

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50126143 (Epacadostat | INCB-024360) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Inhibition of IDO1 in IFNgamma-stimulated human A375 cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysis | J Med Chem 63: 3047-3065 (2020) Article DOI: 10.1021/acs.jmedchem.9b01809 BindingDB Entry DOI: 10.7270/Q29K4FKS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM50514760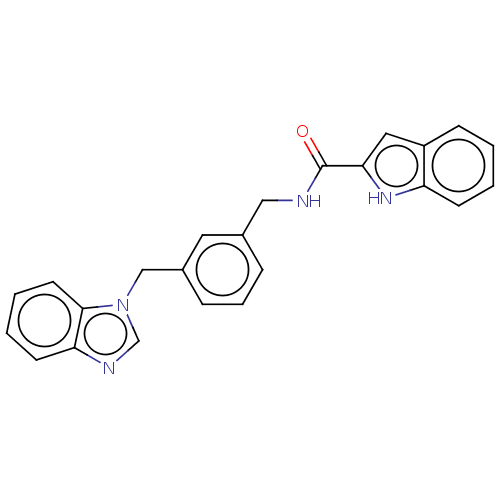 (CHEMBL4448402) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Inhibition of mouse IDO1 transfected in P815 cells assessed as reduction in L-Kyn level measured after 16 hrs by HPLC analysis | J Med Chem 63: 3047-3065 (2020) Article DOI: 10.1021/acs.jmedchem.9b01809 BindingDB Entry DOI: 10.7270/Q29K4FKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50126143 (Epacadostat | INCB-024360) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO1 assessed as reduction in kynurenine production using L-tryptophan incubated for 60 mins by Ehrlich's reagent bas... | Bioorg Med Chem Lett 28: 651-657 (2018) Article DOI: 10.1016/j.bmcl.2018.01.032 BindingDB Entry DOI: 10.7270/Q24T6MZD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM50514753 (CHEMBL4557994) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Inhibition of mouse IDO1 transfected in P815 cells assessed as reduction in L-Kyn level measured after 16 hrs by HPLC analysis | J Med Chem 63: 3047-3065 (2020) Article DOI: 10.1021/acs.jmedchem.9b01809 BindingDB Entry DOI: 10.7270/Q29K4FKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50514753 (CHEMBL4557994) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Inhibition of IDO1 in IFNgamma-stimulated human LXF-289 cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysis | J Med Chem 63: 3047-3065 (2020) Article DOI: 10.1021/acs.jmedchem.9b01809 BindingDB Entry DOI: 10.7270/Q29K4FKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50514753 (CHEMBL4557994) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Inhibition of IDO1 in IFNgamma-stimulated human A375 cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysis | J Med Chem 63: 3047-3065 (2020) Article DOI: 10.1021/acs.jmedchem.9b01809 BindingDB Entry DOI: 10.7270/Q29K4FKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50514759 (CHEMBL4571131) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Inhibition of IDO1 in IFNgamma-stimulated human A375 cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysis | J Med Chem 63: 3047-3065 (2020) Article DOI: 10.1021/acs.jmedchem.9b01809 BindingDB Entry DOI: 10.7270/Q29K4FKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM132093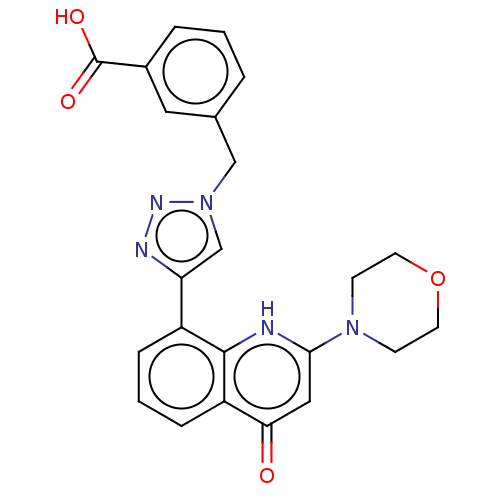 (US8841288, CL27e) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | 25 |
