

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glucocorticoid receptor (RAT) | BDBM19235 (2-{5-[4-({[(2,4-difluorophenyl)methyl](3-methanesu...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 8.0 | 4 |
Abbott Laboratories | Assay Description For compounds able to displace the radiolabeled ligand (competitor) from the receptor an IC50 value (the concentration required to inhibit 50% of the... | J Med Chem 48: 5295-304 (2005) Article DOI: 10.1021/jm050205o BindingDB Entry DOI: 10.7270/Q21C1V5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM19234 (4-{4-[3-(4-{[benzyl(3-methanesulfonamido-2-methylp...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | 86 | n/a | n/a | 8.0 | 4 |
Abbott Laboratories | Assay Description For compounds able to displace the 3(H)-dexamethasone from the receptor an IC50 value (the concentration required to inhibit 50% of the binding of 3(... | J Med Chem 48: 5295-304 (2005) Article DOI: 10.1021/jm050205o BindingDB Entry DOI: 10.7270/Q21C1V5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (RAT) | BDBM19234 (4-{4-[3-(4-{[benzyl(3-methanesulfonamido-2-methylp...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 8.0 | 4 |
Abbott Laboratories | Assay Description For compounds able to displace the radiolabeled ligand (competitor) from the receptor an IC50 value (the concentration required to inhibit 50% of the... | J Med Chem 48: 5295-304 (2005) Article DOI: 10.1021/jm050205o BindingDB Entry DOI: 10.7270/Q21C1V5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18627 ((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 1.10 | n/a | 4.80 | n/a | n/a | 8.0 | 4 |
Abbott Laboratories | Assay Description For compounds able to displace the 3(H)-dexamethasone from the receptor an IC50 value (the concentration required to inhibit 50% of the binding of 3(... | J Med Chem 48: 5295-304 (2005) Article DOI: 10.1021/jm050205o BindingDB Entry DOI: 10.7270/Q21C1V5P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glucocorticoid receptor (RAT) | BDBM18627 ((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | 8.0 | 4 |
Abbott Laboratories | Assay Description For compounds able to displace the radiolabeled ligand (competitor) from the receptor an IC50 value (the concentration required to inhibit 50% of the... | J Med Chem 48: 5295-304 (2005) Article DOI: 10.1021/jm050205o BindingDB Entry DOI: 10.7270/Q21C1V5P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM19236 (2-{2-bromo-5-[4-({[(2,4-difluorophenyl)methyl](3-m...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | 330 | n/a | n/a | 8.0 | 4 |
Abbott Laboratories | Assay Description For compounds able to displace the 3(H)-dexamethasone from the receptor an IC50 value (the concentration required to inhibit 50% of the binding of 3(... | J Med Chem 48: 5295-304 (2005) Article DOI: 10.1021/jm050205o BindingDB Entry DOI: 10.7270/Q21C1V5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (RAT) | BDBM19238 (2-{2-chloro-5-[4-({[(2,4-difluorophenyl)methyl](3-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | 8.0 | 4 |
