo[5,1- a]i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
NewLink Genetics Corporation Curated by ChEMBL | Assay Description Inhibition of purified human IDO1 using L-tryptophan as substrate preincubated for 5 mins followed by substrate addition and measured after 15 mins b... | J Med Chem 62: 6705-6733 (2019) Article DOI: 10.1021/acs.jmedchem.9b00662 BindingDB Entry DOI: 10.7270/Q21G0QNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM370488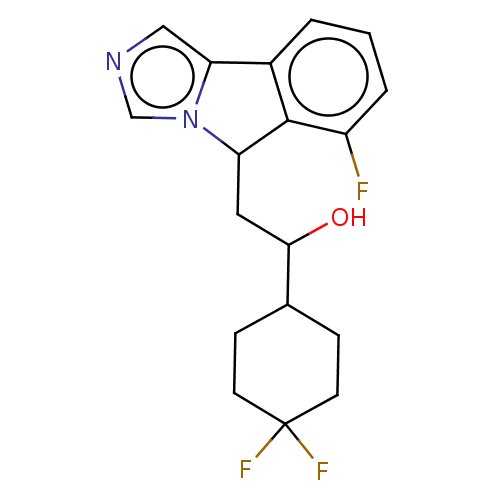 (1-(4,4-difluorocyclohexyl)-2-(6-fluoro-5H- imidazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
NewLink Genetics Corporation Curated by ChEMBL | Assay Description Inhibition of purified human IDO1 using L-tryptophan as substrate preincubated for 5 mins followed by substrate addition and measured after 15 mins b... | J Med Chem 62: 6705-6733 (2019) Article DOI: 10.1021/acs.jmedchem.9b00662 BindingDB Entry DOI: 10.7270/Q21G0QNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50138827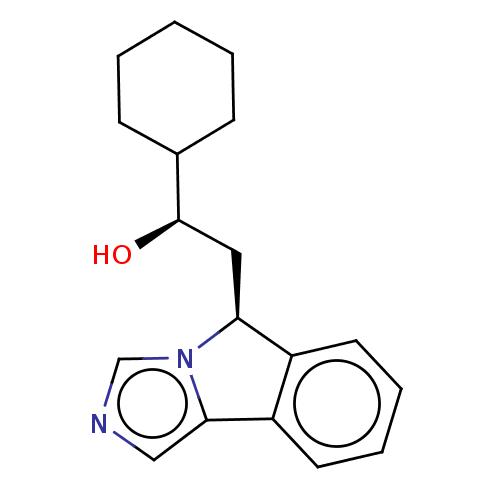 (CHEMBL3752711 | US10233190, Example 1418) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
NewLink Genetics Corporation Curated by ChEMBL | Assay Description Inhibition of purified human IDO1 using L-tryptophan as substrate preincubated for 5 mins followed by substrate addition and measured after 15 mins b... | J Med Chem 62: 6705-6733 (2019) Article DOI: 10.1021/acs.jmedchem.9b00662 BindingDB Entry DOI: 10.7270/Q21G0QNZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM370487 (1-(4-(1-hydroxy-2-(5H-imidazo[5,1-a]isoindol- 5-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
NewLink Genetics Corporation Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) using midazolam as substrate | J Med Chem 62: 6705-6733 (2019) Article DOI: 10.1021/acs.jmedchem.9b00662 BindingDB Entry DOI: 10.7270/Q21G0QNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50516665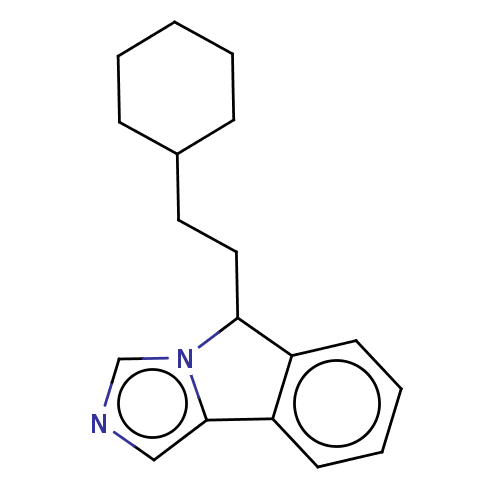 (CHEMBL4454031) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 135 | n/a | n/a | n/a | n/a | n/a | n/a |
NewLink Genetics Corporation Curated by ChEMBL | Assay Description Inhibition of purified human IDO1 using L-tryptophan as substrate preincubated for 5 mins followed by substrate addition and measured after 15 mins b... | J Med Chem 62: 6705-6733 (2019) Article DOI: 10.1021/acs.jmedchem.9b00662 BindingDB Entry DOI: 10.7270/Q21G0QNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM370455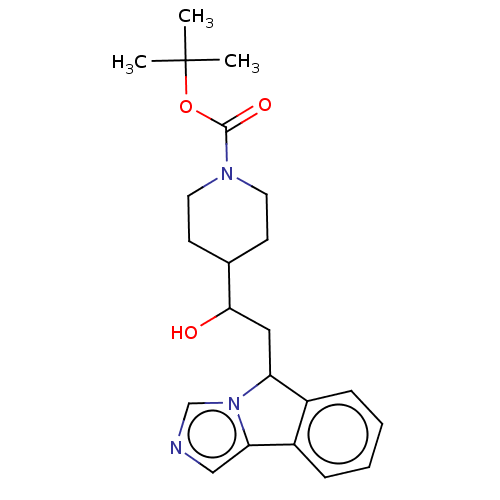 (US10233190, Example 1363 | tert-butyl 4-(1-hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