Universtà Degli Studi di Torino; Università Degli Studi del Piemonte Orientale “Amedeo Avogadro” US Patent | Assay Description Some compounds of formula (I) described herein were assayed in vitro for their ability to inhibit the lipid kinase activity of PI3Ks, by using a non-... | US Patent US8841288 (2014) BindingDB Entry DOI: 10.7270/Q2RJ4H5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50514753 (CHEMBL4557994) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Inhibition of IDO1 in IFNgamma-stimulated human MCF7 cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysis | J Med Chem 63: 3047-3065 (2020) Article DOI: 10.1021/acs.jmedchem.9b01809 BindingDB Entry DOI: 10.7270/Q29K4FKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50514753 (CHEMBL4557994) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Inhibition of IDO1 in IFNgamma-stimulated human HepG2 cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysis | J Med Chem 63: 3047-3065 (2020) Article DOI: 10.1021/acs.jmedchem.9b01809 BindingDB Entry DOI: 10.7270/Q29K4FKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50514753 (CHEMBL4557994) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Inhibition of IDO1 in IFNgamma-stimulated human HeLa cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysis | J Med Chem 63: 3047-3065 (2020) Article DOI: 10.1021/acs.jmedchem.9b01809 BindingDB Entry DOI: 10.7270/Q29K4FKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50514760 (CHEMBL4448402) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Inhibition of IDO1 in IFNgamma-stimulated human A375 cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysis | J Med Chem 63: 3047-3065 (2020) Article DOI: 10.1021/acs.jmedchem.9b01809 BindingDB Entry DOI: 10.7270/Q29K4FKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50514760 (CHEMBL4448402) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Inhibition of IDO1 in IFNgamma-stimulated human HeLa cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysis | J Med Chem 63: 3047-3065 (2020) Article DOI: 10.1021/acs.jmedchem.9b01809 BindingDB Entry DOI: 10.7270/Q29K4FKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50030792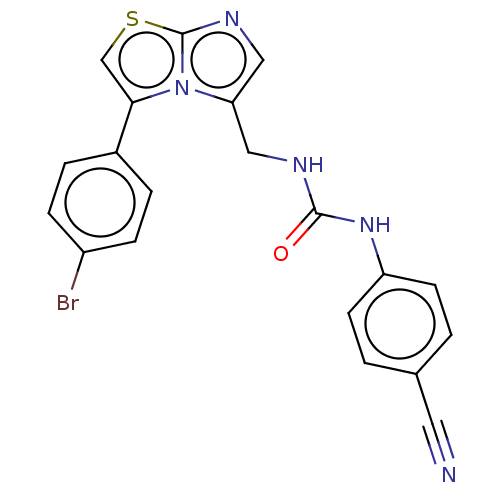 (CHEMBL3342401) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO1 assessed as reduction in kynurenine production using L-tryptophan incubated for 60 mins by Ehrlich's reagent bas... | Bioorg Med Chem Lett 28: 651-657 (2018) Article DOI: 10.1016/j.bmcl.2018.01.032 BindingDB Entry DOI: 10.7270/Q24T6MZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50514760 (CHEMBL4448402) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Inhibition of IDO1 in IFNgamma-stimulated human MCF7 cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysis | J Med Chem 63: 3047-3065 (2020) Article DOI: 10.1021/acs.jmedchem.9b01809 BindingDB Entry DOI: 10.7270/Q29K4FKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50514752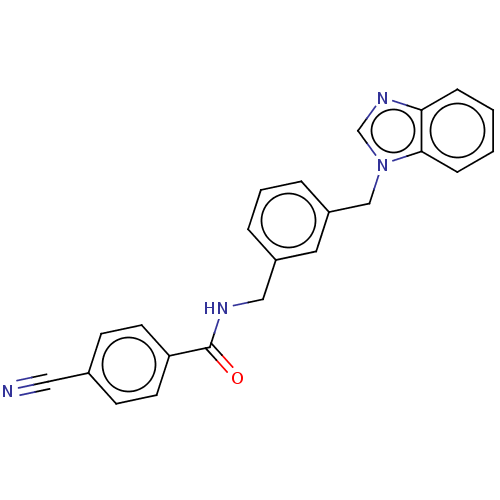 (CHEMBL4458549) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Inhibition of IDO1 in IFNgamma-stimulated human A375 cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysis | J Med Chem 63: 3047-3065 (2020) Article DOI: 10.1021/acs.jmedchem.9b01809 BindingDB Entry DOI: 10.7270/Q29K4FKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50461837 (CHEMBL4226608) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Antagonist activity at TRPV1 (unknown origin) expressed in human SH-SY5Y cells assessed as inhibition of capsaicin-induced calcium increase after 25 ... | J Med Chem 61: 4436-4455 (2018) Article DOI: 10.1021/acs.jmedchem.8b00109 BindingDB Entry DOI: 10.7270/Q2PK0JSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50514752 (CHEMBL4458549) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Inhibition of IDO1 in IFNgamma-stimulated human HeLa cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysis | J Med Chem 63: 3047-3065 (2020) Article DOI: 10.1021/acs.jmedchem.9b01809 BindingDB Entry DOI: 10.7270/Q29K4FKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM132092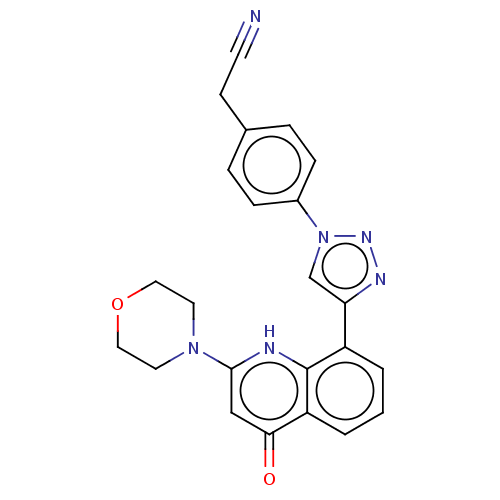 (US8841288, CL12) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | 25 |
Universtà Degli Studi di Torino; Università Degli Studi del Piemonte Orientale “Amedeo Avogadro” US Patent | Assay Description Some compounds of formula (I) described herein were assayed in vitro for their ability to inhibit the lipid kinase activity of PI3Ks, by using a non-... | US Patent US8841288 (2014) BindingDB Entry DOI: 10.7270/Q2RJ4H5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM132097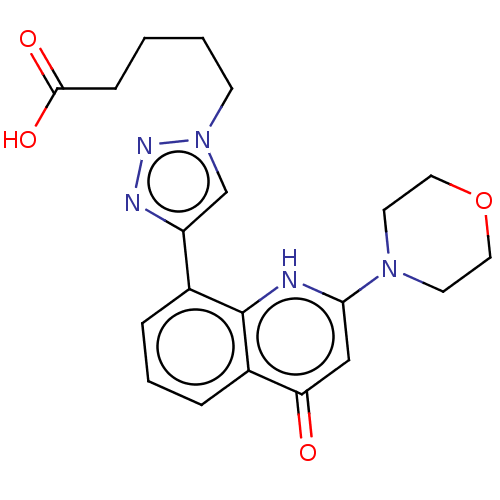 (US8841288, CL 64a) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | 25 |
Universtà Degli Studi di Torino; Università Degli Studi del Piemonte Orientale “Amedeo Avogadro” US Patent | Assay Description Some compounds of formula (I) described herein were assayed in vitro for their ability to inhibit the lipid kinase activity of PI3Ks, by using a non-... | US Patent US8841288 (2014) BindingDB Entry DOI: 10.7270/Q2RJ4H5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM132096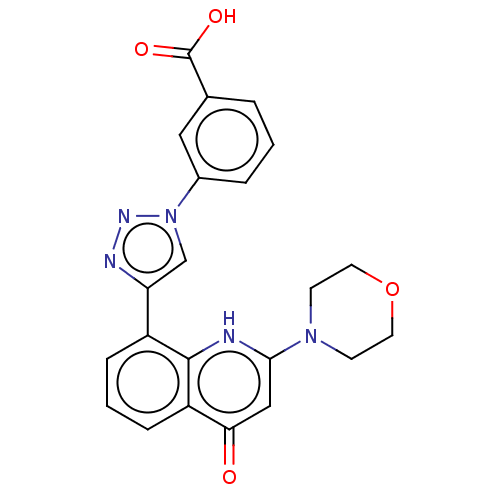 (US8841288, CL55b) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | 25 |