Abbott Laboratories | Assay Description For compounds able to displace the radiolabeled ligand (competitor) from the receptor an IC50 value (the concentration required to inhibit 50% of the... | J Med Chem 48: 5295-304 (2005) Article DOI: 10.1021/jm050205o BindingDB Entry DOI: 10.7270/Q21C1V5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (RAT) | BDBM19237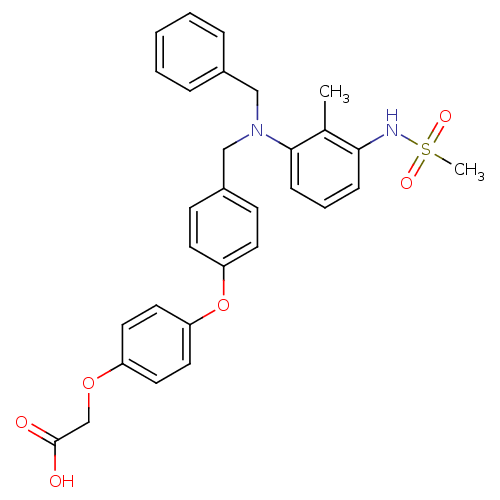 (2-[4-(4-{[benzyl(3-methanesulfonamido-2-methylphen...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | 8.0 | 4 |
Abbott Laboratories | Assay Description For compounds able to displace the radiolabeled ligand (competitor) from the receptor an IC50 value (the concentration required to inhibit 50% of the... | J Med Chem 48: 5295-304 (2005) Article DOI: 10.1021/jm050205o BindingDB Entry DOI: 10.7270/Q21C1V5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (RAT) | BDBM19233 ((2R,10S,11S,14S,15S)-14-hydroxy-15-methyl-2-[(4-me...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | 8.0 | 4 |
Abbott Laboratories | Assay Description For compounds able to displace the radiolabeled ligand (competitor) from the receptor an IC50 value (the concentration required to inhibit 50% of the... | J Med Chem 48: 5295-304 (2005) Article DOI: 10.1021/jm050205o BindingDB Entry DOI: 10.7270/Q21C1V5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM19233 ((2R,10S,11S,14S,15S)-14-hydroxy-15-methyl-2-[(4-me...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | 43 | n/a | n/a | 8.0 | 4 |
Abbott Laboratories | Assay Description For compounds able to displace the 3(H)-dexamethasone from the receptor an IC50 value (the concentration required to inhibit 50% of the binding of 3(... | J Med Chem 48: 5295-304 (2005) Article DOI: 10.1021/jm050205o BindingDB Entry DOI: 10.7270/Q21C1V5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM19238 (2-{2-chloro-5-[4-({[(2,4-difluorophenyl)methyl](3-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60 | n/a | 500 | n/a | n/a | 8.0 | 4 |
Abbott Laboratories | Assay Description For compounds able to displace the 3(H)-dexamethasone from the receptor an IC50 value (the concentration required to inhibit 50% of the binding of 3(... | J Med Chem 48: 5295-304 (2005) Article DOI: 10.1021/jm050205o BindingDB Entry DOI: 10.7270/Q21C1V5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM18627 ((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | 8.0 | 4 |
Abbott Laboratories | Assay Description For compounds able to displace the radiolabeled ligand (competitor) from the receptor an IC50 value (the concentration required to inhibit 50% of the... | J Med Chem 48: 5295-304 (2005) Article DOI: 10.1021/jm050205o BindingDB Entry DOI: 10.7270/Q21C1V5P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM19235 (2-{5-[4-({[(2,4-difluorophenyl)methyl](3-methanesu...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80 | n/a | 440 | n/a | n/a | 8.0 | 4 |
