

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50559715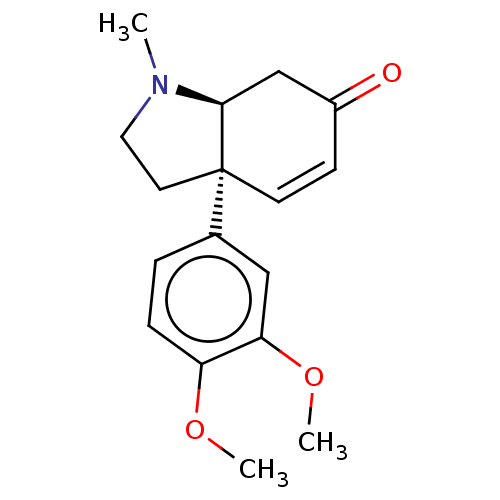 (CHEMBL4781238) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]imipramine from recombinant human 5'-HT transporter expressed in CHO cells incubated for 60 mins by Scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.9b02170 BindingDB Entry DOI: 10.7270/Q2W95DVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D (Homo sapiens (Human)) | BDBM50415001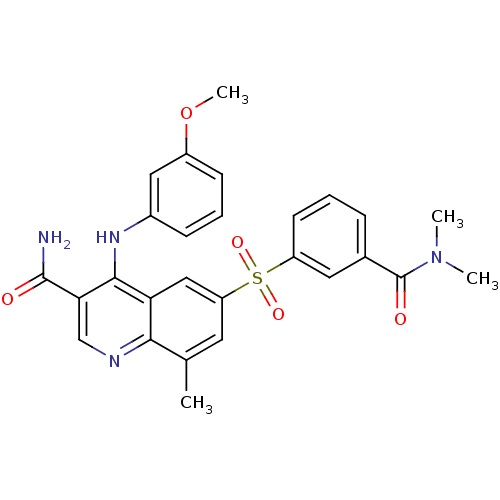 (CHEMBL570015 | GSK-256066 | GSK-256066 (3)) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PDE4 expressed in Saccharomyces cerevisiae preincubated for 30 mins followed by cAMP substrate addition | Citation and Details Article DOI: 10.1021/acs.jmedchem.9b02170 BindingDB Entry DOI: 10.7270/Q2W95DVV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50559714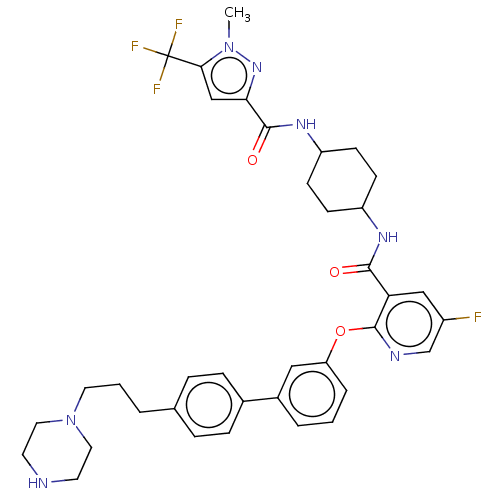 (CHEMBL4794267) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human PDE4B2 preincubated for 30 mins followed by [3H]cyclic AMP addition and measured after 20 mins by radiometric assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.9b02170 BindingDB Entry DOI: 10.7270/Q2W95DVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50559706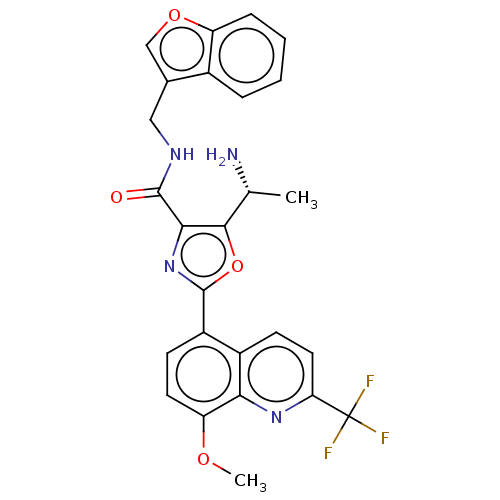 (CHEMBL4744560) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human PDE4B2 using cAMP as substrate preincubated for 15 mins followed by substrate addition and measured after 60 mins by ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.9b02170 BindingDB Entry DOI: 10.7270/Q2W95DVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM14775 (3-(cyclopentyloxy)-N-(3,5-dichloropyridin-4-yl)-4-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PDE4D by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.9b02170 BindingDB Entry DOI: 10.7270/Q2W95DVV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D (Homo sapiens (Human)) | BDBM50495704 (Chf-6001 | Chf6001 | J3.309.618F) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PDE4 in human U937 cell lysate using cAMP as substrate by LANCE assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.9b02170 BindingDB Entry DOI: 10.7270/Q2W95DVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D (Homo sapiens (Human)) | BDBM50199701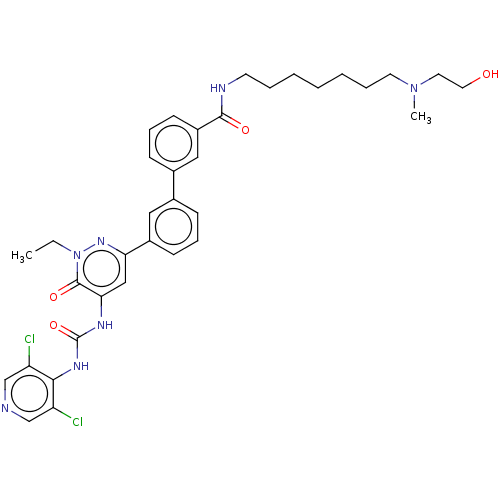 (CHEMBL3968147) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PDE4 (unknown origin) expressed in Saccharomyces cerevisiae using [3H] cAMP as substrate incubated for 1 hr by scintillation proximity ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.9b02170 BindingDB Entry DOI: 10.7270/Q2W95DVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM14775 (3-(cyclopentyloxy)-N-(3,5-dichloropyridin-4-yl)-4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.0410 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PDE4B by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.9b02170 BindingDB Entry DOI: 10.7270/Q2W95DVV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D (Homo sapiens (Human)) | BDBM50559711 (CHEMBL4800045) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PDE4 (unknown origin) expressed in Saccharomyces cerevisiae using [3H] cAMP as substrate incubated for 1 hr by scintillation proximity ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.9b02170 BindingDB Entry DOI: 10.7270/Q2W95DVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Streptococcus pneumoniae serotype 4 (strain ATCC B...) | BDBM50383996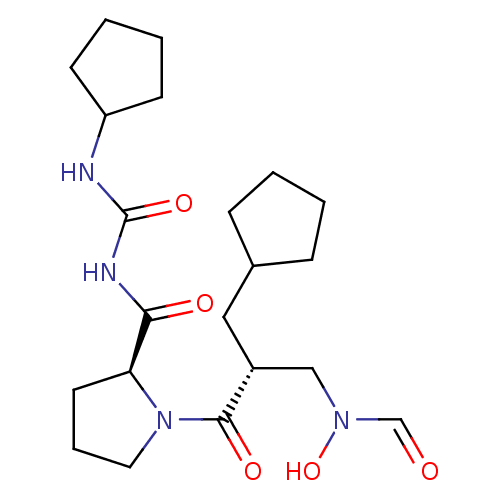 (CHEMBL2032134) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae PDF assessed as formate release from fMAS peptide substrate after 20 mins by formate dehydrogenase coupled ass... | Bioorg Med Chem Lett 22: 4028-32 (2012) Article DOI: 10.1016/j.bmcl.2012.04.086 BindingDB Entry DOI: 10.7270/Q2K64K3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Streptococcus pneumoniae serotype 4 (strain ATCC B...) | BDBM50383999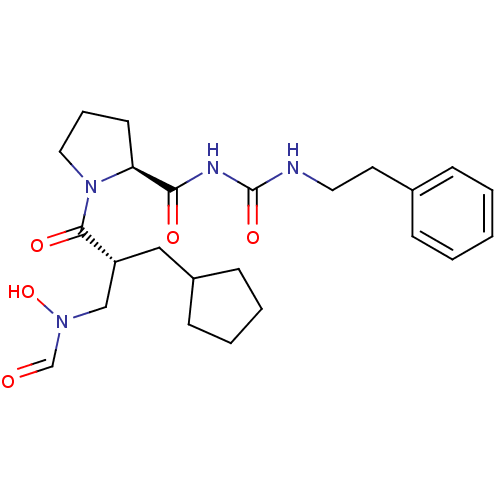 (CHEMBL2032137) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae PDF assessed as formate release from fMAS peptide substrate after 20 mins by formate dehydrogenase coupled ass... | Bioorg Med Chem Lett 22: 4028-32 (2012) Article DOI: 10.1016/j.bmcl.2012.04.086 BindingDB Entry DOI: 10.7270/Q2K64K3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50559713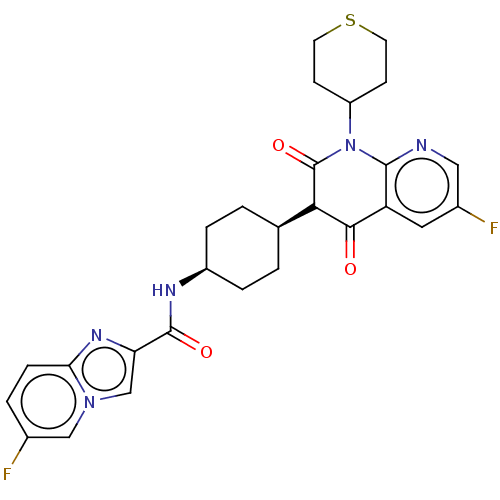 (CHEMBL4759356) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human PDE4B2 preincubated for 30 mins followed by [3H]cyclic AMP addition and measured after 20 mins by radiometric assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.9b02170 BindingDB Entry DOI: 10.7270/Q2W95DVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Streptococcus pneumoniae serotype 4 (strain ATCC B...) | BDBM50383966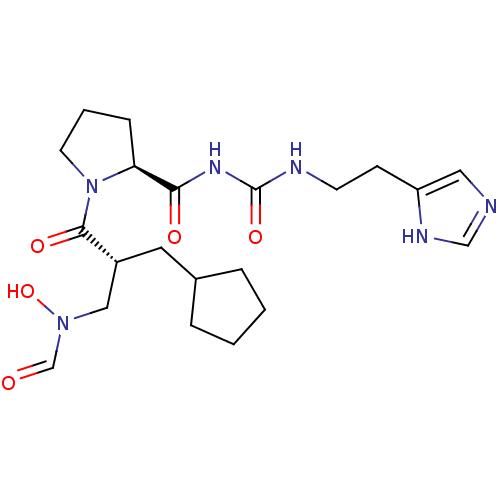 (CHEMBL2032148) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae PDF assessed as formate release from fMAS peptide substrate after 20 mins by formate dehydrogenase coupled ass... | Bioorg Med Chem Lett 22: 4028-32 (2012) Article DOI: 