 Found 222 hits with Last Name = 'kirkland' and Initial = 't'
Found 222 hits with Last Name = 'kirkland' and Initial = 't' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase HCK
(Homo sapiens (Human)) | BDBM50322535

(3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N...)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cnc4cccnn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-5-6-22(16-21(20)8-10-25-18-33-27-4-3-11-34-38(25)27)28(39)35-24-9-7-23(26(17-24)29(30,31)32)19-37-14-12-36(2)13-15-37/h3-7,9,11,16-18H,12-15,19H2,1-2H3,(H,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human HCK using KVEKIGEGTYGVVYK as substrate by radiometric hotspot kinase assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.8b00081
BindingDB Entry DOI: 10.7270/Q2WM1J37 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM50322535

(3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N...)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cnc4cccnn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-5-6-22(16-21(20)8-10-25-18-33-27-4-3-11-34-38(25)27)28(39)35-24-9-7-23(26(17-24)29(30,31)32)19-37-14-12-36(2)13-15-37/h3-7,9,11,16-18H,12-15,19H2,1-2H3,(H,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to LYN (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.8b00081
BindingDB Entry DOI: 10.7270/Q2WM1J37 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50322535

(3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N...)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cnc4cccnn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-5-6-22(16-21(20)8-10-25-18-33-27-4-3-11-34-38(25)27)28(39)35-24-9-7-23(26(17-24)29(30,31)32)19-37-14-12-36(2)13-15-37/h3-7,9,11,16-18H,12-15,19H2,1-2H3,(H,35,39) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human LCK using poly[Glu:Tyr] (4:1) as substrate by radiometric hotspot kinase assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.8b00081
BindingDB Entry DOI: 10.7270/Q2WM1J37 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Fyn
(Homo sapiens (Human)) | BDBM50322535

(3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N...)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cnc4cccnn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-5-6-22(16-21(20)8-10-25-18-33-27-4-3-11-34-38(25)27)28(39)35-24-9-7-23(26(17-24)29(30,31)32)19-37-14-12-36(2)13-15-37/h3-7,9,11,16-18H,12-15,19H2,1-2H3,(H,35,39) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human FYN using poly[Glu:Tyr] (4:1) as substrate by radiometric hotspot kinase assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.8b00081
BindingDB Entry DOI: 10.7270/Q2WM1J37 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50322535

(3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N...)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cnc4cccnn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-5-6-22(16-21(20)8-10-25-18-33-27-4-3-11-34-38(25)27)28(39)35-24-9-7-23(26(17-24)29(30,31)32)19-37-14-12-36(2)13-15-37/h3-7,9,11,16-18H,12-15,19H2,1-2H3,(H,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human ABL1 using EAIYAAPFAKKK as substrate by radiometric hotspot kinase assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.8b00081
BindingDB Entry DOI: 10.7270/Q2WM1J37 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM50322535

(3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N...)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cnc4cccnn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-5-6-22(16-21(20)8-10-25-18-33-27-4-3-11-34-38(25)27)28(39)35-24-9-7-23(26(17-24)29(30,31)32)19-37-14-12-36(2)13-15-37/h3-7,9,11,16-18H,12-15,19H2,1-2H3,(H,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human YES using poly[Glu:Tyr] (4:1) as substrate by radiometric hotspot kinase assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.8b00081
BindingDB Entry DOI: 10.7270/Q2WM1J37 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50251744
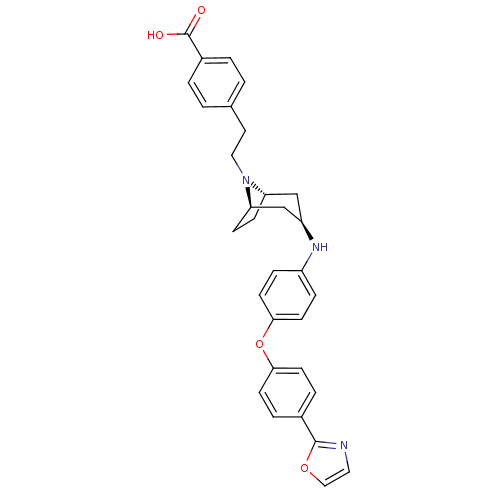
(CHEMBL481672 | endo-4-(2-(3-(4-(4-(oxazol-2-yl)phe...)Show SMILES OC(=O)c1ccc(CCN2[C@H]3CC[C@@H]2C[C@@H](C3)Nc2ccc(Oc3ccc(cc3)-c3ncco3)cc2)cc1 |r,THB:8:9:16.15.14:11.12| Show InChI InChI=1S/C31H31N3O4/c35-31(36)23-3-1-21(2-4-23)15-17-34-26-9-10-27(34)20-25(19-26)33-24-7-13-29(14-8-24)38-28-11-5-22(6-12-28)30-32-16-18-37-30/h1-8,11-14,16,18,25-27,33H,9-10,15,17,19-20H2,(H,35,36)/t25-,26+,27- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of LTA4 hydrolase (unknown origin) |
Bioorg Med Chem Lett 18: 3895-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.041
BindingDB Entry DOI: 10.7270/Q2GQ6XK8 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha
(Homo sapiens (Human)) | BDBM50322535

(3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N...)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cnc4cccnn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-5-6-22(16-21(20)8-10-25-18-33-27-4-3-11-34-38(25)27)28(39)35-24-9-7-23(26(17-24)29(30,31)32)19-37-14-12-36(2)13-15-37/h3-7,9,11,16-18H,12-15,19H2,1-2H3,(H,35,39) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human PDGFRalpha using poly[Glu:Tyr] (4:1) as substrate by radiometric hotspot kinase assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.8b00081
BindingDB Entry DOI: 10.7270/Q2WM1J37 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase FRK
(Homo sapiens (Human)) | BDBM50322535

(3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N...)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cnc4cccnn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-5-6-22(16-21(20)8-10-25-18-33-27-4-3-11-34-38(25)27)28(39)35-24-9-7-23(26(17-24)29(30,31)32)19-37-14-12-36(2)13-15-37/h3-7,9,11,16-18H,12-15,19H2,1-2H3,(H,35,39) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human FRK using poly[Glu:Tyr] (4:1) as substrate by radiometric hotspot kinase assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.8b00081
BindingDB Entry DOI: 10.7270/Q2WM1J37 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM345819
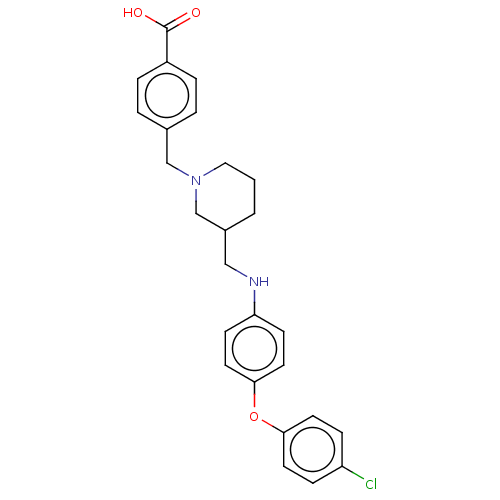
(US10202362, Compound T2.2)Show SMILES OC(=O)c1ccc(CN2CCCC(CNc3ccc(Oc4ccc(Cl)cc4)cc3)C2)cc1 Show InChI InChI=1S/C26H27ClN2O3/c27-22-7-11-24(12-8-22)32-25-13-9-23(10-14-25)28-16-20-2-1-15-29(18-20)17-19-3-5-21(6-4-19)26(30)31/h3-14,20,28H,1-2,15-18H2,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Celtaxsys, Inc.
US Patent
| Assay Description
LTB4 formed was quantified with the HTRF assay in which free LTB4 competes with LTB4-XL665 conjugate (acceptor) for anti-LTB4 monoclonal antibody lab... |
US Patent US10202362 (2019)
BindingDB Entry DOI: 10.7270/Q2MC9241 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50251705