Abbott Laboratories | Assay Description For compounds able to displace the 3(H)-dexamethasone from the receptor an IC50 value (the concentration required to inhibit 50% of the binding of 3(... | J Med Chem 48: 5295-304 (2005) Article DOI: 10.1021/jm050205o BindingDB Entry DOI: 10.7270/Q21C1V5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM19237 (2-[4-(4-{[benzyl(3-methanesulfonamido-2-methylphen...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.40 | n/a | 255 | n/a | n/a | 8.0 | 4 |
Abbott Laboratories | Assay Description For compounds able to displace the 3(H)-dexamethasone from the receptor an IC50 value (the concentration required to inhibit 50% of the binding of 3(... | J Med Chem 48: 5295-304 (2005) Article DOI: 10.1021/jm050205o BindingDB Entry DOI: 10.7270/Q21C1V5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18627 ((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | 8.0 | 4 |
Abbott Laboratories | Assay Description For compounds able to displace the radiolabeled ligand (competitor) from the receptor an IC50 value (the concentration required to inhibit 50% of the... | J Med Chem 48: 5295-304 (2005) Article DOI: 10.1021/jm050205o BindingDB Entry DOI: 10.7270/Q21C1V5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50250183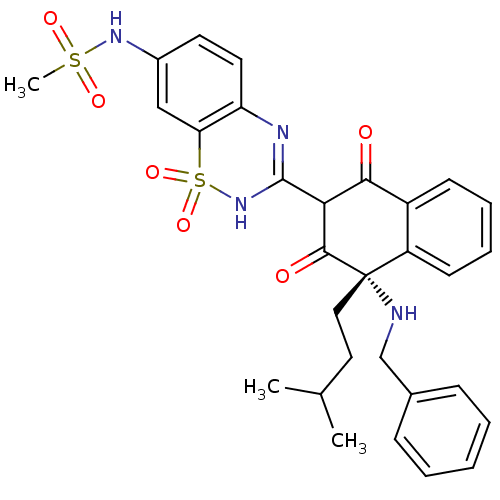 (CHEMBL503188 | N-{3-[(4S)-4-Benzylamino-1-hydroxy-...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of HCV1b Con1 NS5B assessed as [3H]UTP incorporation into RNA by scintillation counting | J Med Chem 52: 3174-83 (2009) Article DOI: 10.1021/jm801485z BindingDB Entry DOI: 10.7270/Q2ZW1MV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50249586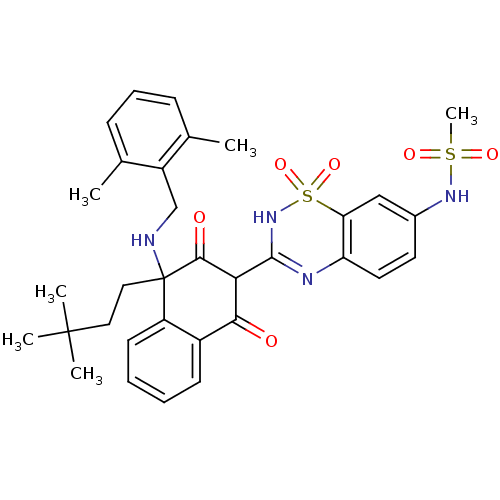 (CHEMBL504729 | N-{3-[4-[(2,6-Dimethylbenzyl)amino]...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of HCV1b Con1 NS5B assessed as [3H]UTP incorporation into RNA by scintillation counting | J Med Chem 52: 3174-83 (2009) Article DOI: 10.1021/jm801485z BindingDB Entry DOI: 10.7270/Q2ZW1MV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM19234 (4-{4-[3-(4-{[benzyl(3-methanesulfonamido-2-methylp...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | 8.0 | 4 |
Abbott Laboratories | Assay Description For compounds able to displace the radiolabeled ligand (competitor) from the receptor an IC50 value (the concentration required to inhibit 50% of the... | J Med Chem 48: 5295-304 (2005) Article DOI: 10.1021/jm050205o BindingDB Entry DOI: 10.7270/Q21C1V5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM19236 (2-{2-bromo-5-[4-({[(2,4-difluorophenyl)methyl](3-m...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | 8.0 | 4 |
Abbott Laboratories | Assay Description For compounds able to displace the radiolabeled ligand (competitor) from the receptor an IC50 value (the concentration required to inhibit 50% of the... | J Med Chem 48: 5295-304 (2005) Article DOI: 10.1021/jm050205o BindingDB Entry DOI: 10.7270/Q21C1V5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM19238 (2-{2-chloro-5-[4-({[(2,4-difluorophenyl)methyl](3-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | 8.0 | 4 |