10.1016/j.bmcl.2012.04.086 BindingDB Entry DOI: 10.7270/Q2K64K3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Streptococcus pneumoniae serotype 4 (strain ATCC B...) | BDBM50383998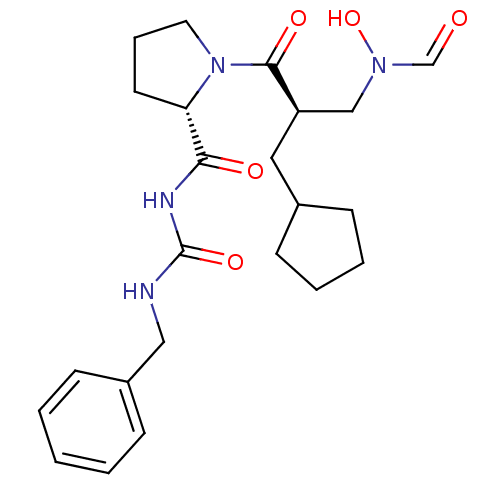 (CHEMBL2032136) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae PDF assessed as formate release from fMAS peptide substrate after 20 mins by formate dehydrogenase coupled ass... | Bioorg Med Chem Lett 22: 4028-32 (2012) Article DOI: 10.1016/j.bmcl.2012.04.086 BindingDB Entry DOI: 10.7270/Q2K64K3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Streptococcus pneumoniae serotype 4 (strain ATCC B...) | BDBM50384000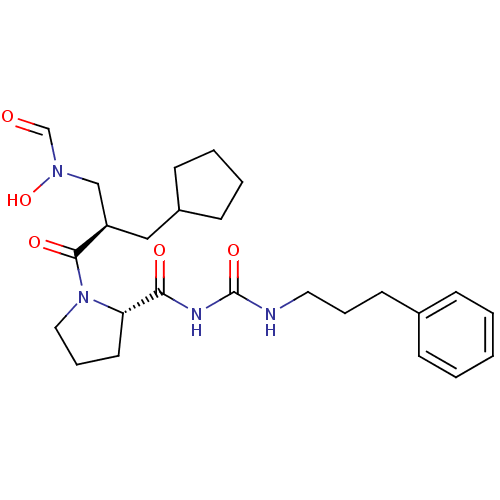 (CHEMBL2032138) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae PDF assessed as formate release from fMAS peptide substrate after 20 mins by formate dehydrogenase coupled ass... | Bioorg Med Chem Lett 22: 4028-32 (2012) Article DOI: 10.1016/j.bmcl.2012.04.086 BindingDB Entry DOI: 10.7270/Q2K64K3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50322823 ((S)-N-(4-(3-chloro-4-fluorophenylamino)-7-(tetrahy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of wild type EGFR (unknown origin) expressed in baculovirus expression system using poly (Glu, Tyr) 4:1 as substrate after 60 mins by ELIS... | Bioorg Med Chem 21: 7988-98 (2013) Article DOI: 10.1016/j.bmc.2013.09.049 BindingDB Entry DOI: 10.7270/Q2ZK5J4G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peptide deformylase (Streptococcus pneumoniae serotype 4 (strain ATCC B...) | BDBM50383997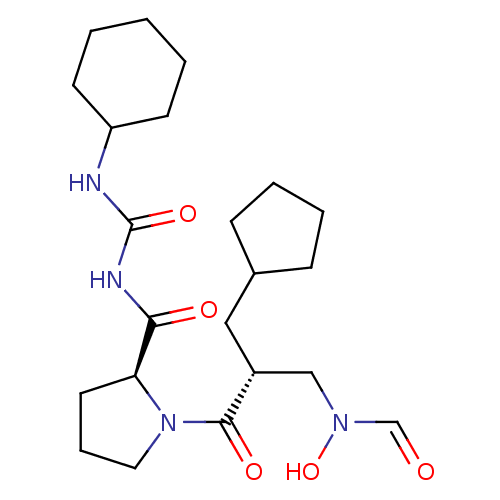 (CHEMBL2032135) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae PDF assessed as formate release from fMAS peptide substrate after 20 mins by formate dehydrogenase coupled ass... | Bioorg Med Chem Lett 22: 4028-32 (2012) Article DOI: 10.1016/j.bmcl.2012.04.086 BindingDB Entry DOI: 10.7270/Q2K64K3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D (Homo sapiens (Human)) | BDBM50559690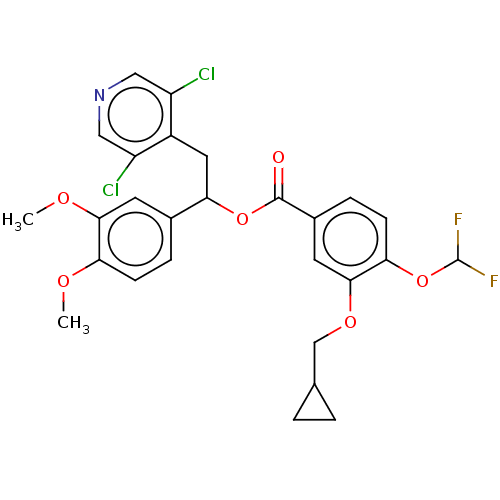 (CHEMBL4799033) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PDE4 (unknown origin) | Citation and Details Article DOI: 10.1021/acs.jmedchem.9b02170 BindingDB Entry DOI: 10.7270/Q2W95DVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Streptococcus pneumoniae serotype 4 (strain ATCC B...) | BDBM50383994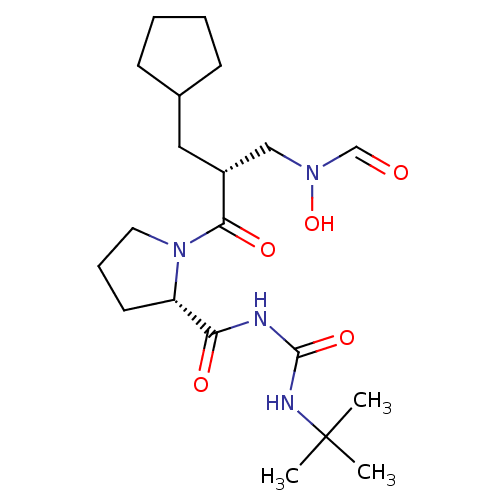 (CHEMBL2032132) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae PDF assessed as formate release from fMAS peptide substrate after 20 mins by