Universtà Degli Studi di Torino; Università Degli Studi del Piemonte Orientale “Amedeo Avogadro” US Patent | Assay Description Some compounds of formula (I) described herein were assayed in vitro for their ability to inhibit the lipid kinase activity of PI3Ks, by using a non-... | US Patent US8841288 (2014) BindingDB Entry DOI: 10.7270/Q2RJ4H5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM132095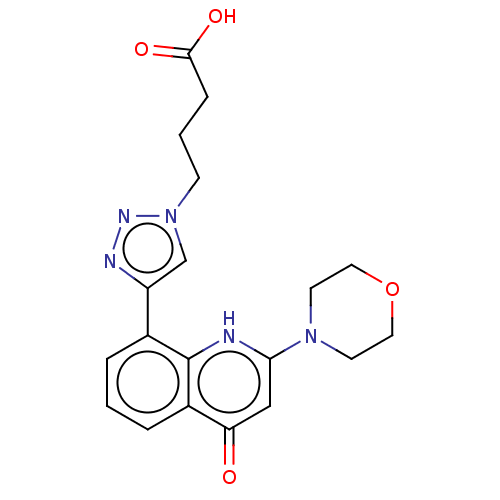 (US8841288, CL55a) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | 25 |
Universtà Degli Studi di Torino; Università Degli Studi del Piemonte Orientale “Amedeo Avogadro” US Patent | Assay Description Some compounds of formula (I) described herein were assayed in vitro for their ability to inhibit the lipid kinase activity of PI3Ks, by using a non-... | US Patent US8841288 (2014) BindingDB Entry DOI: 10.7270/Q2RJ4H5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50514752 (CHEMBL4458549) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Inhibition of IDO1 in IFNgamma-stimulated human MCF7 cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysis | J Med Chem 63: 3047-3065 (2020) Article DOI: 10.1021/acs.jmedchem.9b01809 BindingDB Entry DOI: 10.7270/Q29K4FKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM50514752 (CHEMBL4458549) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 112 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Inhibition of mouse IDO1 transfected in P815 cells assessed as reduction in L-Kyn level measured after 16 hrs by HPLC analysis | J Med Chem 63: 3047-3065 (2020) Article DOI: 10.1021/acs.jmedchem.9b01809 BindingDB Entry DOI: 10.7270/Q29K4FKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50514757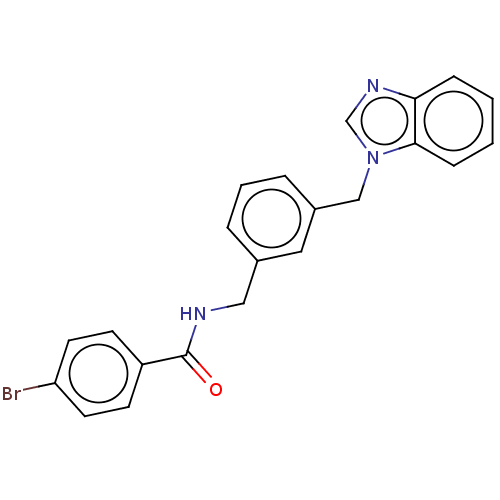 (CHEMBL4466117) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 134 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Inhibition of IDO1 in IFNgamma-stimulated human A375 cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysis | J Med Chem 63: 3047-3065 (2020) Article DOI: 10.1021/acs.jmedchem.9b01809 BindingDB Entry DOI: 10.7270/Q29K4FKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM132098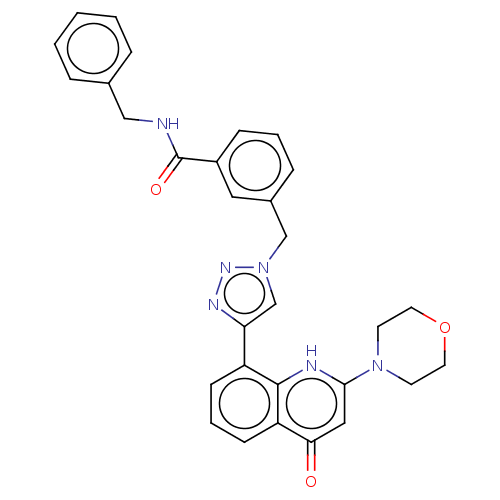 (US8841288, CL 129A) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | 25 |
Universtà Degli Studi di Torino; Università Degli Studi del Piemonte Orientale “Amedeo Avogadro” US Patent | Assay Description Some compounds of formula (I) described herein were assayed in vitro for their ability to inhibit the lipid kinase activity of PI3Ks, by using a non-... | US Patent US8841288 (2014) BindingDB Entry DOI: 10.7270/Q2RJ4H5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50514760 (CHEMBL4448402) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Inhibition