Abbott Laboratories | Assay Description For compounds able to displace the radiolabeled ligand (competitor) from the receptor an IC50 value (the concentration required to inhibit 50% of the... | J Med Chem 48: 5295-304 (2005) Article DOI: 10.1021/jm050205o BindingDB Entry DOI: 10.7270/Q21C1V5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM19235 (2-{5-[4-({[(2,4-difluorophenyl)methyl](3-methanesu...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | 8.0 | 4 |
Abbott Laboratories | Assay Description For compounds able to displace the radiolabeled ligand (competitor) from the receptor an IC50 value (the concentration required to inhibit 50% of the... | J Med Chem 48: 5295-304 (2005) Article DOI: 10.1021/jm050205o BindingDB Entry DOI: 10.7270/Q21C1V5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM19234 (4-{4-[3-(4-{[benzyl(3-methanesulfonamido-2-methylp...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 550 | n/a | n/a | n/a | n/a | 8.0 | 4 |
Abbott Laboratories | Assay Description For compounds able to displace the radiolabeled ligand (competitor) from the receptor an IC50 value (the concentration required to inhibit 50% of the... | J Med Chem 48: 5295-304 (2005) Article DOI: 10.1021/jm050205o BindingDB Entry DOI: 10.7270/Q21C1V5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM19237 (2-[4-(4-{[benzyl(3-methanesulfonamido-2-methylphen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 690 | n/a | n/a | n/a | n/a | 8.0 | 4 |
Abbott Laboratories | Assay Description For compounds able to displace the radiolabeled ligand (competitor) from the receptor an IC50 value (the concentration required to inhibit 50% of the... | J Med Chem 48: 5295-304 (2005) Article DOI: 10.1021/jm050205o BindingDB Entry DOI: 10.7270/Q21C1V5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM19238 (2-{2-chloro-5-[4-({[(2,4-difluorophenyl)methyl](3-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | 8.0 | 4 |
Abbott Laboratories | Assay Description For compounds able to displace the radiolabeled ligand (competitor) from the receptor an IC50 value (the concentration required to inhibit 50% of the... | J Med Chem 48: 5295-304 (2005) Article DOI: 10.1021/jm050205o BindingDB Entry DOI: 10.7270/Q21C1V5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM19234 (4-{4-[3-(4-{[benzyl(3-methanesulfonamido-2-methylp...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | 8.0 | 4 |
Abbott Laboratories | Assay Description For compounds able to displace the radiolabeled ligand (competitor) from the receptor an IC50 value (the concentration required to inhibit 50% of the... | J Med Chem 48: 5295-304 (2005) Article DOI: 10.1021/jm050205o BindingDB Entry DOI: 10.7270/Q21C1V5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM19237 (2-[4-(4-{[benzyl(3-methanesulfonamido-2-methylphen...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | 8.0 | 4 |