formate dehydrogenase coupled ass... | Bioorg Med Chem Lett 22: 4028-32 (2012) Article DOI: 10.1016/j.bmcl.2012.04.086 BindingDB Entry DOI: 10.7270/Q2K64K3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Streptococcus pneumoniae serotype 4 (strain ATCC B...) | BDBM50383987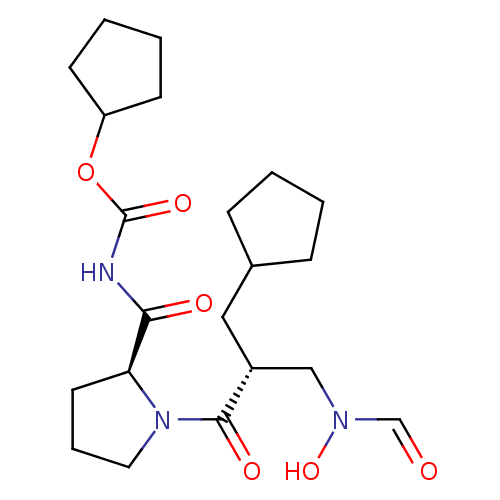 (CHEMBL2032125) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae PDF assessed as formate release from fMAS peptide substrate after 20 mins by formate dehydrogenase coupled ass... | Bioorg Med Chem Lett 22: 4028-32 (2012) Article DOI: 10.1016/j.bmcl.2012.04.086 BindingDB Entry DOI: 10.7270/Q2K64K3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Streptococcus pneumoniae serotype 4 (strain ATCC B...) | BDBM50383982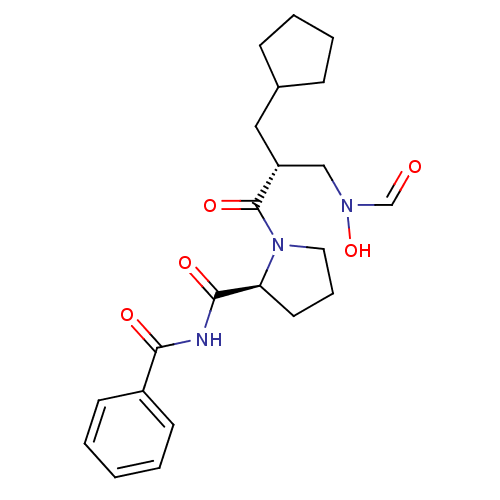 (CHEMBL2032119) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae PDF assessed as formate release from fMAS peptide substrate after 20 mins by formate dehydrogenase coupled ass... | Bioorg Med Chem Lett 22: 4028-32 (2012) Article DOI: 10.1016/j.bmcl.2012.04.086 BindingDB Entry DOI: 10.7270/Q2K64K3V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peptide deformylase (Streptococcus pneumoniae serotype 4 (strain ATCC B...) | BDBM50383995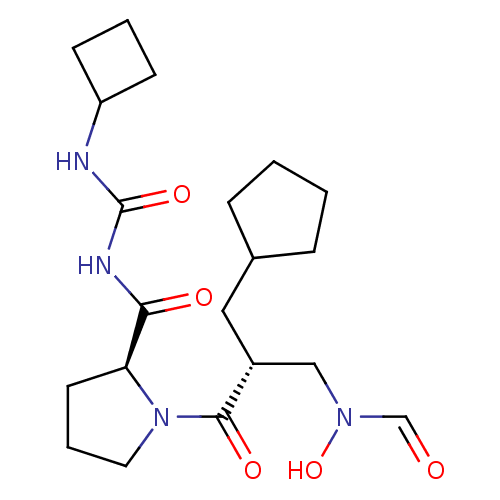 (CHEMBL2032133) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae PDF assessed as formate release from fMAS peptide substrate after 20 mins by formate dehydrogenase coupled ass... | Bioorg Med Chem Lett 22: 4028-32 (2012) Article DOI: 10.1016/j.bmcl.2012.04.086 BindingDB Entry DOI: 10.7270/Q2K64K3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Streptococcus pneumoniae serotype 4 (strain ATCC B...) | BDBM50383988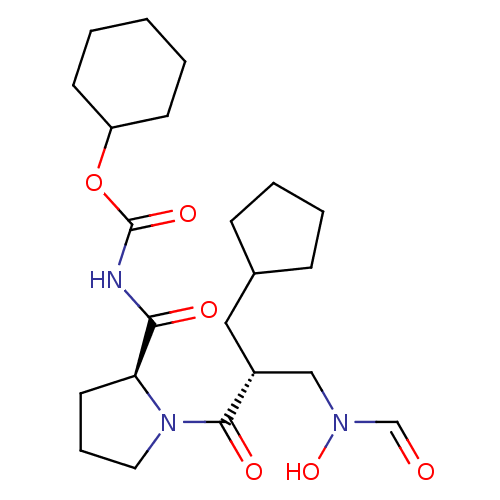 (CHEMBL2032126) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae PDF assessed as formate release from fMAS peptide substrate after 20 mins by formate dehydrogenase coupled ass... | Bioorg Med Chem Lett 22: 4028-32 (2012) Article DOI: 10.1016/j.bmcl.2012.04.086 BindingDB Entry DOI: 10.7270/Q2K64K3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Streptococcus pneumoniae serotype 4 (strain ATCC B...) | BDBM50383993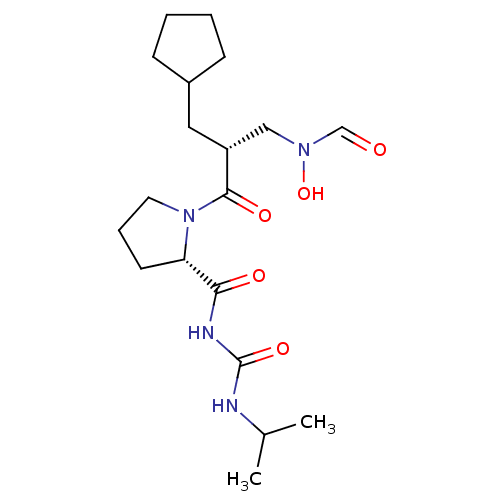 (CHEMBL2032131) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae PDF assessed as formate release from fMAS peptide substrate after 20 mins by formate dehydrogenase coupled ass... | Bioorg Med Chem Lett 22: 4028-32 (2012) Article DOI: 10.1016/j.bmcl.2012.04.086 BindingDB Entry DOI: 10.7270/Q2K64K3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Streptococcus