NewLink Genetics Corporation Curated by ChEMBL | Assay Description Inhibition of purified human IDO1 using L-tryptophan as substrate preincubated for 5 mins followed by substrate addition and measured after 15 mins b... | J Med Chem 62: 6705-6733 (2019) Article DOI: 10.1021/acs.jmedchem.9b00662 BindingDB Entry DOI: 10.7270/Q21G0QNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM370454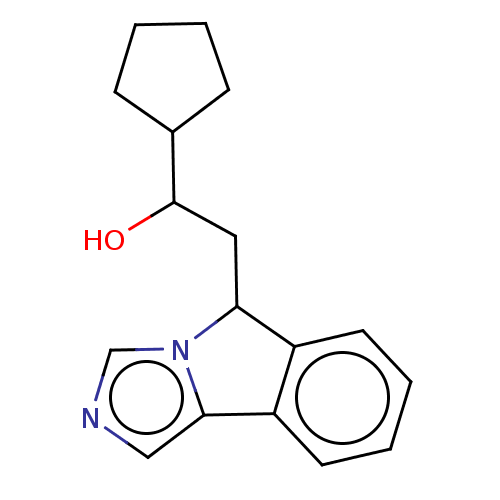 (1-cyclopentyl-2-(5H-imidazo[5,1-a]isoindol-5- yl)e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
NewLink Genetics Corporation Curated by ChEMBL | Assay Description Inhibition of purified human IDO1 using L-tryptophan as substrate preincubated for 5 mins followed by substrate addition and measured after 15 mins b... | J Med Chem 62: 6705-6733 (2019) Article DOI: 10.1021/acs.jmedchem.9b00662 BindingDB Entry DOI: 10.7270/Q21G0QNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50126144 (CHEMBL3629569 | US10155972, Compound NewLink 1 | U...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
NewLink Genetics Corporation Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) using midazolam as substrate | J Med Chem 62: 6705-6733 (2019) Article DOI: 10.1021/acs.jmedchem.9b00662 BindingDB Entry DOI: 10.7270/Q21G0QNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM370460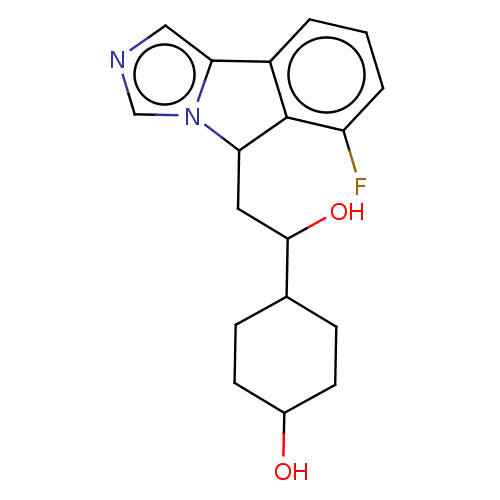 (4-(2-(6-fluoro-5H-imidazo[5,1-a]isoindol-5-yl)- 1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
NewLink Genetics Corporation Curated by ChEMBL | Assay Description Inhibition of purified human IDO1 using L-tryptophan as substrate preincubated for 5 mins followed by substrate addition and measured after 15 mins b... | J Med Chem 62: 6705-6733 (2019) Article DOI: 10.1021/acs.jmedchem.9b00662 BindingDB Entry DOI: 10.7270/Q21G0QNZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM370542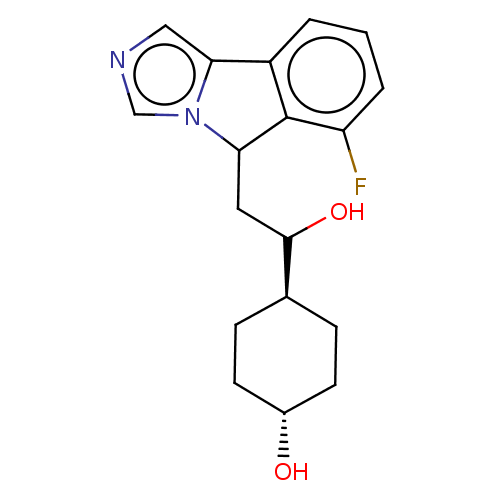 ((1r,4r)-4-(2-(6-fluoro-5H-imidazo[5,1- a]isoindol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
NewLink Genetics Corporation Curated by ChEMBL | Assay Description Inhibition of purified human IDO1 using L-tryptophan as substrate preincubated for 5 mins followed by substrate addition and measured after 15 mins b... | J Med Chem 62: 6705-6733 (2019) Article DOI: 10.1021/acs.jmedchem.9b00662 BindingDB Entry DOI: 10.7270/Q21G0QNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM370452 (2-(7-chloro-5H-imidazo[5,1-a]isoindol-5-yl)-1- cyc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