Abbott Laboratories | Assay Description For compounds able to displace the radiolabeled ligand (competitor) from the receptor an IC50 value (the concentration required to inhibit 50% of the... | J Med Chem 48: 5295-304 (2005) Article DOI: 10.1021/jm050205o BindingDB Entry DOI: 10.7270/Q21C1V5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM19233 ((2R,10S,11S,14S,15S)-14-hydroxy-15-methyl-2-[(4-me...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | 8.0 | 4 |
Abbott Laboratories | Assay Description For compounds able to displace the radiolabeled ligand (competitor) from the receptor an IC50 value (the concentration required to inhibit 50% of the... | J Med Chem 48: 5295-304 (2005) Article DOI: 10.1021/jm050205o BindingDB Entry DOI: 10.7270/Q21C1V5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM19238 (2-{2-chloro-5-[4-({[(2,4-difluorophenyl)methyl](3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | 8.0 | 4 |
Abbott Laboratories | Assay Description For compounds able to displace the radiolabeled ligand (competitor) from the receptor an IC50 value (the concentration required to inhibit 50% of the... | J Med Chem 48: 5295-304 (2005) Article DOI: 10.1021/jm050205o BindingDB Entry DOI: 10.7270/Q21C1V5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM18627 ((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | 8.0 | 4 |
Abbott Laboratories | Assay Description For compounds able to displace the radiolabeled ligand (competitor) from the receptor an IC50 value (the concentration required to inhibit 50% of the... | J Med Chem 48: 5295-304 (2005) Article DOI: 10.1021/jm050205o BindingDB Entry DOI: 10.7270/Q21C1V5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM19233 ((2R,10S,11S,14S,15S)-14-hydroxy-15-methyl-2-[(4-me...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | 8.0 | 4 |
Abbott Laboratories | Assay Description For compounds able to displace the radiolabeled ligand (competitor) from the receptor an IC50 value (the concentration required to inhibit 50% of the... | J Med Chem 48: 5295-304 (2005) Article DOI: 10.1021/jm050205o BindingDB Entry DOI: 10.7270/Q21C1V5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM19233 ((2R,10S,11S,14S,15S)-14-hydroxy-15-methyl-2-[(4-me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | 8.0 | 4 |
Abbott Laboratories | Assay Description For compounds able to displace the radiolabeled ligand (competitor) from the receptor an IC50 value (the concentration required to inhibit 50% of the... | J Med Chem 48: 5295-304 (2005) Article DOI: 10.1021/jm050205o BindingDB Entry DOI: 10.7270/Q21C1V5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM19237 (2-[4-(4-{[benzyl(3-methanesulfonamido-2-methylphen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.17E+4 | n/a | n/a | n/a | n/a | 8.0 | 4 |