pneumoniae serotype 4 (strain ATCC B...) | BDBM50383968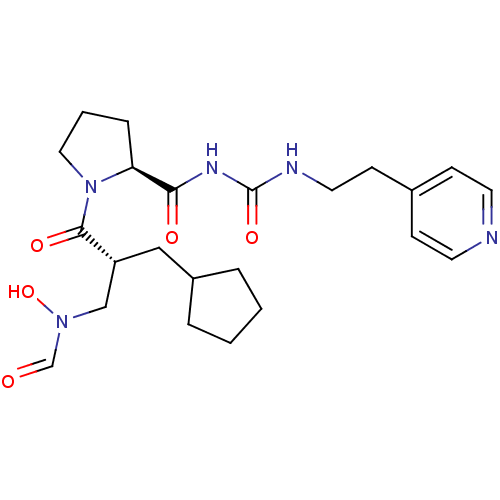 (CHEMBL2032140) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae PDF assessed as formate release from fMAS peptide substrate after 20 mins by formate dehydrogenase coupled ass... | Bioorg Med Chem Lett 22: 4028-32 (2012) Article DOI: 10.1016/j.bmcl.2012.04.086 BindingDB Entry DOI: 10.7270/Q2K64K3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Streptococcus pneumoniae serotype 4 (strain ATCC B...) | BDBM50384003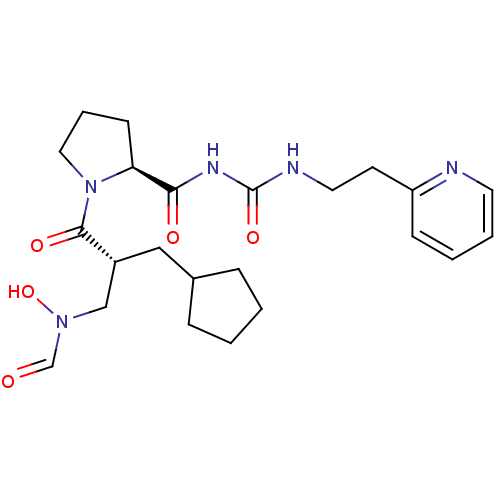 (CHEMBL2032142) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae PDF assessed as formate release from fMAS peptide substrate after 20 mins by formate dehydrogenase coupled ass... | Bioorg Med Chem Lett 22: 4028-32 (2012) Article DOI: 10.1016/j.bmcl.2012.04.086 BindingDB Entry DOI: 10.7270/Q2K64K3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D (Homo sapiens (Human)) | BDBM50559694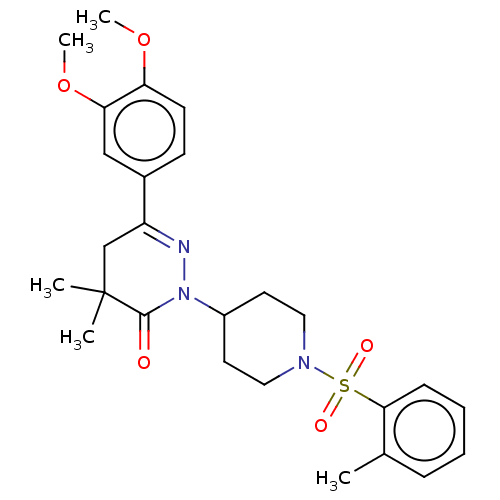 (CHEMBL4752721) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.355 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PDE4 (unknown origin) | Citation and Details Article DOI: 10.1021/acs.jmedchem.9b02170 BindingDB Entry DOI: 10.7270/Q2W95DVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Streptococcus pneumoniae serotype 4 (strain ATCC B...) | BDBM50384002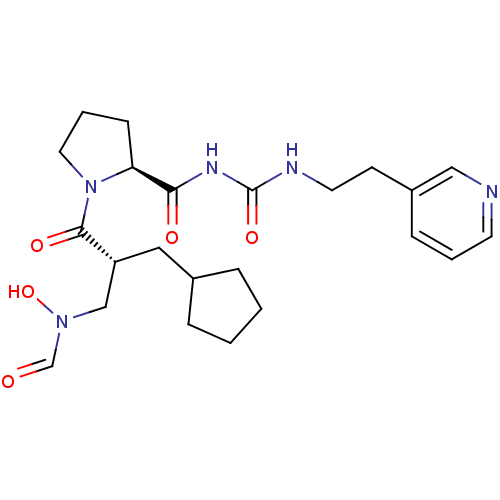 (CHEMBL2032141) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae PDF assessed as formate release from fMAS peptide substrate after 20 mins by formate dehydrogenase coupled ass... | Bioorg Med Chem Lett 22: 4028-32 (2012) Article DOI: 10.1016/j.bmcl.2012.04.086 BindingDB Entry DOI: 10.7270/Q2K64K3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM50017340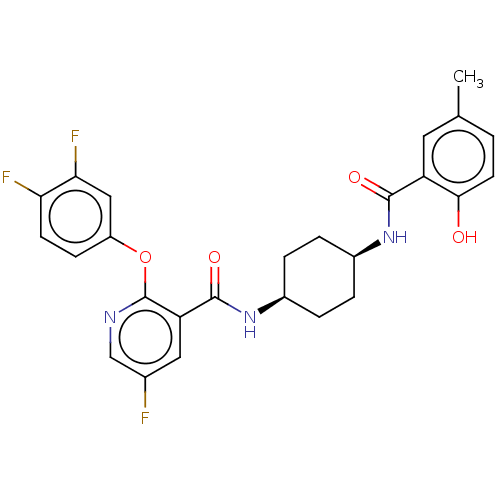 (CHEMBL3113734) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PDE4A (unknown origin) | Citation and Details Article DOI: 10.1021/acs.jmedchem.9b02170 BindingDB Entry DOI: 10.7270/Q2W95DVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50604104 (CHEMBL5182567) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114249 BindingDB Entry DOI: 10.7270/Q2Z89HHD | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Streptococcus pneumoniae serotype 4 (strain ATCC B...) | BDBM50383972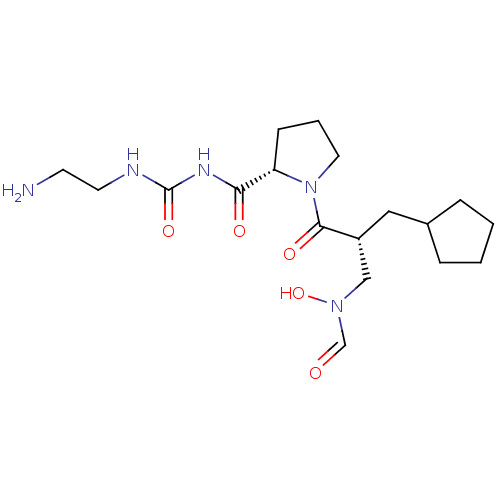 (CHEMBL2032146) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae PDF assessed as formate release from fMAS peptide substrate after 20 mins by formate