(4-(2-(3-(4-(4-(oxazol-2-yl)phenoxy)phenylamino)pip...)Show SMILES OC(=O)c1ccc(CCN2CCCC(C2)Nc2ccc(Oc3ccc(cc3)-c3ncco3)cc2)cc1 Show InChI InChI=1S/C29H29N3O4/c33-29(34)23-5-3-21(4-6-23)15-18-32-17-1-2-25(20-32)31-24-9-13-27(14-10-24)36-26-11-7-22(8-12-26)28-30-16-19-35-28/h3-14,16,19,25,31H,1-2,15,17-18,20H2,(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of LTA4 hydrolase (unknown origin) |
Bioorg Med Chem Lett 18: 3895-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.041
BindingDB Entry DOI: 10.7270/Q2GQ6XK8 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM345818
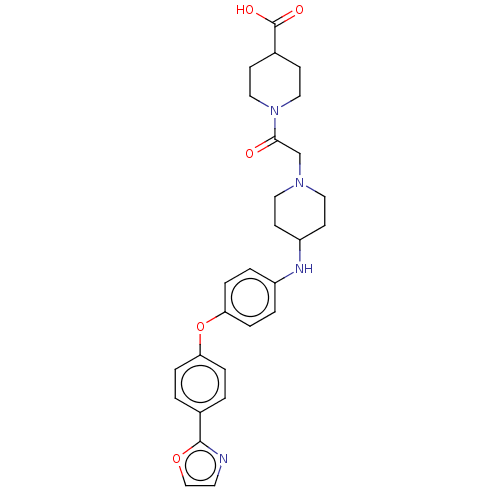
(US10202362, Compound T2.1)Show SMILES OC(=O)C1CCN(CC1)C(=O)CN1CCC(CC1)Nc1ccc(Oc2ccc(cc2)-c2ncco2)cc1 Show InChI InChI=1S/C28H32N4O5/c33-26(32-16-9-21(10-17-32)28(34)35)19-31-14-11-23(12-15-31)30-22-3-7-25(8-4-22)37-24-5-1-20(2-6-24)27-29-13-18-36-27/h1-8,13,18,21,23,30H,9-12,14-17,19H2,(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Celtaxsys, Inc.
US Patent
| Assay Description
LTB4 formed was quantified with the HTRF assay in which free LTB4 competes with LTB4-XL665 conjugate (acceptor) for anti-LTB4 monoclonal antibody lab... |
US Patent US10202362 (2019)
BindingDB Entry DOI: 10.7270/Q2MC9241 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50322535

(3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N...)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cnc4cccnn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-5-6-22(16-21(20)8-10-25-18-33-27-4-3-11-34-38(25)27)28(39)35-24-9-7-23(26(17-24)29(30,31)32)19-37-14-12-36(2)13-15-37/h3-7,9,11,16-18H,12-15,19H2,1-2H3,(H,35,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human FGFR1 using poly[Glu:Tyr] (4:1) as substrate by radiometric hotspot kinase assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.8b00081
BindingDB Entry DOI: 10.7270/Q2WM1J37 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50251397
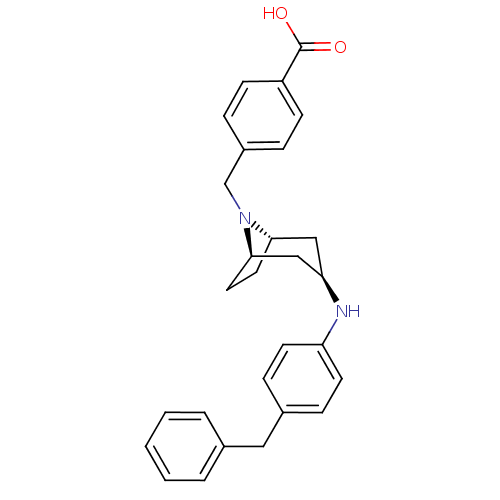
(CHEMBL480334 | endo-4-((3-(4-benzylphenylamino)-8-...)Show SMILES OC(=O)c1ccc(CN2[C@H]3CC[C@@H]2C[C@@H](C3)Nc2ccc(Cc3ccccc3)cc2)cc1 |r,THB:7:8:15.14.13:10.11| Show InChI InChI=1S/C28H30N2O2/c31-28(32)23-10-6-22(7-11-23)19-30-26-14-15-27(30)18-25(17-26)29-24-12-8-21(9-13-24)16-20-4-2-1-3-5-20/h1-13,25-27,29H,14-19H2,(H,31,32)/t25-,26+,27- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of LTA4 hydrolase (unknown origin) |
Bioorg Med Chem Lett 18: 3895-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.041
BindingDB Entry DOI: 10.7270/Q2GQ6XK8 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50251386