of IDO1 in IFNgamma-stimulated human DAN-G cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysis | J Med Chem 63: 3047-3065 (2020) Article DOI: 10.1021/acs.jmedchem.9b01809 BindingDB Entry DOI: 10.7270/Q29K4FKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50454819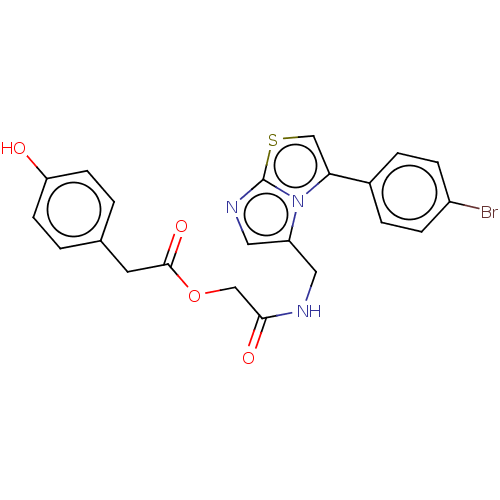 (CHEMBL4208781) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO1 assessed as reduction in kynurenine production using L-tryptophan incubated for 60 mins by Ehrlich's reagent bas... | Bioorg Med Chem Lett 28: 651-657 (2018) Article DOI: 10.1016/j.bmcl.2018.01.032 BindingDB Entry DOI: 10.7270/Q24T6MZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcium release-activated calcium channel protein/Stromal interaction molecule 1 (Homo sapiens (Human)) | BDBM50549365 (CHEMBL3403742) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 228 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of STIM1/Orai1 (unknown origin) expressed in HEK293 cells assessed as reduction in tBHQ-induced calcium response | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01305 BindingDB Entry DOI: 10.7270/Q2HH6PPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM132091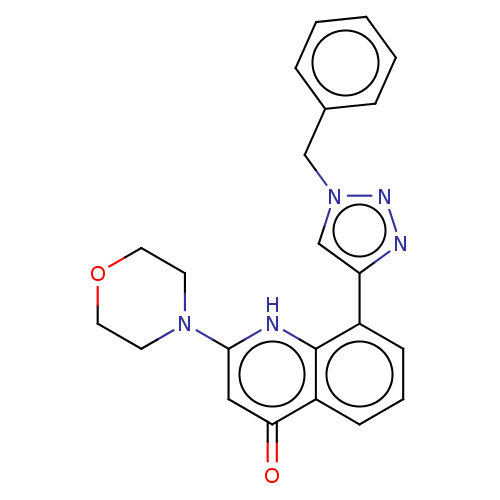 (US8841288, CL5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | 25 |
Universtà Degli Studi di Torino; Università Degli Studi del Piemonte Orientale “Amedeo Avogadro” US Patent | Assay Description Some compounds of formula (I) described herein were assayed in vitro for their ability to inhibit the lipid kinase activity of PI3Ks, by using a non-... | US Patent US8841288 (2014) BindingDB Entry DOI: 10.7270/Q2RJ4H5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM132089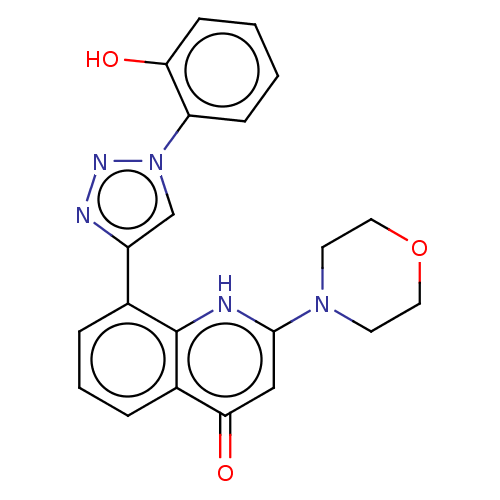 (US8841288, TP714) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | 25 |
Universtà Degli Studi di Torino; Università Degli Studi del Piemonte Orientale “Amedeo Avogadro” US Patent | Assay Description Some compounds of formula (I) described herein were assayed in vitro for their ability to inhibit the lipid kinase activity of PI3Ks, by using a non-... | US Patent US8841288 (2014) BindingDB Entry DOI: 10.7270/Q2RJ4H5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Short transient receptor potential channel 1 (Homo sapiens) | BDBM50450630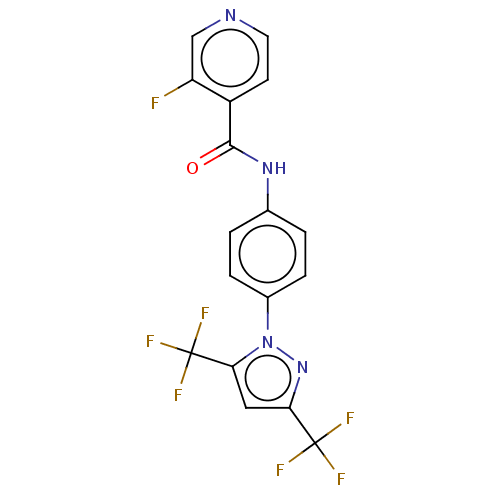 (CHEMBL101896) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Modulation of TRPC1/TRPC3/STIM1/Orai1 in HEK cells assessed as induction of store-operated calcium entry by measuring residual activity preincubated ... | J Med Chem 61: 9756-9783 (2018) Article DOI: 10.1021/acs.jmedchem.8b01512 BindingDB Entry DOI: 10.7270/Q2QV3Q2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM132094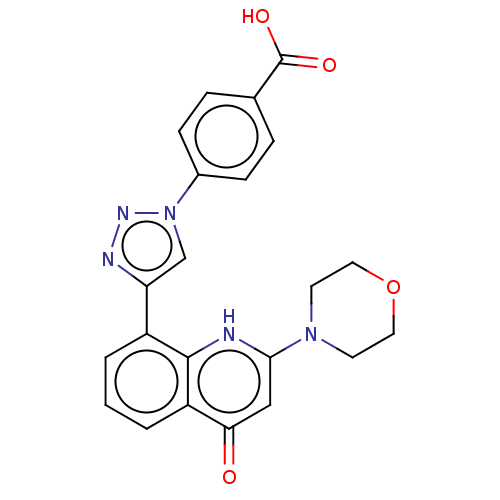 (US8841288, CL29a) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | 25 |