NewLink Genetics Corporation Curated by ChEMBL | Assay Description Inhibition of purified human IDO1 using L-tryptophan as substrate preincubated for 5 mins followed by substrate addition and measured after 15 mins b... | J Med Chem 62: 6705-6733 (2019) Article DOI: 10.1021/acs.jmedchem.9b00662 BindingDB Entry DOI: 10.7270/Q21G0QNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM370517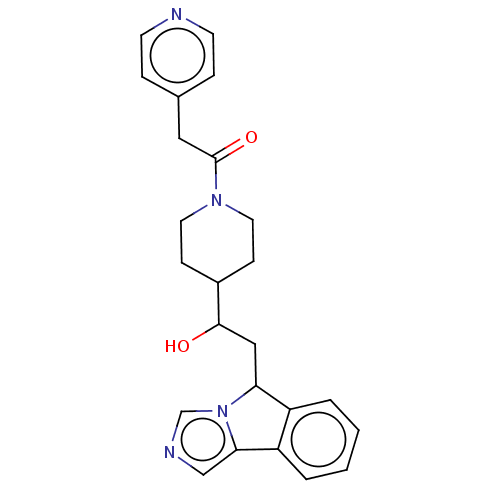 (1-(4-(1-hydroxy-2-(5H-imidazo[5,1-a]isoindol- 5-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
NewLink Genetics Corporation Curated by ChEMBL | Assay Description Inhibition of purified human IDO1 using L-tryptophan as substrate preincubated for 5 mins followed by substrate addition and measured after 15 mins b... | J Med Chem 62: 6705-6733 (2019) Article DOI: 10.1021/acs.jmedchem.9b00662 BindingDB Entry DOI: 10.7270/Q21G0QNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50516659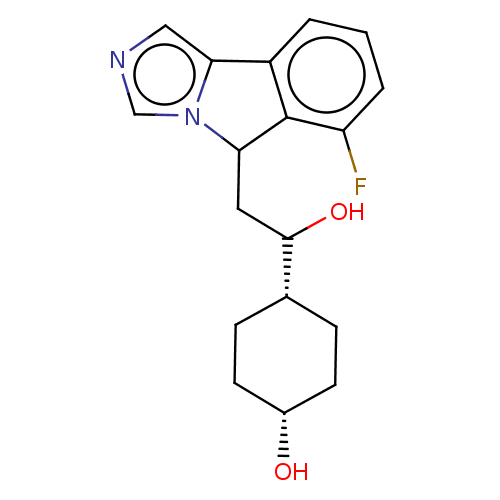 (CHEMBL4562289) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
NewLink Genetics Corporation Curated by ChEMBL | Assay Description Inhibition of purified human IDO1 using L-tryptophan as substrate preincubated for 5 mins followed by substrate addition and measured after 15 mins b... | J Med Chem 62: 6705-6733 (2019) Article DOI: 10.1021/acs.jmedchem.9b00662 BindingDB Entry DOI: 10.7270/Q21G0QNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50516664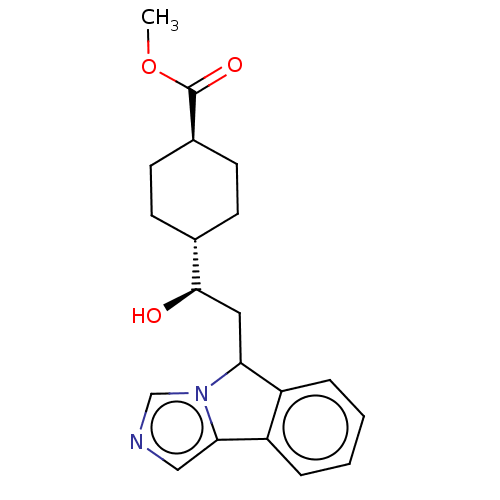 (CHEMBL4461858) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
NewLink Genetics Corporation Curated by ChEMBL | Assay Description Inhibition of purified human IDO1 using L-tryptophan as substrate preincubated for 5 mins followed by substrate addition and measured after 15 mins b... | J Med Chem 62: 6705-6733 (2019) Article DOI: 10.1021/acs.jmedchem.9b00662 BindingDB Entry DOI: 10.7270/Q21G0QNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50516671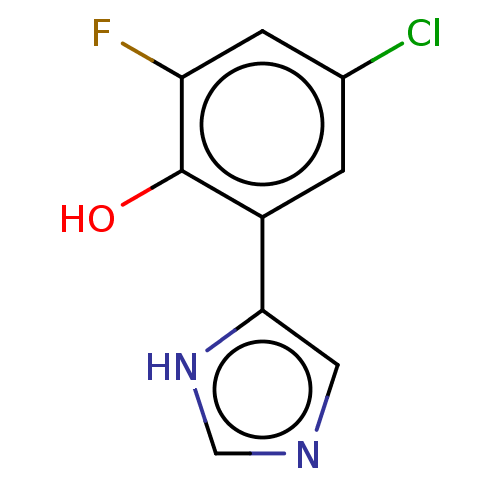 (CHEMBL4579820) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