(4-(2-(methyl(3-(methyl(2-oxo-2-(4-phenoxyphenylami...)Show SMILES CN(CCCN(C)CC(=O)Nc1ccc(Oc2ccccc2)cc1)CCc1ccc(cc1)C(O)=O Show InChI InChI=1S/C28H33N3O4/c1-30(20-17-22-9-11-23(12-10-22)28(33)34)18-6-19-31(2)21-27(32)29-24-13-15-26(16-14-24)35-25-7-4-3-5-8-25/h3-5,7-16H,6,17-21H2,1-2H3,(H,29,32)(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of LTA4 hydrolase (unknown origin) by hydrolase assay |
Bioorg Med Chem Lett 18: 3891-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.046
BindingDB Entry DOI: 10.7270/Q20P0ZTV |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50251627
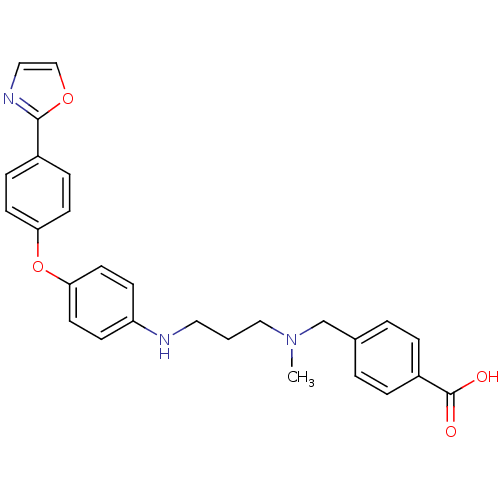
(4-((methyl(3-(4-(4-(oxazol-2-yl)phenoxy)phenylamin...)Show SMILES CN(CCCNc1ccc(Oc2ccc(cc2)-c2ncco2)cc1)Cc1ccc(cc1)C(O)=O Show InChI InChI=1S/C27H27N3O4/c1-30(19-20-3-5-22(6-4-20)27(31)32)17-2-15-28-23-9-13-25(14-10-23)34-24-11-7-21(8-12-24)26-29-16-18-33-26/h3-14,16,18,28H,2,15,17,19H2,1H3,(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of LTA4 hydrolase (unknown origin) |
Bioorg Med Chem Lett 18: 3895-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.041
BindingDB Entry DOI: 10.7270/Q2GQ6XK8 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50251665
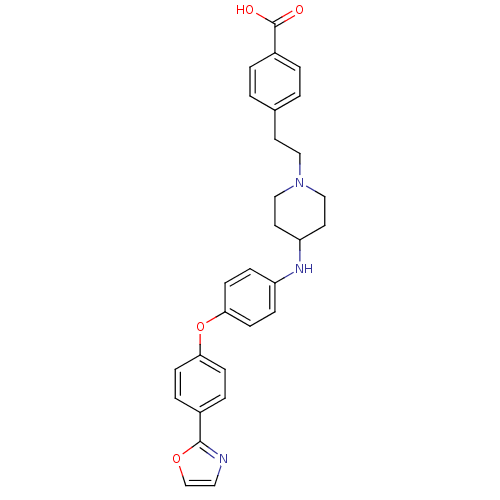
(4-(2-(4-(4-(4-(oxazol-2-yl)phenoxy)phenylamino)pip...)Show SMILES OC(=O)c1ccc(CCN2CCC(CC2)Nc2ccc(Oc3ccc(cc3)-c3ncco3)cc2)cc1 Show InChI InChI=1S/C29H29N3O4/c33-29(34)23-3-1-21(2-4-23)13-17-32-18-14-25(15-19-32)31-24-7-11-27(12-8-24)36-26-9-5-22(6-10-26)28-30-16-20-35-28/h1-12,16,20,25,31H,13-15,17-19H2,(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of LTA4 hydrolase (unknown origin) |
Bioorg Med Chem Lett 18: 3895-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.041
BindingDB Entry DOI: 10.7270/Q2GQ6XK8 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50251448

(4-(((3-((2-(4-benzylphenylamino)-2-oxoethyl)(methy...)Show SMILES CN(CCCN(C)Cc1ccc(cc1)C(O)=O)CC(=O)Nc1ccc(Cc2ccccc2)cc1 Show InChI InChI=1S/C28H33N3O3/c1-30(20-24-9-13-25(14-10-24)28(33)34)17-6-18-31(2)21-27(32)29-26-15-11-23(12-16-26)19-22-7-4-3-5-8-22/h3-5,7-16H,6,17-21H2,1-2H3,(H,29,32)(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of LTA4 hydrolase (unknown origin) by hydrolase assay |
Bioorg Med Chem Lett 18: 3891-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.046
BindingDB Entry DOI: 10.7270/Q20P0ZTV |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 1
(Homo sapiens (Human)) | BDBM50322535

(3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N...)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cnc4cccnn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-5-6-22(16-21(20)8-10-25-18-33-27-4-3-11-34-38(25)27)28(39)35-24-9-7-23(26(17-24)29(30,31)32)19-37-14-12-36(2)13-15-37/h3-7,9,11,16-18H,12-15,19H2,1-2H3,(H,35,39) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to FLT1 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.8b00081
BindingDB Entry DOI: 10.7270/Q2WM1J37 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50251805

(CHEMBL481171 | endo-4-((3-(4-(4-(1H-pyrrol-1-yl)ph...)Show SMILES OC(=O)c1ccc(CN2[C@H]3CC[C@@H]2C[C@@H](C3)Nc2ccc(Oc3ccc(cc3)-n3cccc3)cc2)cc1 |r,THB:7:8:15.14.13:10.11| Show InChI InChI=1S/C31H31N3O3/c35-31(36)23-5-3-22(4-6-23)21-34-27-9-10-28(34)20-25(19-27)32-24-7-13-29(14-8-24)37-30-15-11-26(12-16-30)33-17-1-2-18-33/h1-8,11-18,25,27-28,32H,9-10,19-21H2,(H,35,36)/t25-,27+,28- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of LTA4 hydrolase (unknown origin) |
Bioorg Med Chem Lett 18: 3895-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.041
BindingDB Entry DOI: 10.7270/Q2GQ6XK8 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50251420
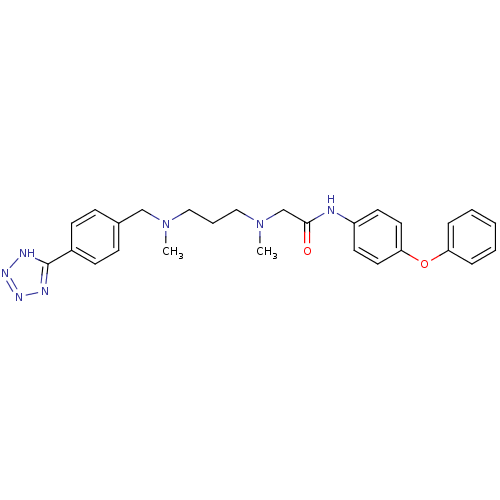
(2-((3-((4-(1H-tetrazol-5-yl)benzyl)(methyl)amino)p...)Show SMILES CN(CCCN(C)Cc1ccc(cc1)-c1nnn[nH]1)CC(=O)Nc1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C27H31N7O2/c1-33(19-21-9-11-22(12-10-21)27-29-31-32-30-27)17-6-18-34(2)20-26(35)28-23-13-15-25(16-14-23)36-24-7-4-3-5-8-24/h3-5,7-16H,6,17-20H2,1-2H3,(H,28,35)(H,29,30,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of LTA4 hydrolase (unknown origin) by hydrolase assay |
Bioorg Med Chem Lett 18: 3891-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.046
BindingDB Entry DOI: 10.7270/Q20P0ZTV |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50251743