dehydrogenase coupled ass... | Bioorg Med Chem Lett 22: 4028-32 (2012) Article DOI: 10.1016/j.bmcl.2012.04.086 BindingDB Entry DOI: 10.7270/Q2K64K3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50017340 (CHEMBL3113734) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PDE4B (unknown origin) | Citation and Details Article DOI: 10.1021/acs.jmedchem.9b02170 BindingDB Entry DOI: 10.7270/Q2W95DVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50017340 (CHEMBL3113734) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PDE4D (unknown origin) | Citation and Details Article DOI: 10.1021/acs.jmedchem.9b02170 BindingDB Entry DOI: 10.7270/Q2W95DVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D (Homo sapiens (Human)) | BDBM50559710 (CHEMBL4793329) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human U937 cell derived PDE4 preincubated for 15 mins followed by [3H]AMP and cAMP addition and measured after 20 mins by liquid scinti... | Citation and Details Article DOI: 10.1021/acs.jmedchem.9b02170 BindingDB Entry DOI: 10.7270/Q2W95DVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Streptococcus pneumoniae serotype 4 (strain ATCC B...) | BDBM50383969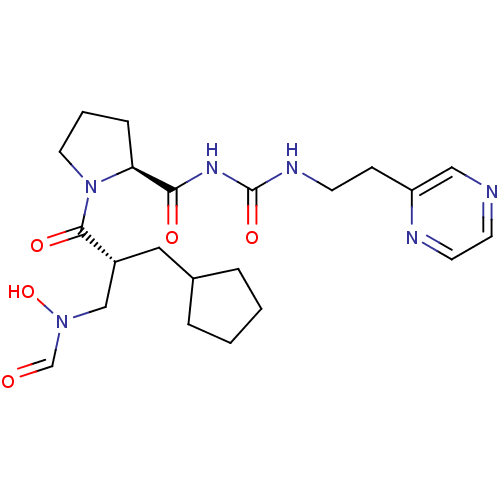 (CHEMBL2032143) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae PDF assessed as formate release from fMAS peptide substrate after 20 mins by formate dehydrogenase coupled ass... | Bioorg Med Chem Lett 22: 4028-32 (2012) Article DOI: 10.1016/j.bmcl.2012.04.086 BindingDB Entry DOI: 10.7270/Q2K64K3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Streptococcus pneumoniae serotype 4 (strain ATCC B...) | BDBM50383980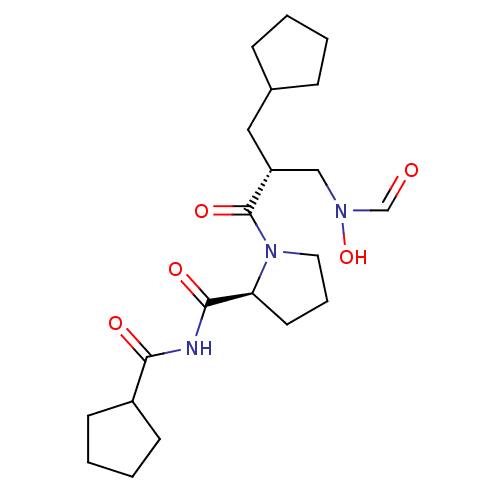 (CHEMBL2032117) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae PDF assessed as formate release from fMAS peptide substrate after 20 mins by formate dehydrogenase coupled ass... | Bioorg Med Chem Lett 22: 4028-32 (2012) Article DOI: 10.1016/j.bmcl.2012.04.086 BindingDB Entry DOI: 10.7270/Q2K64K3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Streptococcus pneumoniae serotype 4 (strain ATCC B...) | BDBM50383992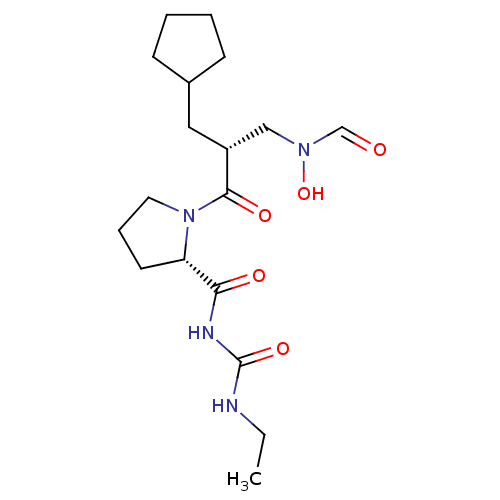 (CHEMBL2032130) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae PDF assessed as formate release from fMAS peptide substrate after 20 mins by formate dehydrogenase coupled ass... | Bioorg Med Chem Lett 22: 4028-32 (2012) Article DOI: 10.1016/j.bmcl.2012.04.086 BindingDB Entry DOI: 10.7270/Q2K64K3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Streptococcus pneumoniae serotype 4 (strain ATCC B...) | BDBM50383965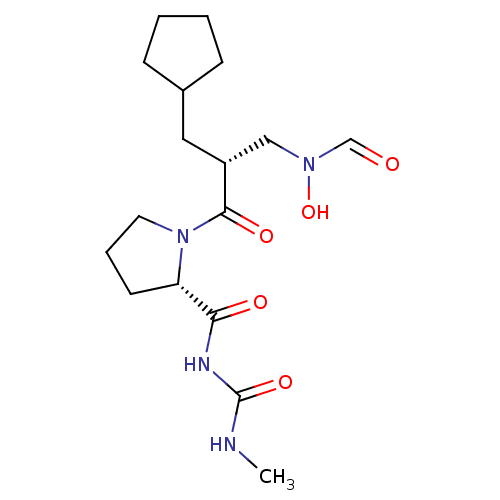 (CHEMBL2032129) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae PDF assessed as formate release from fMAS peptide substrate after 20 mins by formate dehydrogenase coupled ass... | Bioorg Med Chem Lett 22: 4028-32 (2012) Article DOI: 10.1016/j.bmcl.2012.04.086 BindingDB Entry DOI: 10.7270/Q2K64K3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Streptococcus pneumoniae serotype 4 (strain ATCC B...) | BDBM50383967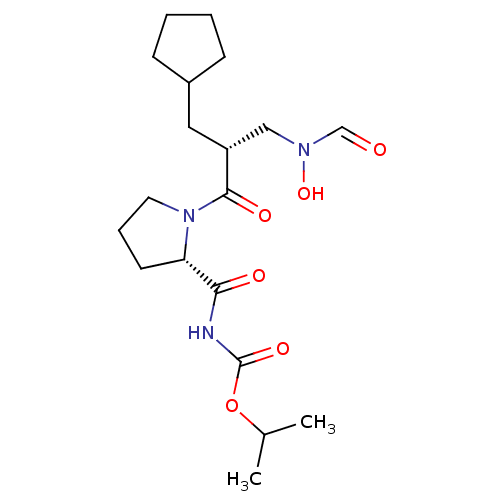 (CHEMBL2032122) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae PDF assessed as formate release from fMAS peptide substrate after 20 mins by formate dehydrogenase coupled ass... | Bioorg Med Chem Lett 22: 4028-32 (2012) Article DOI: 10.1016/j.bmcl.2012.04.086 BindingDB Entry DOI: 10.7270/Q2K64K3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Streptococcus pneumoniae serotype 4 (strain ATCC B...) | BDBM50383981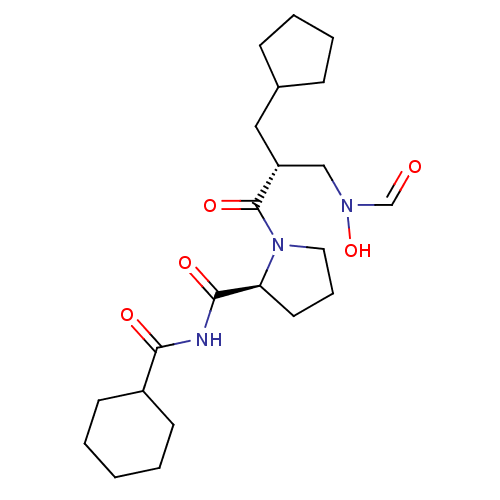 (CHEMBL2032118) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae PDF assessed as formate release from fMAS peptide substrate after 20 mins by formate dehydrogenase coupled ass... | Bioorg Med Chem Lett 22: 4028-32 (2012) Article DOI: 10.1016/j.bmcl.2012.04.086 BindingDB Entry DOI: 10.7270/Q2K64K3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Streptococcus pneumoniae serotype 4 (strain ATCC B...) | BDBM50383986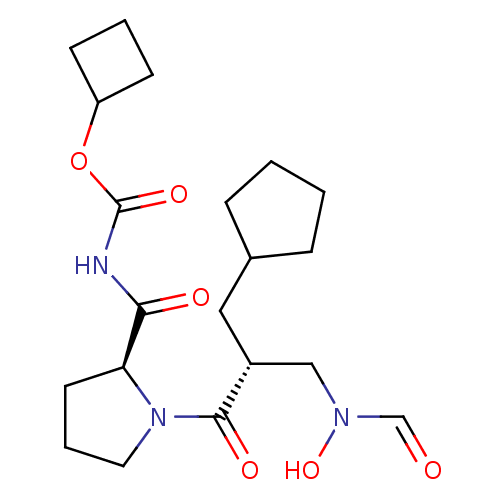 (CHEMBL2032124) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae PDF assessed as formate release from fMAS peptide substrate after 20 mins by formate dehydrogenase coupled ass... | Bioorg Med Chem Lett 22: 4028-32 (2012) Article DOI: 10.1016/j.bmcl.2012.04.086 BindingDB Entry DOI: 10.7270/Q2K64K3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Staphylococcus aureus) | BDBM50383996 (CHEMBL2032134) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus PDF assessed as formate release from fMAS peptide substrate after 20 mins by formate dehydrogenase coupled assay | Bioorg Med Chem Lett 22: 4028-32 (2012) Article DOI: 10.1016/j.bmcl.2012.04.086 BindingDB Entry DOI: 10.7270/Q2K64K3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Streptococcus pneumoniae serotype 4 (strain ATCC B...) | BDBM50383985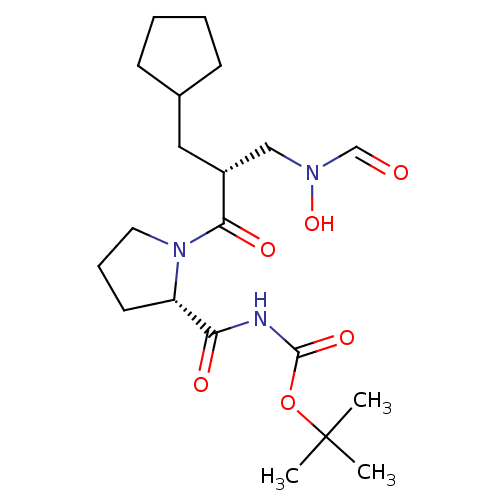 (CHEMBL2032123) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae PDF assessed as formate release from fMAS peptide substrate after 20 mins by formate dehydrogenase coupled ass... | Bioorg Med Chem Lett 