NewLink Genetics Corporation Curated by ChEMBL | Assay Description Inhibition of purified human IDO1 using L-tryptophan as substrate preincubated for 5 mins followed by substrate addition and measured after 15 mins b... | J Med Chem 62: 6705-6733 (2019) Article DOI: 10.1021/acs.jmedchem.9b00662 BindingDB Entry DOI: 10.7270/Q21G0QNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM370446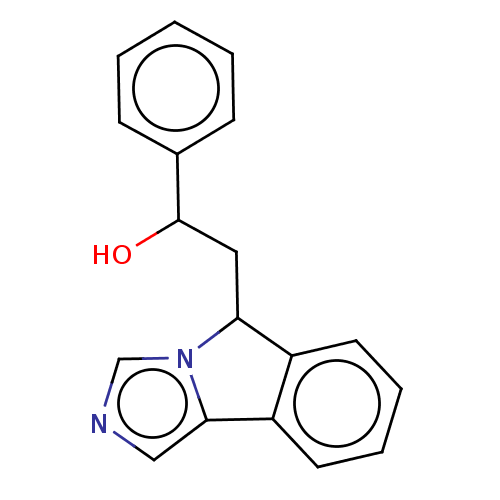 (2-(5H-imidazo[5,1-a]isoindol-5-yl)-1- phenylethano...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
NewLink Genetics Corporation Curated by ChEMBL | Assay Description Inhibition of purified human IDO1 using L-tryptophan as substrate preincubated for 5 mins followed by substrate addition and measured after 15 mins b... | J Med Chem 62: 6705-6733 (2019) Article DOI: 10.1021/acs.jmedchem.9b00662 BindingDB Entry DOI: 10.7270/Q21G0QNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM370568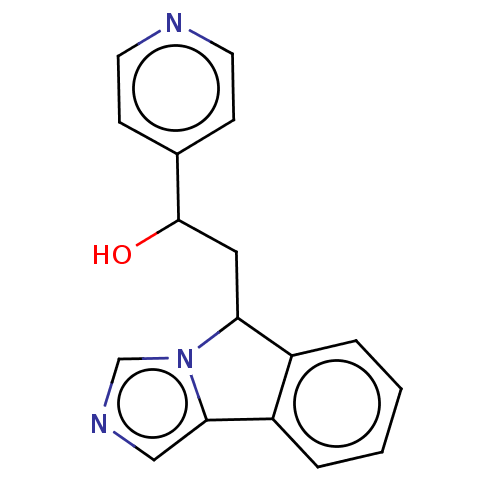 (2-(5H-imidazo[5,1-a]isoindol-5-yl)-1-(pyridin-4- y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
NewLink Genetics Corporation Curated by ChEMBL | Assay Description Inhibition of purified human IDO1 using L-tryptophan as substrate preincubated for 5 mins followed by substrate addition and measured after 15 mins b... | J Med Chem 62: 6705-6733 (2019) Article DOI: 10.1021/acs.jmedchem.9b00662 BindingDB Entry DOI: 10.7270/Q21G0QNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50516644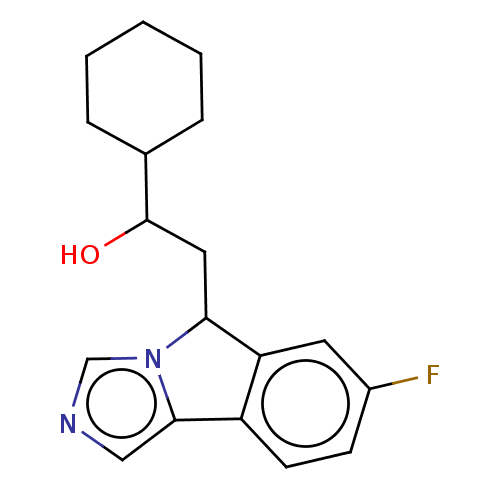 (CHEMBL4563021) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
NewLink Genetics Corporation Curated by ChEMBL | Assay Description Inhibition of purified human IDO1 using L-tryptophan as substrate preincubated for 5 mins followed by substrate addition and measured after 15 mins b... | J Med Chem 62: 6705-6733 (2019) Article DOI: 10.1021/acs.jmedchem.9b00662 BindingDB Entry DOI: 10.7270/Q21G0QNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM370552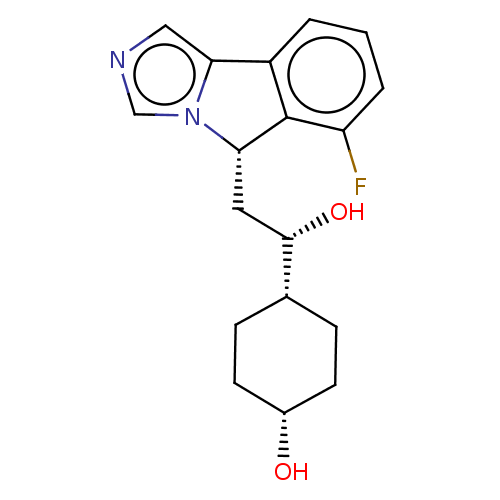 ((1R,4s)-4-((S)-2-((S)-6-fluoro-5H-imidazo[5,1- a]i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
NewLink Genetics Corporation Curated by ChEMBL | Assay Description Inhibition of purified human IDO1 using L-tryptophan as substrate preincubated for 5 mins followed by substrate addition and measured after 15 mins b... | J Med Chem 62: 6705-6733 (2019) Article DOI: 10.1021/acs.jmedchem.9b00662 BindingDB Entry DOI: 10.7270/Q21G0QNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50516641 (CHEMBL4455007) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