(CHEMBL481671 | endo-4-((3-(4-(4-(oxazol-2-yl)pheno...)Show SMILES OC(=O)c1ccc(CN2[C@H]3CC[C@@H]2C[C@@H](C3)Nc2ccc(Oc3ccc(cc3)-c3ncco3)cc2)cc1 |r,THB:7:8:15.14.13:10.11| Show InChI InChI=1S/C30H29N3O4/c34-30(35)22-3-1-20(2-4-22)19-33-25-9-10-26(33)18-24(17-25)32-23-7-13-28(14-8-23)37-27-11-5-21(6-12-27)29-31-15-16-36-29/h1-8,11-16,24-26,32H,9-10,17-19H2,(H,34,35)/t24-,25+,26- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of LTA4 hydrolase (unknown origin) |
Bioorg Med Chem Lett 18: 3895-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.041
BindingDB Entry DOI: 10.7270/Q2GQ6XK8 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50251666
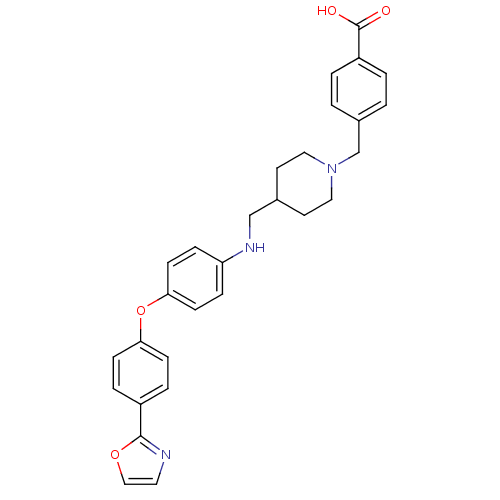
(4-((4-((4-(4-(oxazol-2-yl)phenoxy)phenylamino)meth...)Show SMILES OC(=O)c1ccc(CN2CCC(CNc3ccc(Oc4ccc(cc4)-c4ncco4)cc3)CC2)cc1 Show InChI InChI=1S/C29H29N3O4/c33-29(34)24-3-1-22(2-4-24)20-32-16-13-21(14-17-32)19-31-25-7-11-27(12-8-25)36-26-9-5-23(6-10-26)28-30-15-18-35-28/h1-12,15,18,21,31H,13-14,16-17,19-20H2,(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of LTA4 hydrolase (unknown origin) |
Bioorg Med Chem Lett 18: 3895-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.041
BindingDB Entry DOI: 10.7270/Q2GQ6XK8 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50251839
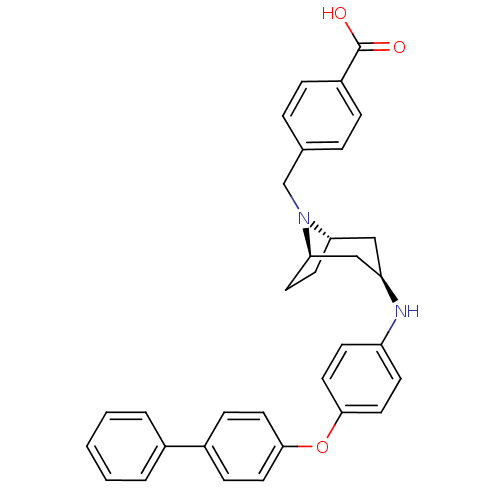
(CHEMBL480949 | endo-4-{3-[4-(Biphenyl-4-yloxy)-phe...)Show SMILES OC(=O)c1ccc(CN2[C@H]3CC[C@@H]2C[C@@H](C3)Nc2ccc(Oc3ccc(cc3)-c3ccccc3)cc2)cc1 |r,THB:7:8:15.14.13:10.11| Show InChI InChI=1S/C33H32N2O3/c36-33(37)26-8-6-23(7-9-26)22-35-29-14-15-30(35)21-28(20-29)34-27-12-18-32(19-13-27)38-31-16-10-25(11-17-31)24-4-2-1-3-5-24/h1-13,16-19,28-30,34H,14-15,20-22H2,(H,36,37)/t28-,29+,30- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of LTA4 hydrolase (unknown origin) |
Bioorg Med Chem Lett 18: 3895-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.041
BindingDB Entry DOI: 10.7270/Q2GQ6XK8 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50251473

(4-((methyl(3-(methyl(2-(4-(4-(oxazol-2-yl)phenoxy)...)Show SMILES CN(CCCN(C)Cc1ccc(cc1)C(O)=O)CC(=O)Nc1ccc(Oc2ccc(cc2)-c2ncco2)cc1 Show InChI InChI=1S/C30H32N4O5/c1-33(20-22-4-6-24(7-5-22)30(36)37)17-3-18-34(2)21-28(35)32-25-10-14-27(15-11-25)39-26-12-8-23(9-13-26)29-31-16-19-38-29/h4-16,19H,3,17-18,20-21H2,1-2H3,(H,32,35)(H,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of LTA4 hydrolase (unknown origin) by hydrolase assay |
Bioorg Med Chem Lett 18: 3891-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.046
BindingDB Entry DOI: 10.7270/Q20P0ZTV |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50322535

(3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N...)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cnc4cccnn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-5-6-22(16-21(20)8-10-25-18-33-27-4-3-11-34-38(25)27)28(39)35-24-9-7-23(26(17-24)29(30,31)32)19-37-14-12-36(2)13-15-37/h3-7,9,11,16-18H,12-15,19H2,1-2H3,(H,35,39) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human SRC using poly[Glu:Tyr] (4:1) as substrate by radiometric hotspot kinase assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.8b00081
BindingDB Entry DOI: 10.7270/Q2WM1J37 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM24285

((3S)-3-amino-5-[(4-phenoxyphenyl)carbamoyl]pentano...)Show SMILES N[C@@H](CCC(=O)Nc1ccc(Oc2ccccc2)cc1)CC(O)=O |r| Show InChI InChI=1S/C18H20N2O4/c19-13(12-18(22)23)6-11-17(21)20-14-7-9-16(10-8-14)24-15-4-2-1-3-5-15/h1-5,7-10,13H,6,11-12,19H2,(H,20,21)(H,22,23)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
| Assay Description
Compound potency against the peptidase activity of LTA4 hydrolase was measured by inhibition of the hydrolysis of L-alanine-p-nitroanilide to L-alani... |
Bioorg Med Chem 16: 4963-83 (2008)
Article DOI: 10.1016/j.bmc.2008.03.042
BindingDB Entry DOI: 10.7270/Q2BP013C |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50251828