Abbott Laboratories | Assay Description For compounds able to displace the radiolabeled ligand (competitor) from the receptor an IC50 value (the concentration required to inhibit 50% of the... | J Med Chem 48: 5295-304 (2005) Article DOI: 10.1021/jm050205o BindingDB Entry DOI: 10.7270/Q21C1V5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50453078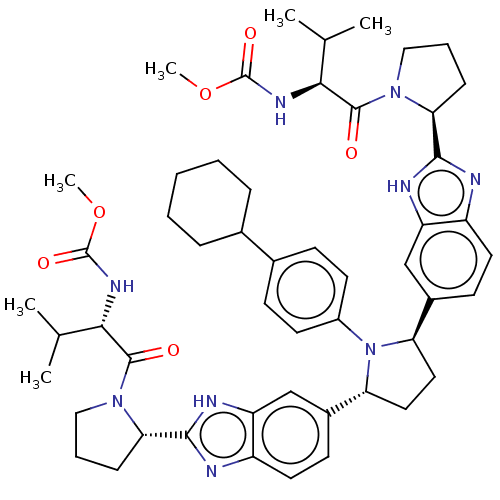 (CHEMBL4215254) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.00700 | n/a | n/a | n/a | n/a |
AbbVie Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1b Con1 NS5A expressed in HuH7 cell infected HCV genotype 2b assessed as decrease in viral replication after 3 days by luc... | J Med Chem 61: 4052-4066 (2018) Article DOI: 10.1021/acs.jmedchem.8b00082 BindingDB Entry DOI: 10.7270/Q269764H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50453100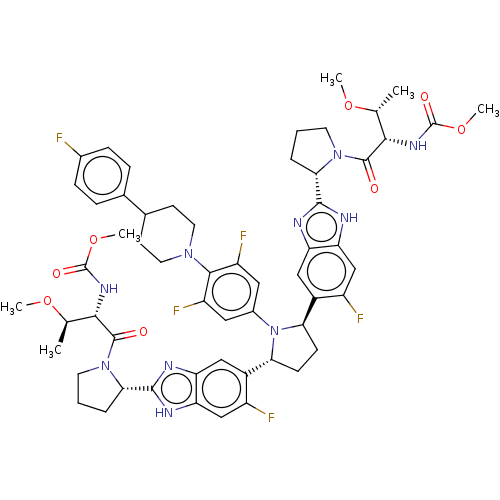 (A-1325912.0 | ABT-530 | Pibrentasvir) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.00190 | n/a | n/a | n/a | n/a |
AbbVie Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1b Con1 NS5A expressed in HuH7 cell infected HCV genotype 2b assessed as decrease in viral replication after 3 days by luc... | J Med Chem 61: 4052-4066 (2018) Article DOI: 10.1021/acs.jmedchem.8b00082 BindingDB Entry DOI: 10.7270/Q269764H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50453101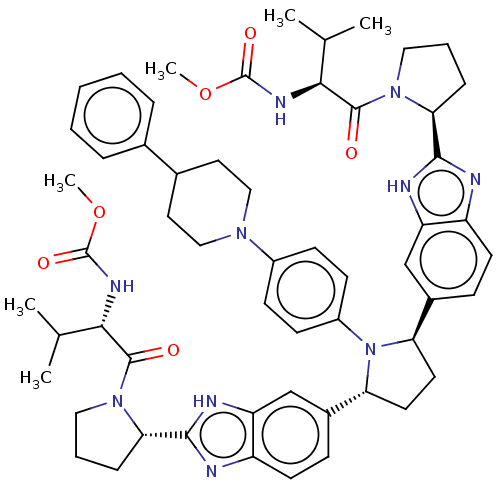 (CHEMBL4208591) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.481 | n/a | n/a | n/a | n/a |
AbbVie Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1b Con1 NS5A expressed in HuH7 cell infected HCV genotype 3a assessed as decrease in viral replication after 3 days by luc... | J Med Chem 61: 4052-4066 (2018) Article DOI: 10.1021/acs.jmedchem.8b00082 BindingDB Entry DOI: 10.7270/Q269764H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50453102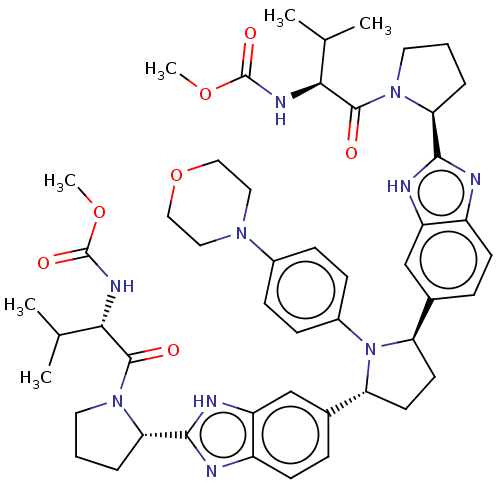 (CHEMBL4206465) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.316 | n/a | n/a | n/a | n/a |
AbbVie Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1b Con1 NS5A expressed in HuH7 cell infected HCV genotype 4a assessed as decrease in viral replication after 3 days by luc... | J Med Chem 61: 4052-4066 (2018) Article DOI: 10.1021/acs.jmedchem.8b00082 BindingDB Entry DOI: 10.7270/Q269764H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50453103 (CHEMBL4218872) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.00100 | n/a | n/a | n/a | n/a |
AbbVie Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1b Con1 NS5A expressed in HuH7 cell infected HCV genotype 5a assessed as decrease in viral replication after 3 days by luc... | J Med Chem 61: 4052-4066 (2018) Article DOI: 10.1021/acs.jmedchem.8b00082 BindingDB Entry DOI: 10.7270/Q269764H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nonstructural protein 5A (Hepatitis C virus) | BDBM50453104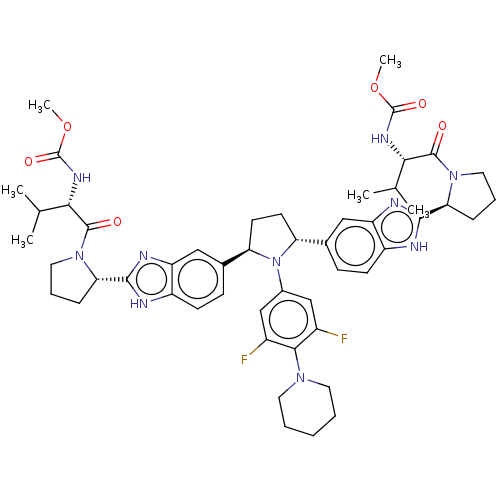 (CHEMBL3915742) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.151 | n/a | n/a | n/a | n/a |