Universtà Degli Studi di Torino; Università Degli Studi del Piemonte Orientale “Amedeo Avogadro” US Patent | Assay Description Some compounds of formula (I) described herein were assayed in vitro for their ability to inhibit the lipid kinase activity of PI3Ks, by using a non-... | US Patent US8841288 (2014) BindingDB Entry DOI: 10.7270/Q2RJ4H5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50514762 (CHEMBL4531545) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 327 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Inhibition of IDO1 in IFNgamma-stimulated human A375 cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysis | J Med Chem 63: 3047-3065 (2020) Article DOI: 10.1021/acs.jmedchem.9b01809 BindingDB Entry DOI: 10.7270/Q29K4FKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50454818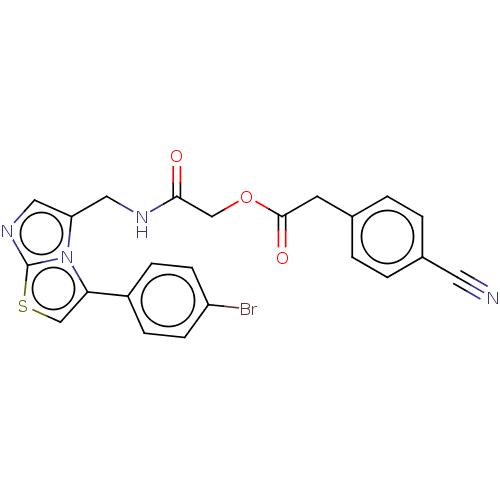 (CHEMBL4206526) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO1 assessed as reduction in kynurenine production using L-tryptophan incubated for 60 mins by Ehrlich's reagent bas... | Bioorg Med Chem Lett 28: 651-657 (2018) Article DOI: 10.1016/j.bmcl.2018.01.032 BindingDB Entry DOI: 10.7270/Q24T6MZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcium release-activated calcium channel protein/Stromal interaction molecule 1 (Homo sapiens (Human)) | BDBM50549358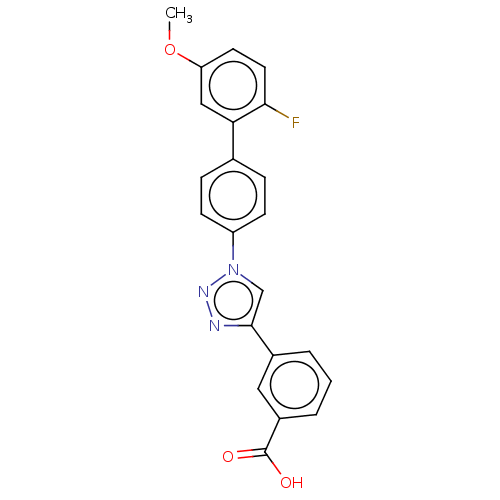 (CHEMBL4753023) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 361 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of STIM1/Orai1 (unknown origin) expressed in HEK293 cells assessed as reduction in tBHQ-induced calcium response | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01305 BindingDB Entry DOI: 10.7270/Q2HH6PPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50514761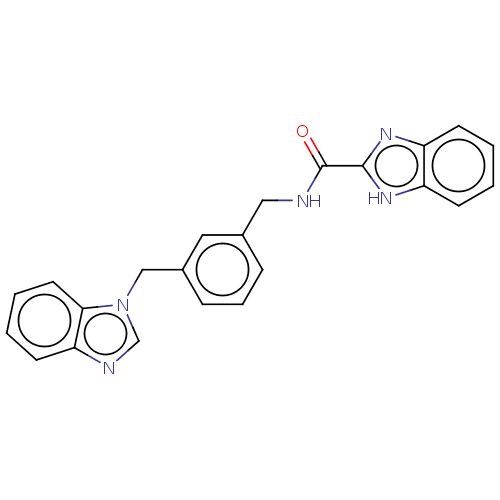 (CHEMBL4472707) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 407 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Inhibition of IDO1 in IFNgamma-stimulated human A375 cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysis | J Med Chem 63: 3047-3065 (2020) Article DOI: 10.1021/acs.jmedchem.9b01809 BindingDB Entry DOI: 10.7270/Q29K4FKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50514754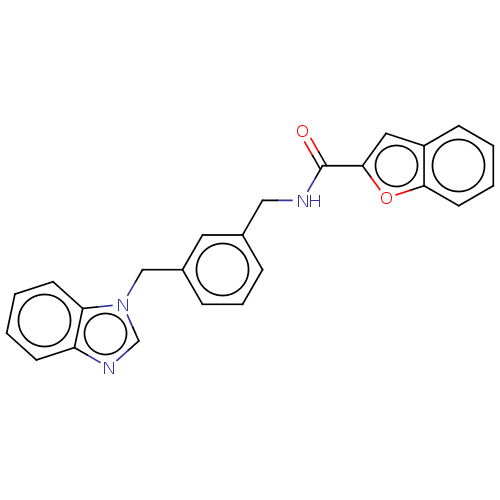 (CHEMBL4588288) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 413 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Inhibition of IDO1 in IFNgamma-stimulated human A375 cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysis | J Med Chem 63: 3047-3065 (2020) Article