(2-fluoro-4-((methyl(3-(methyl(2-oxo-2-(4-phenoxyph...)Show SMILES CN(CCCN(C)Cc1ccc(C(O)=O)c(F)c1)CC(=O)Nc1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C27H30FN3O4/c1-30(18-20-9-14-24(27(33)34)25(28)17-20)15-6-16-31(2)19-26(32)29-21-10-12-23(13-11-21)35-22-7-4-3-5-8-22/h3-5,7-14,17H,6,15-16,18-19H2,1-2H3,(H,29,32)(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of LTA4 hydrolase (unknown origin) by hydrolase assay |
Bioorg Med Chem Lett 18: 3891-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.046
BindingDB Entry DOI: 10.7270/Q20P0ZTV |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50251449
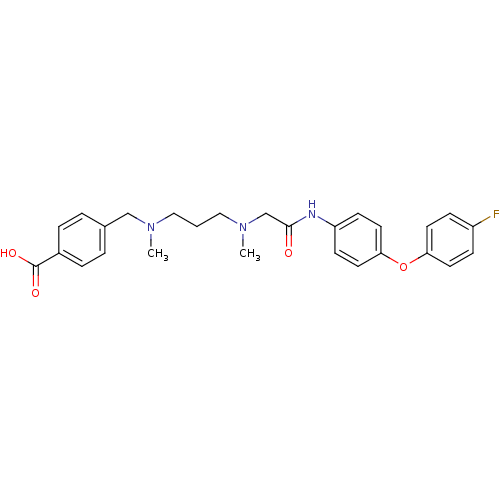
(4-(((3-((2-(4-(4-fluorophenoxy)phenylamino)-2-oxoe...)Show SMILES CN(CCCN(C)Cc1ccc(cc1)C(O)=O)CC(=O)Nc1ccc(Oc2ccc(F)cc2)cc1 Show InChI InChI=1S/C27H30FN3O4/c1-30(18-20-4-6-21(7-5-20)27(33)34)16-3-17-31(2)19-26(32)29-23-10-14-25(15-11-23)35-24-12-8-22(28)9-13-24/h4-15H,3,16-19H2,1-2H3,(H,29,32)(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of LTA4 hydrolase (unknown origin) by hydrolase assay |
Bioorg Med Chem Lett 18: 3891-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.046
BindingDB Entry DOI: 10.7270/Q20P0ZTV |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50251775
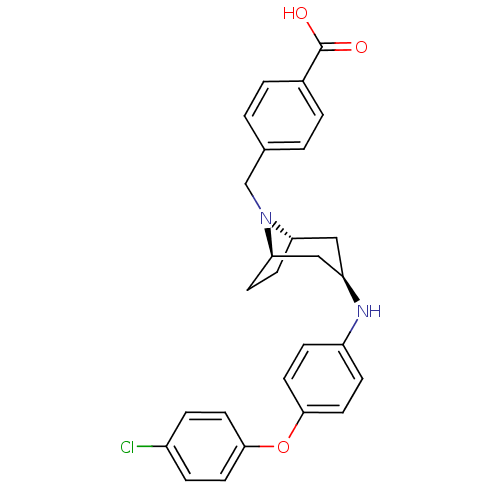
(CHEMBL480347 | endo-4-((3-(4-(4-chlorophenoxy)phen...)Show SMILES OC(=O)c1ccc(CN2[C@H]3CC[C@@H]2C[C@@H](C3)Nc2ccc(Oc3ccc(Cl)cc3)cc2)cc1 |r,THB:7:8:15.14.13:10.11| Show InChI InChI=1S/C27H27ClN2O3/c28-20-5-11-25(12-6-20)33-26-13-7-21(8-14-26)29-22-15-23-9-10-24(16-22)30(23)17-18-1-3-19(4-2-18)27(31)32/h1-8,11-14,22-24,29H,9-10,15-17H2,(H,31,32)/t22-,23+,24- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of LTA4 hydrolase (unknown origin) |
Bioorg Med Chem Lett 18: 3895-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.041
BindingDB Entry DOI: 10.7270/Q2GQ6XK8 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50251446
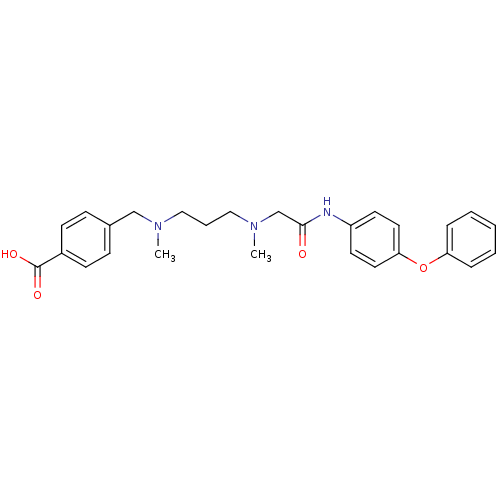
(4-((methyl(3-(methyl(2-oxo-2-(4-phenoxyphenylamino...)Show SMILES CN(CCCN(C)Cc1ccc(cc1)C(O)=O)CC(=O)Nc1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C27H31N3O4/c1-29(19-21-9-11-22(12-10-21)27(32)33)17-6-18-30(2)20-26(31)28-23-13-15-25(16-14-23)34-24-7-4-3-5-8-24/h3-5,7-16H,6,17-20H2,1-2H3,(H,28,31)(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of LTA4 hydrolase (unknown origin) by hydrolase assay |
Bioorg Med Chem Lett 18: 3891-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.046
BindingDB Entry DOI: 10.7270/Q20P0ZTV |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50251778
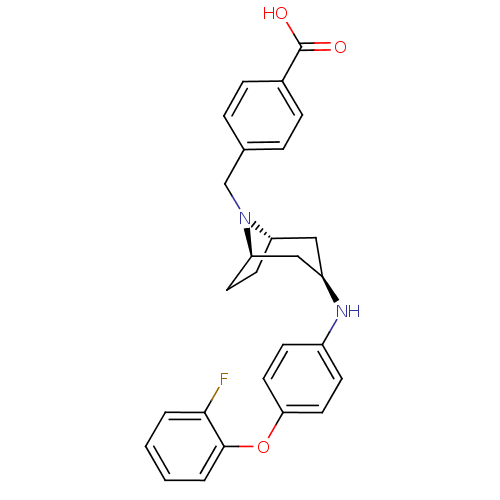
(CHEMBL471954 | endo-4-((3-(4-(2-fluorophenoxy)phen...)Show SMILES OC(=O)c1ccc(CN2[C@H]3CC[C@@H]2C[C@@H](C3)Nc2ccc(Oc3ccccc3F)cc2)cc1 |r,THB:7:8:15.14.13:10.11| Show InChI InChI=1S/C27H27FN2O3/c28-25-3-1-2-4-26(25)33-24-13-9-20(10-14-24)29-21-15-22-11-12-23(16-21)30(22)17-18-5-7-19(8-6-18)27(31)32/h1-10,13-14,21-23,29H,11-12,15-17H2,(H,31,32)/t21-,22+,23- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of LTA4 hydrolase (unknown origin) |
Bioorg Med Chem Lett 18: 3895-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.041
BindingDB Entry DOI: 10.7270/Q2GQ6XK8 |
More data for this
Ligand-Target Pair | |
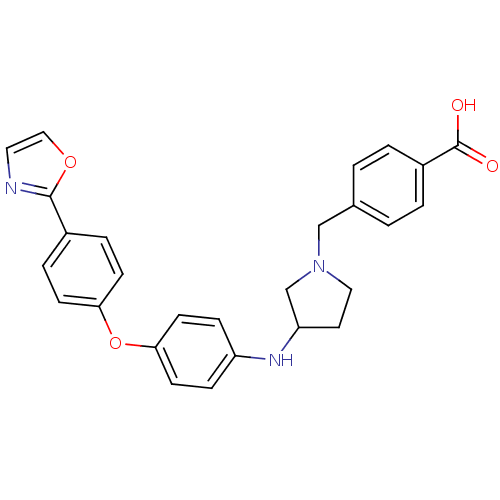
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50251706
(4-((3-(4-(4-(oxazol-2-yl)phenoxy)phenylamino)pyrro...)Show SMILES OC(=O)c1ccc(CN2CCC(C2)Nc2ccc(Oc3ccc(cc3)-c3ncco3)cc2)cc1 Show InChI InChI=1S/C27H25N3O4/c31-27(32)21-3-1-19(2-4-21)17-30-15-13-23(18-30)29-22-7-11-25(12-8-22)34-24-9-5-20(6-10-24)26-28-14-16-33-26/h1-12,14,16,23,29H,13,15,17-18H2,(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of LTA4 hydrolase (unknown origin) |
Bioorg Med Chem Lett 18: 3895-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.041
BindingDB Entry DOI: 10.7270/Q2GQ6XK8 |
More data for this
Ligand-Target Pair | |
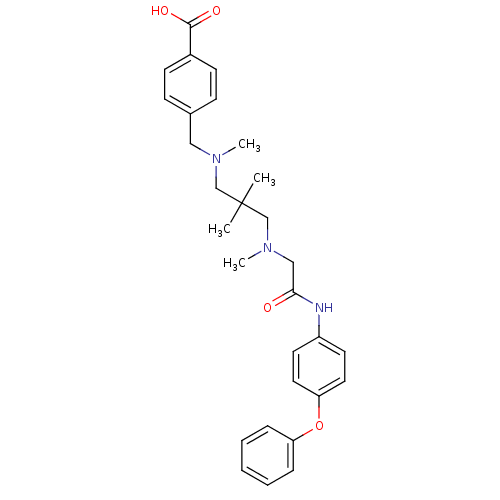
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50251500
(4-(((2,2-dimethyl-3-(methyl(2-oxo-2-(4-phenoxyphen...)Show SMILES CN(CC(=O)Nc1ccc(Oc2ccccc2)cc1)CC(C)(C)CN(C)Cc1ccc(cc1)C(O)=O Show InChI InChI=1S/C29H35N3O4/c1-29(2,20-31(3)18-22-10-12-23(13-11-22)28(34)35)21-32(4)19-27(33)30-24-14-16-26(17-15-24)36-25-8-6-5-7-9-25/h5-17H,18-21H2,1-4H3,(H,30,33)(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of LTA4 hydrolase (unknown origin) by hydrolase assay |
Bioorg Med Chem Lett 18: 3891-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.046
BindingDB Entry DOI: 10.7270/Q20P0ZTV |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50251826