NewLink Genetics Corporation Curated by ChEMBL | Assay Description Inhibition of purified human IDO1 using L-tryptophan as substrate preincubated for 5 mins followed by substrate addition and measured after 15 mins b... | J Med Chem 62: 6705-6733 (2019) Article DOI: 10.1021/acs.jmedchem.9b00662 BindingDB Entry DOI: 10.7270/Q21G0QNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM370526 (4-(1-hydroxy-2-(5H-imidazo[5,1-a]isoindol-5- yl)et...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
NewLink Genetics Corporation Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) using midazolam as substrate | J Med Chem 62: 6705-6733 (2019) Article DOI: 10.1021/acs.jmedchem.9b00662 BindingDB Entry DOI: 10.7270/Q21G0QNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM370569 (2-(5H-imidazo[5,1-a]isoindol-5-yl)-1-(pyridin-2- y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Pharmaceuticals Corporation | Assay Description The IC50 values for each compound were determined by testing the activity of IDO in a mixture containing 50 mM potassium phosphate buffer at pH 6.5; ... | J Med Chem 50: 984-1000 (2007) BindingDB Entry DOI: 10.7270/Q26H4KQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50138830 (CHEMBL3752659 | US10233190, Example 1415) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Pharmaceuticals Corporation | Assay Description The IC50 values for each compound were determined by testing the activity of IDO in a mixture containing 50 mM potassium phosphate buffer at pH 6.5; ... | J Med Chem 50: 984-1000 (2007) BindingDB Entry DOI: 10.7270/Q26H4KQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50138829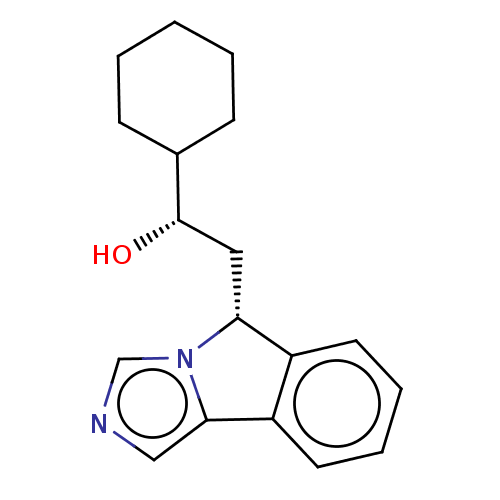 (CHEMBL3754097 | US10233190, Example 1416) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Pharmaceuticals Corporation | Assay Description The IC50 values for each compound were determined by testing the activity of IDO in a mixture containing 50 mM potassium phosphate buffer at pH 6.5; ... | J Med Chem 50: 984-1000 (2007) BindingDB Entry DOI: 10.7270/Q26H4KQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM370500 (1-cyclohexyl-2-(5H-imidazo[5,1-a]isoindol-5- ylide...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Pharmaceuticals Corporation | Assay Description The IC50 values for each compound were determined by testing the activity of IDO in a mixture containing 50 mM potassium phosphate buffer at pH 6.5; ... | J Med Chem 50: 984-1000 (2007) BindingDB Entry DOI: 10.7270/Q26H4KQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM370508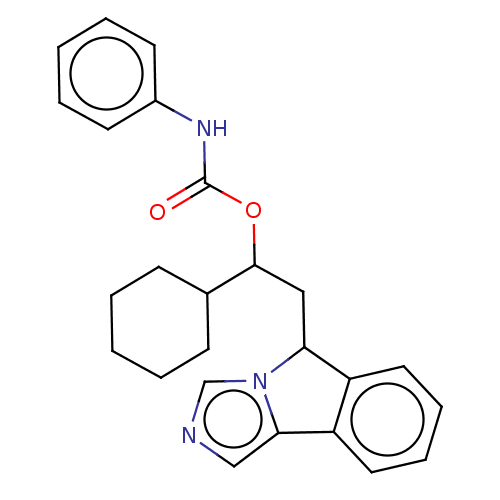 (1-cyclohexyl-2-(5H-imidazo[5,1-a]isoindol-5- yl)et...