DOI: 10.1021/acs.jmedchem.9b01809 BindingDB Entry DOI: 10.7270/Q29K4FKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50454816 (CHEMBL4210198) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO1 assessed as reduction in kynurenine production using L-tryptophan incubated for 60 mins by Ehrlich's reagent bas... | Bioorg Med Chem Lett 28: 651-657 (2018) Article DOI: 10.1016/j.bmcl.2018.01.032 BindingDB Entry DOI: 10.7270/Q24T6MZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50514758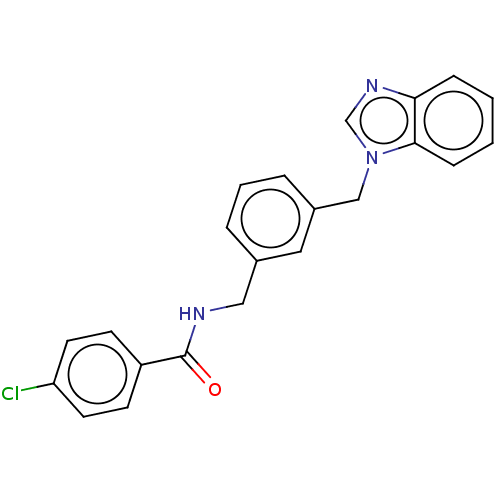 (CHEMBL4451614) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 477 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Inhibition of IDO1 in IFNgamma-stimulated human A375 cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysis | J Med Chem 63: 3047-3065 (2020) Article DOI: 10.1021/acs.jmedchem.9b01809 BindingDB Entry DOI: 10.7270/Q29K4FKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi del Piemonte Orientale A. Avogadro Curated by ChEMBL | Assay Description Inhibition of HDAC in human SHSY5Y cells by fluorimetric cellular activity assay | J Med Chem 52: 2776-85 (2009) Article DOI: 10.1021/jm801529c BindingDB Entry DOI: 10.7270/Q2D221F1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Short transient receptor potential channel 1 (Homo sapiens) | BDBM50450629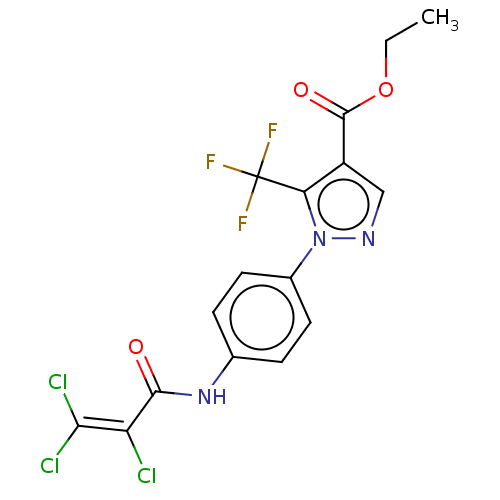 (CHEMBL4177187) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Modulation of TRPC1/TRPC3/STIM1/Orai1 in HEK cells assessed as induction of store-operated calcium entry by measuring residual activity preincubated ... | J Med Chem 61: 9756-9783 (2018) Article DOI: 10.1021/acs.jmedchem.8b01512 BindingDB Entry DOI: 10.7270/Q2QV3Q2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50454821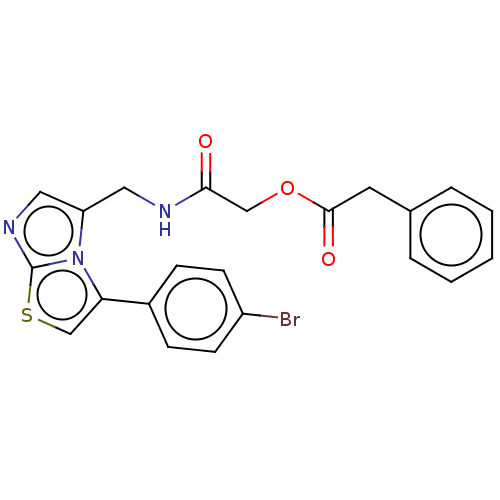 (CHEMBL4209779) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO1 assessed as reduction in kynurenine production using L-tryptophan incubated for 60 mins by Ehrlich's reagent bas... | Bioorg Med Chem Lett 28: 651-657 (2018) Article DOI: 10.1016/j.bmcl.2018.01.032 BindingDB Entry DOI: 10.7270/Q24T6MZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcium release-activated calcium channel protein 1/Short transient receptor potential channel 1/Short transient receptor potential channel 3/Stromal interaction molecule 1 (Homo sapiens (Human)) | BDBM50450626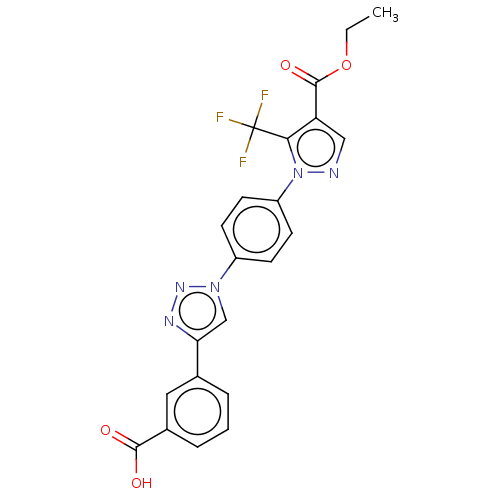 (CHEMBL4162723) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Modulation of TRPC1/TRPC3/STIM1/Orai1 in HEK cells assessed as induction of store-operated calcium entry by measuring residual activity preincubated ... | J Med Chem 61: 9756-9783 (2018) Article DOI: 10.1021/acs.jmedchem.8b01512 