(3-fluoro-4-((methyl(3-(methyl(2-oxo-2-(4-phenoxyph...)Show SMILES CN(CCCN(C)Cc1ccc(cc1F)C(O)=O)CC(=O)Nc1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C27H30FN3O4/c1-30(18-21-10-9-20(27(33)34)17-25(21)28)15-6-16-31(2)19-26(32)29-22-11-13-24(14-12-22)35-23-7-4-3-5-8-23/h3-5,7-14,17H,6,15-16,18-19H2,1-2H3,(H,29,32)(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of LTA4 hydrolase (unknown origin) by hydrolase assay |
Bioorg Med Chem Lett 18: 3891-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.046
BindingDB Entry DOI: 10.7270/Q20P0ZTV |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50251587

((R)-4-((2-((methyl(2-oxo-2-(4-phenoxyphenylamino)e...)Show SMILES CN(C[C@H]1CCCN1Cc1ccc(cc1)C(O)=O)CC(=O)Nc1ccc(Oc2ccccc2)cc1 |r| Show InChI InChI=1S/C28H31N3O4/c1-30(19-24-6-5-17-31(24)18-21-9-11-22(12-10-21)28(33)34)20-27(32)29-23-13-15-26(16-14-23)35-25-7-3-2-4-8-25/h2-4,7-16,24H,5-6,17-20H2,1H3,(H,29,32)(H,33,34)/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of LTA4 hydrolase (unknown origin) by hydrolase assay |
Bioorg Med Chem Lett 18: 3891-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.046
BindingDB Entry DOI: 10.7270/Q20P0ZTV |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 1
(Homo sapiens (Human)) | BDBM345818

(US10202362, Compound T2.1)Show SMILES OC(=O)C1CCN(CC1)C(=O)CN1CCC(CC1)Nc1ccc(Oc2ccc(cc2)-c2ncco2)cc1 Show InChI InChI=1S/C28H32N4O5/c33-26(32-16-9-21(10-17-32)28(34)35)19-31-14-11-23(12-15-31)30-22-3-7-25(8-4-22)37-24-5-1-20(2-6-24)27-29-13-18-36-27/h1-8,13,18,21,23,30H,9-12,14-17,19H2,(H,34,35) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Celtaxsys, Inc.
US Patent
| Assay Description
In brief, the enzyme (29 nM) was incubated with L-alanine-p-nitroanilide (1 mM) in 50 mM HEPES (pH 7.5), 100 mM KCL, 1% DMSO in the absence or presen... |
US Patent US10202362 (2019)
BindingDB Entry DOI: 10.7270/Q2MC9241 |
More data for this
Ligand-Target Pair | |
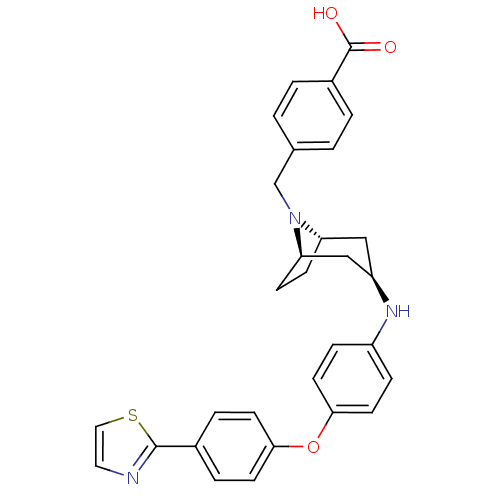
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50251838
(CHEMBL481531 | endo-4-((3-(4-(4-(thiazol-2-yl)phen...)Show SMILES OC(=O)c1ccc(CN2[C@H]3CC[C@@H]2C[C@@H](C3)Nc2ccc(Oc3ccc(cc3)-c3nccs3)cc2)cc1 |r,THB:7:8:15.14.13:10.11| Show InChI InChI=1S/C30H29N3O3S/c34-30(35)22-3-1-20(2-4-22)19-33-25-9-10-26(33)18-24(17-25)32-23-7-13-28(14-8-23)36-27-11-5-21(6-12-27)29-31-15-16-37-29/h1-8,11-16,24-26,32H,9-10,17-19H2,(H,34,35)/t24-,25+,26- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of LTA4 hydrolase (unknown origin) |
Bioorg Med Chem Lett 18: 3895-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.041
BindingDB Entry DOI: 10.7270/Q2GQ6XK8 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50251840
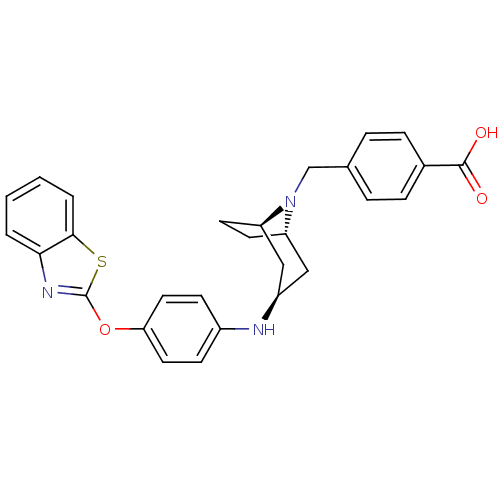
(CHEMBL481110 | endo-4-((3-(4-(benzo[d]thiazol-2-yl...)Show SMILES OC(=O)c1ccc(CN2[C@H]3CC[C@@H]2C[C@@H](C3)Nc2ccc(Oc3nc4ccccc4s3)cc2)cc1 |r,THB:7:8:15.14.13:10.11| Show InChI InChI=1S/C28H27N3O3S/c32-27(33)19-7-5-18(6-8-19)17-31-22-11-12-23(31)16-21(15-22)29-20-9-13-24(14-10-20)34-28-30-25-3-1-2-4-26(25)35-28/h1-10,13-14,21-23,29H,11-12,15-17H2,(H,32,33)/t21-,22+,23- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of LTA4 hydrolase (unknown origin) |
Bioorg Med Chem Lett 18: 3895-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.041
BindingDB Entry DOI: 10.7270/Q2GQ6XK8 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50251668
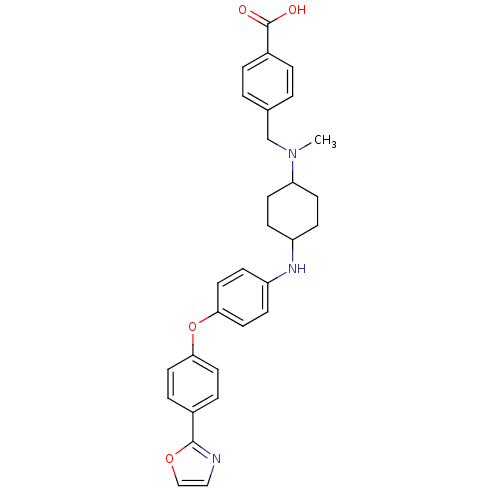
(4-((methyl(4-(4-(4-(oxazol-2-yl)phenoxy)phenylamin...)Show SMILES CN(Cc1ccc(cc1)C(O)=O)C1CCC(CC1)Nc1ccc(Oc2ccc(cc2)-c2ncco2)cc1 |(36.8,-1.72,;36.79,-.18,;38.13,.59,;39.48,-.18,;39.49,-1.72,;40.83,-2.47,;42.15,-1.69,;42.13,-.15,;40.79,.6,;43.5,-2.45,;43.51,-3.99,;44.81,-1.67,;35.45,.58,;34.14,-.2,;32.8,.56,;32.79,2.1,;34.11,2.88,;35.45,2.13,;31.44,2.88,;30.1,2.1,;30.11,.56,;28.79,-.22,;27.43,.56,;26.07,-.2,;24.74,.6,;23.4,-.16,;22.07,.62,;22.1,2.17,;23.44,2.92,;24.76,2.15,;20.78,2.96,;19.33,2.42,;18.38,3.62,;19.24,4.91,;20.71,4.49,;27.43,2.1,;28.76,2.88,)| Show InChI InChI=1S/C30H31N3O4/c1-33(20-21-2-4-23(5-3-21)30(34)35)26-12-8-24(9-13-26)32-25-10-16-28(17-11-25)37-27-14-6-22(7-15-27)29-31-18-19-36-29/h2-7,10-11,14-19,24,26,32H,8-9,12-13,20H2,1H3,(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of LTA4 hydrolase (unknown origin) |
Bioorg Med Chem Lett 18: 3895-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.041
BindingDB Entry DOI: 10.7270/Q2GQ6XK8 |
More data for this
Ligand-Target Pair | |
Epithelial discoidin domain-containing receptor 1
(Homo sapiens (Human)) | BDBM50322535