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Pharmaceuticals Corporation | Assay Description The IC50 values for each compound were determined by testing the activity of IDO in a mixture containing 50 mM potassium phosphate buffer at pH 6.5; ... | J Med Chem 50: 984-1000 (2007) BindingDB Entry DOI: 10.7270/Q26H4KQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM370509 (4-(1-cyclohexyl-2-(5H-imidazo[5,1-a]isoindol-5- yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Pharmaceuticals Corporation | Assay Description The IC50 values for each compound were determined by testing the activity of IDO in a mixture containing 50 mM potassium phosphate buffer at pH 6.5; ... | J Med Chem 50: 984-1000 (2007) BindingDB Entry DOI: 10.7270/Q26H4KQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM370513 (4-(1-hydroxy-2-(5H-imidazo[5,1-a]isoindol-5- yl)et...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Pharmaceuticals Corporation | Assay Description The IC50 values for each compound were determined by testing the activity of IDO in a mixture containing 50 mM potassium phosphate buffer at pH 6.5; ... | J Med Chem 50: 984-1000 (2007) BindingDB Entry DOI: 10.7270/Q26H4KQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM370516 (4-(1-hydroxy-2-(5H-imidazo[5,1-a]isoindol-5- yl)et...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Pharmaceuticals Corporation | Assay Description The IC50 values for each compound were determined by testing the activity of IDO in a mixture containing 50 mM potassium phosphate buffer at pH 6.5; ... | J Med Chem 50: 984-1000 (2007) BindingDB Entry DOI: 10.7270/Q26H4KQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM370522 ((2S)-1-cyclohexyl-2-(5H-imidazo[5,1-a]isoindol- 5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Pharmaceuticals Corporation | Assay Description The IC50 values for each compound were determined by testing the activity of IDO in a mixture containing 50 mM potassium phosphate buffer at pH 6.5; ... | J Med Chem 50: 984-1000 (2007) BindingDB Entry DOI: 10.7270/Q26H4KQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM370527 ((4-fluorophenyl)(4-(1-hydroxy-2-(5H- imidazo[5,1-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Pharmaceuticals Corporation | Assay Description The IC50 values for each compound were determined by testing the