BindingDB Entry DOI: 10.7270/Q2QV3Q2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50514753 (CHEMBL4557994) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 605 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Inhibition of IDO1 in IFNgamma-stimulated human DAN-G cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysis | J Med Chem 63: 3047-3065 (2020) Article DOI: 10.1021/acs.jmedchem.9b01809 BindingDB Entry DOI: 10.7270/Q29K4FKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50454820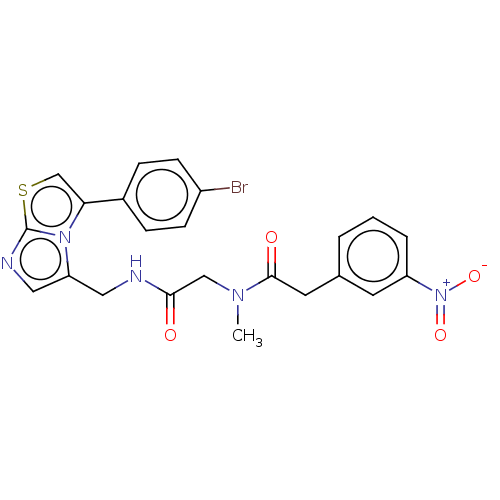 (CHEMBL4214662) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO1 assessed as reduction in kynurenine production using L-tryptophan incubated for 60 mins by Ehrlich's reagent bas... | Bioorg Med Chem Lett 28: 651-657 (2018) Article DOI: 10.1016/j.bmcl.2018.01.032 BindingDB Entry DOI: 10.7270/Q24T6MZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50514755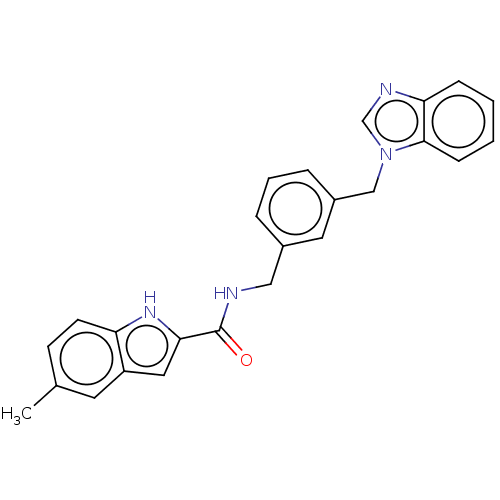 (CHEMBL4443904) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 636 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Inhibition of IDO1 in IFNgamma-stimulated human A375 cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysis | J Med Chem 63: 3047-3065 (2020) Article DOI: 10.1021/acs.jmedchem.9b01809 BindingDB Entry DOI: 10.7270/Q29K4FKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50454815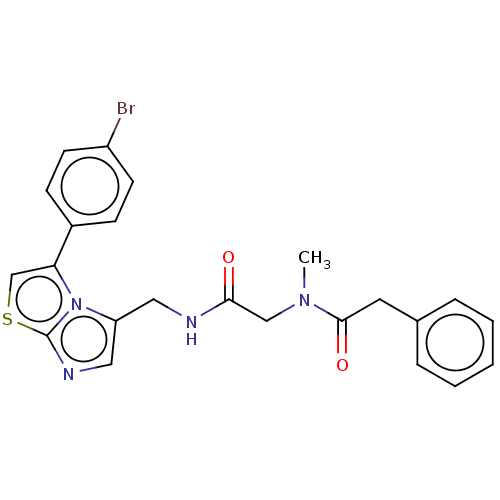 (CHEMBL4218468) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO1 assessed as reduction in kynurenine production using L-tryptophan incubated for 60 mins by Ehrlich's reagent bas... | Bioorg Med Chem Lett 28: 651-657 (2018) Article DOI: 10.1016/j.bmcl.2018.01.032 BindingDB Entry DOI: 10.7270/Q24T6MZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50454814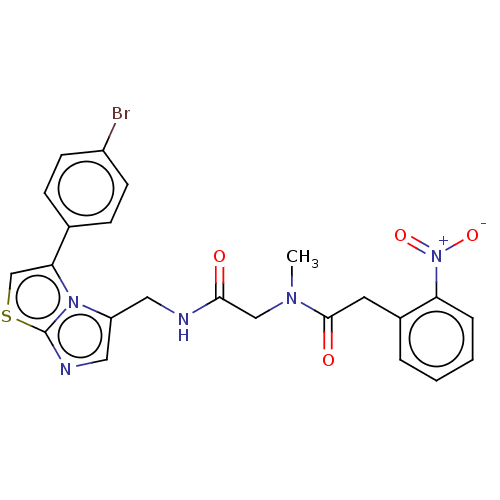 (CHEMBL4214873) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO1 assessed as reduction in kynurenine production using L-tryptophan incubated for 60 mins by Ehrlich's reagent bas... | Bioorg Med Chem Lett 28: 651-657 (2018) Article DOI: 10.1016/j.bmcl.2018.01.032 BindingDB Entry DOI: 10.7270/Q24T6MZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM132090 (US8841288, CL1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | 25 |
Universtà Degli Studi di Torino; Università Degli Studi del Piemonte Orientale “Amedeo Avogadro” US Patent | Assay Description Some compounds of formula (I) described herein were assayed in vitro for their ability to inhibit the lipid kinase activity of PI3Ks, by using a non-... | US Patent US8841288 (2014) BindingDB Entry DOI: 10.7270/Q2RJ4H5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 89 total ) | Next | Last >> |