(3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N...)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cnc4cccnn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-5-6-22(16-21(20)8-10-25-18-33-27-4-3-11-34-38(25)27)28(39)35-24-9-7-23(26(17-24)29(30,31)32)19-37-14-12-36(2)13-15-37/h3-7,9,11,16-18H,12-15,19H2,1-2H3,(H,35,39) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to DDR1 (unknown origin) at 5 uM |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.8b00081
BindingDB Entry DOI: 10.7270/Q2WM1J37 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50322535

(3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N...)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cnc4cccnn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-5-6-22(16-21(20)8-10-25-18-33-27-4-3-11-34-38(25)27)28(39)35-24-9-7-23(26(17-24)29(30,31)32)19-37-14-12-36(2)13-15-37/h3-7,9,11,16-18H,12-15,19H2,1-2H3,(H,35,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to MAPK14 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.8b00081
BindingDB Entry DOI: 10.7270/Q2WM1J37 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50251384

(2-bromo-4-((methyl(3-(methyl(2-oxo-2-(4-phenoxyphe...)Show SMILES CN(CCCN(C)Cc1ccc(C(O)=O)c(Br)c1)CC(=O)Nc1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C27H30BrN3O4/c1-30(18-20-9-14-24(27(33)34)25(28)17-20)15-6-16-31(2)19-26(32)29-21-10-12-23(13-11-21)35-22-7-4-3-5-8-22/h3-5,7-14,17H,6,15-16,18-19H2,1-2H3,(H,29,32)(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of LTA4 hydrolase (unknown origin) by hydrolase assay |
Bioorg Med Chem Lett 18: 3891-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.046
BindingDB Entry DOI: 10.7270/Q20P0ZTV |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50251667
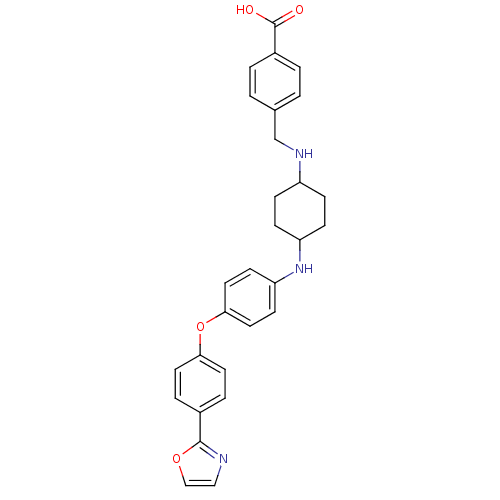
(4-((4-(4-(4-(oxazol-2-yl)phenoxy)phenylamino)cyclo...)Show SMILES OC(=O)c1ccc(CNC2CCC(CC2)Nc2ccc(Oc3ccc(cc3)-c3ncco3)cc2)cc1 |(15.61,-3.03,;15.6,-1.48,;16.91,-.7,;14.25,-.72,;12.93,-1.51,;11.59,-.75,;11.58,.78,;10.23,1.56,;8.89,.78,;7.55,1.55,;6.24,.76,;4.9,1.52,;4.89,3.07,;6.21,3.85,;7.55,3.1,;3.54,3.85,;2.2,3.07,;2.21,1.53,;.89,.75,;-.48,1.53,;-1.83,.77,;-3.16,1.57,;-4.5,.81,;-5.83,1.59,;-5.8,3.13,;-4.46,3.89,;-3.14,3.11,;-7.12,3.92,;-8.57,3.38,;-9.52,4.58,;-8.66,5.87,;-7.19,5.46,;-.47,3.07,;.86,3.85,;12.89,1.57,;14.23,.82,)| Show InChI InChI=1S/C29H29N3O4/c33-29(34)22-3-1-20(2-4-22)19-31-23-7-9-24(10-8-23)32-25-11-15-27(16-12-25)36-26-13-5-21(6-14-26)28-30-17-18-35-28/h1-6,11-18,23-24,31-32H,7-10,19H2,(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of LTA4 hydrolase (unknown origin) |
Bioorg Med Chem Lett 18: 3895-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.041
BindingDB Entry DOI: 10.7270/Q2GQ6XK8 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50251804
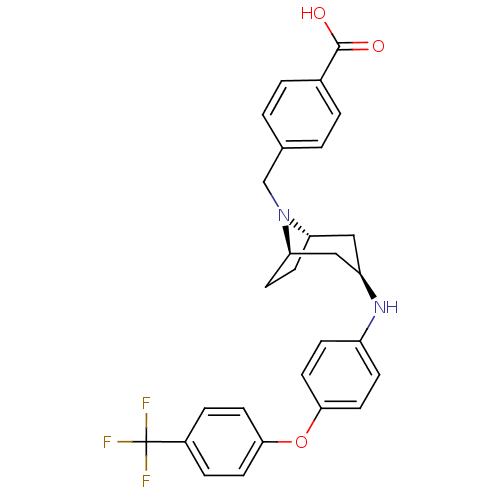
(CHEMBL481165 | endo-4-((3-(4-(4-(trifluoromethyl)p...)Show SMILES OC(=O)c1ccc(CN2[C@H]3CC[C@@H]2C[C@@H](C3)Nc2ccc(Oc3ccc(cc3)C(F)(F)F)cc2)cc1 |r,THB:7:8:15.14.13:10.11| Show InChI InChI=1S/C28H27F3N2O3/c29-28(30,31)20-5-11-25(12-6-20)36-26-13-7-21(8-14-26)32-22-15-23-9-10-24(16-22)33(23)17-18-1-3-19(4-2-18)27(34)35/h1-8,11-14,22-24,32H,9-10,15-17H2,(H,34,35)/t22-,23+,24- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of LTA4 hydrolase (unknown origin) |
Bioorg Med Chem Lett 18: 3895-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.041
BindingDB Entry DOI: 10.7270/Q2GQ6XK8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase CSK
(Homo sapiens (Human)) | BDBM50322535