activity of IDO in a mixture containing 50 mM potassium phosphate buffer at pH 6.5; ... | J Med Chem 50: 984-1000 (2007) BindingDB Entry DOI: 10.7270/Q26H4KQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM370528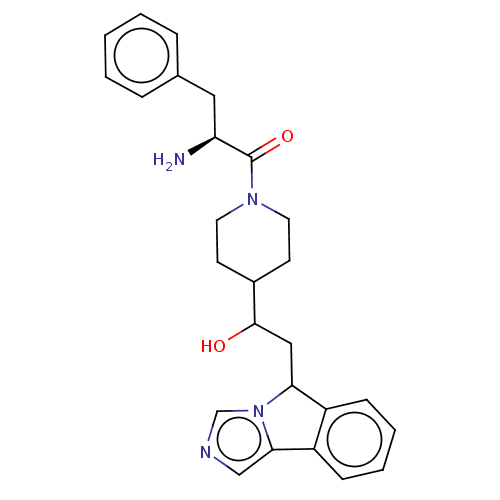 ((2S)-2-amino-1-(4-(1-hydroxy-2-(5H- imidazo[5,1-a]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Pharmaceuticals Corporation | Assay Description The IC50 values for each compound were determined by testing the activity of IDO in a mixture containing 50 mM potassium phosphate buffer at pH 6.5; ... | J Med Chem 50: 984-1000 (2007) BindingDB Entry DOI: 10.7270/Q26H4KQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM370536 (US10233190, Example 1469 | tert-butyl 4-((S)-1-hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Pharmaceuticals Corporation | Assay Description The IC50 values for each compound were determined by testing the activity of IDO in a mixture containing 50 mM potassium phosphate buffer at pH 6.5; ... | J Med Chem 50: 984-1000 (2007) BindingDB Entry DOI: 10.7270/Q26H4KQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM370537 (US10233190, Example 1470 | tert-butyl 4-((R)-1-hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Pharmaceuticals Corporation | Assay Description The IC50 values for each compound were determined by testing the activity of IDO in a mixture containing 50 mM potassium phosphate buffer at pH 6.5; ... | J Med Chem 50: 984-1000 (2007) BindingDB Entry DOI: 10.7270/Q26H4KQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM370541 (2-(5H-imidazo[5,1-a]isoindol-5-yl)-1-(pyridin-3- y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Pharmaceuticals Corporation | Assay Description The IC50 values for each compound were determined by testing the activity of IDO in a mixture containing 50 mM potassium phosphate buffer at pH 6.5; ... | J Med Chem 50: 984-1000 (2007) BindingDB Entry DOI: 10.7270/Q26H4KQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM370543 (4-((S)-1-hydroxy-2-((R)-5H-imidazo[5,1- a]isoindol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Pharmaceuticals Corporation | Assay Description The IC50 values for each compound were determined by testing the activity of IDO in a mixture containing 50 mM potassium phosphate buffer at pH 6.5; ... | J Med Chem 50: 984-1000 (2007) BindingDB Entry DOI: 10.7270/Q26H4KQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 391 total ) | Next | Last >> |