22: 4028-32 (2012) Article DOI: 10.1016/j.bmcl.2012.04.086 BindingDB Entry DOI: 10.7270/Q2K64K3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Streptococcus pneumoniae serotype 4 (strain ATCC B...) | BDBM50383984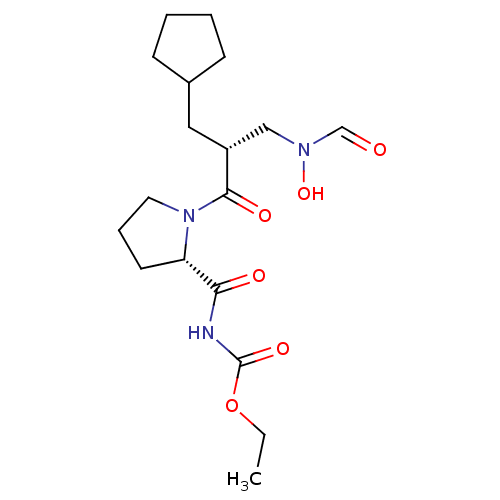 (CHEMBL2032121) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae PDF assessed as formate release from fMAS peptide substrate after 20 mins by formate dehydrogenase coupled ass... | Bioorg Med Chem Lett 22: 4028-32 (2012) Article DOI: 10.1016/j.bmcl.2012.04.086 BindingDB Entry DOI: 10.7270/Q2K64K3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D (Homo sapiens (Human)) | BDBM50347348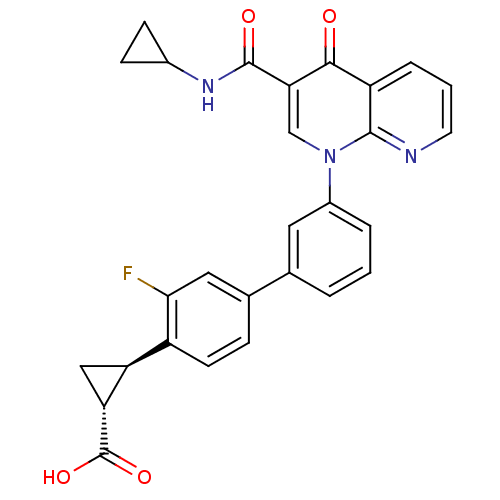 (CHEMBL1801160) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PDE4 (unknown origin) | Citation and Details Article DOI: 10.1021/acs.jmedchem.9b02170 BindingDB Entry DOI: 10.7270/Q2W95DVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50604114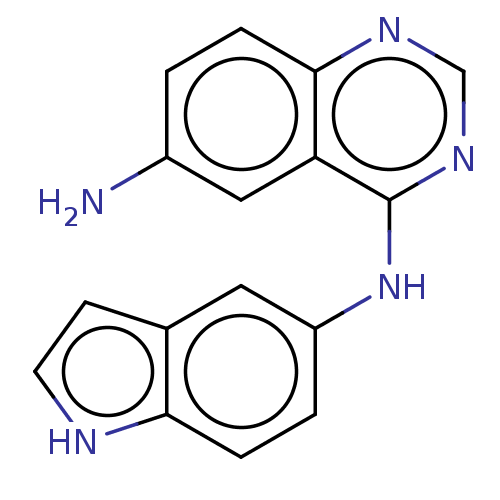 (CHEMBL5180251) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114249 BindingDB Entry DOI: 10.7270/Q2Z89HHD | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Streptococcus pneumoniae serotype 4 (strain ATCC B...) | BDBM50383989 (CHEMBL2032127) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae PDF assessed as formate release from fMAS peptide substrate after 20 mins by formate dehydrogenase coupled ass... | Bioorg Med Chem Lett 22: 4028-32 (2012) Article DOI: 10.1016/j.bmcl.2012.04.086 BindingDB Entry DOI: 10.7270/Q2K64K3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50322823 ((S)-N-(4-(3-chloro-4-fluorophenylamino)-7-(tetrahy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114249 BindingDB Entry DOI: 10.7270/Q2Z89HHD | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM14774 (3-(cyclopropylmethoxy)-N-(3,5-dichloropyridin-4-yl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PDE4D by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.9b02170 BindingDB Entry DOI: 10.7270/Q2W95DVV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peptide deformylase (Streptococcus pneumoniae serotype 4 (strain ATCC B...) | BDBM50383978 (CHEMBL2032115) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae PDF assessed as formate release from fMAS peptide substrate after 20 mins by formate dehydrogenase coupled ass... | Bioorg Med Chem Lett 22: 4028-32 (2012) Article DOI: 10.1016/j.bmcl.2012.04.086 BindingDB Entry DOI: 10.7270/Q2K64K3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 634 total ) | Next | Last >> |