(3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N...)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cnc4cccnn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-5-6-22(16-21(20)8-10-25-18-33-27-4-3-11-34-38(25)27)28(39)35-24-9-7-23(26(17-24)29(30,31)32)19-37-14-12-36(2)13-15-37/h3-7,9,11,16-18H,12-15,19H2,1-2H3,(H,35,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human CSK using poly[Glu:Tyr] (4:1) as substrate by radiometric hotspot kinase assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.8b00081
BindingDB Entry DOI: 10.7270/Q2WM1J37 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM24282
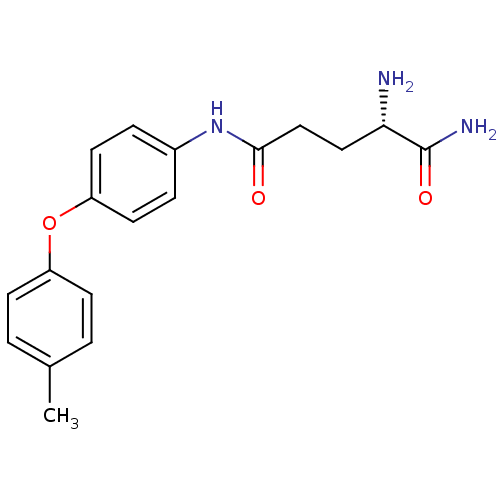
((2S)-2-amino-N-[4-(4-methylphenoxy)phenyl]pentaned...)Show SMILES Cc1ccc(Oc2ccc(NC(=O)CC[C@H](N)C(N)=O)cc2)cc1 |r| Show InChI InChI=1S/C18H21N3O3/c1-12-2-6-14(7-3-12)24-15-8-4-13(5-9-15)21-17(22)11-10-16(19)18(20)23/h2-9,16H,10-11,19H2,1H3,(H2,20,23)(H,21,22)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
| Assay Description
Compound potency against the peptidase activity of LTA4 hydrolase was measured by inhibition of the hydrolysis of L-alanine-p-nitroanilide to L-alani... |
Bioorg Med Chem 16: 4963-83 (2008)
Article DOI: 10.1016/j.bmc.2008.03.042
BindingDB Entry DOI: 10.7270/Q2BP013C |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM24291
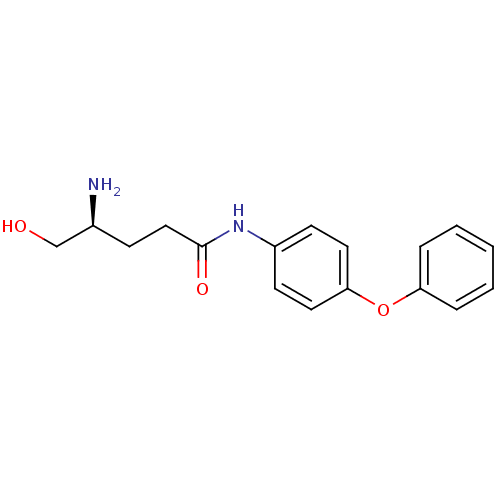
((4S)-4-amino-5-hydroxy-N-(4-phenoxyphenyl)pentanam...)Show InChI InChI=1S/C17H20N2O3/c18-13(12-20)6-11-17(21)19-14-7-9-16(10-8-14)22-15-4-2-1-3-5-15/h1-5,7-10,13,20H,6,11-12,18H2,(H,19,21)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
| Assay Description
Compound potency against the peptidase activity of LTA4 hydrolase was measured by inhibition of the hydrolysis of L-alanine-p-nitroanilide to L-alani... |
Bioorg Med Chem 16: 4963-83 (2008)
Article DOI: 10.1016/j.bmc.2008.03.042
BindingDB Entry DOI: 10.7270/Q2BP013C |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50251745

(CHEMBL520596 | endo-4-((3-(4-phenoxyphenylamino)-8...)Show SMILES OC(=O)c1ccc(CN2[C@H]3CC[C@@H]2C[C@@H](C3)Nc2ccc(Oc3ccccc3)cc2)cc1 |r,THB:7:8:15.14.13:10.11| Show InChI InChI=1S/C27H28N2O3/c30-27(31)20-8-6-19(7-9-20)18-29-23-12-13-24(29)17-22(16-23)28-21-10-14-26(15-11-21)32-25-4-2-1-3-5-25/h1-11,14-15,22-24,28H,12-13,16-18H2,(H,30,31)/t22-,23+,24- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of LTA4 hydrolase (unknown origin) |
Bioorg Med Chem Lett 18: 3895-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.041
BindingDB Entry DOI: 10.7270/Q2GQ6XK8 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50251707

(4-((4-(4-(4-(oxazol-2-yl)phenoxy)phenylamino)azepa...)Show SMILES OC(=O)c1ccc(CN2CCCC(CC2)Nc2ccc(Oc3ccc(cc3)-c3ncco3)cc2)cc1 Show InChI InChI=1S/C29H29N3O4/c33-29(34)23-5-3-21(4-6-23)20-32-17-1-2-24(15-18-32)31-25-9-13-27(14-10-25)36-26-11-7-22(8-12-26)28-30-16-19-35-28/h3-14,16,19,24,31H,1-2,15,17-18,20H2,(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of LTA4 hydrolase (unknown origin) |
Bioorg Med Chem Lett 18: 3895-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.041
BindingDB Entry DOI: 10.7270/Q2GQ6XK8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data