AbbVie Curated by ChEMBL | Assay Description Inhibition of NS5A in HuH7 cell infected HCV genotype 1a H77 assessed as decrease in viral replication after 3 days in presence of 40% human plasma b... | J Med Chem 61: 4052-4066 (2018) Article DOI: 10.1021/acs.jmedchem.8b00082 BindingDB Entry DOI: 10.7270/Q269764H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50453105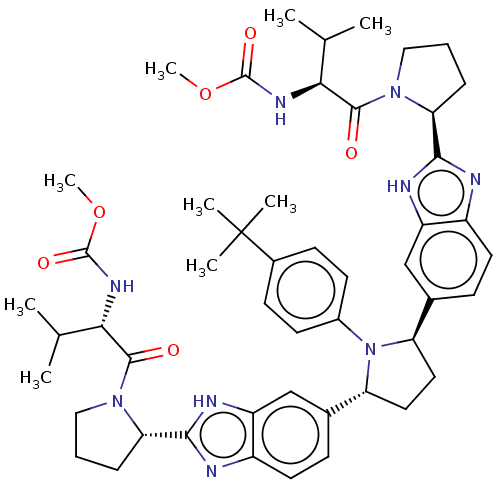 (CHEMBL3960757) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.00600 | n/a | n/a | n/a | n/a |
AbbVie Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1b Con1 NS5A expressed in HuH7 cell infected HCV genotype 4a assessed as decrease in viral replication after 3 days by luc... | J Med Chem 61: 4052-4066 (2018) Article DOI: 10.1021/acs.jmedchem.8b00082 BindingDB Entry DOI: 10.7270/Q269764H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50453105 (CHEMBL3960757) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.152 | n/a | n/a | n/a | n/a |
AbbVie Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1b Con1 NS5A expressed in HuH7 cell infected HCV genotype 2a assessed as decrease in viral replication after 3 days by luc... | J Med Chem 61: 4052-4066 (2018) Article DOI: 10.1021/acs.jmedchem.8b00082 BindingDB Entry DOI: 10.7270/Q269764H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50453106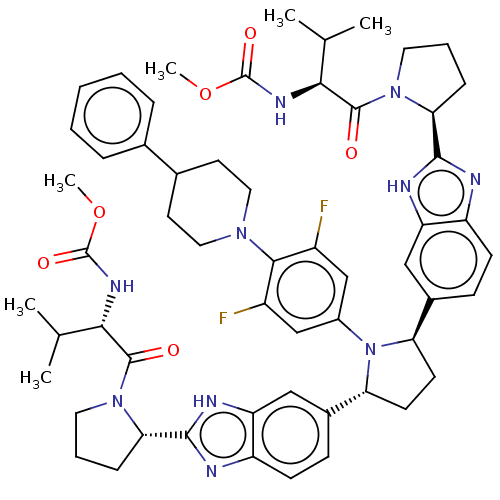 (CHEMBL4215923) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.183 | n/a | n/a | n/a | n/a |
AbbVie Curated by ChEMBL | Assay Description Inhibition of NS5A in HuH7 cell infected HCV genotype 1b Con1 assessed as decrease in viral replication after 3 days in presence of 40% human plasma ... | J Med Chem 61: 4052-4066 (2018) Article DOI: 10.1021/acs.jmedchem.8b00082 BindingDB Entry DOI: 10.7270/Q269764H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50453107 (CHEMBL4215241) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a |
AbbVie Curated by ChEMBL | Assay Description Inhibition of NS5A in HuH7 cell infected HCV genotype 1b Con1 assessed as decrease in viral replication after 3 days in presence of 40% human plasma ... | J Med Chem 61: 4052-4066 (2018) Article DOI: 10.1021/acs.jmedchem.8b00082 BindingDB Entry DOI: 10.7270/Q269764H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nonstructural protein 5A (Hepatitis C virus) | BDBM50453108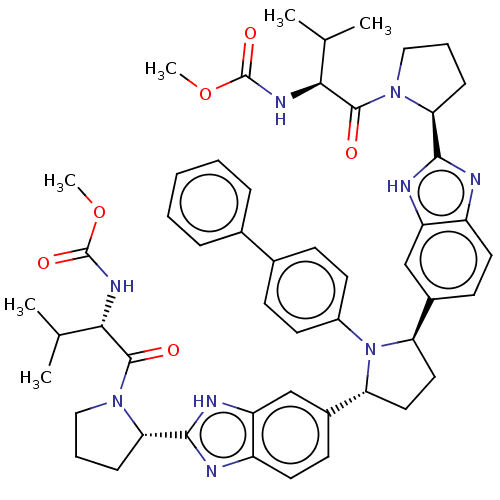 (CHEMBL4202479) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.0750 | n/a | n/a | n/a | n/a |
AbbVie Curated by ChEMBL | Assay Description Inhibition of NS5A in HuH7 cell infected HCV genotype 1a H77 assessed as decrease in viral replication after 3 days in presence of 40% human plasma b... | J Med Chem 61: 4052-4066 (2018) Article DOI: 10.1021/acs.jmedchem.8b00082 BindingDB Entry DOI: 10.7270/Q269764H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50453109 (CHEMBL4205352) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.00300 | n/a | n/a | n/a | n/a |
AbbVie Curated by ChEMBL | Assay Description Inhibition of NS5A in HuH7 cell infected HCV genotype 1b Con1 assessed as decrease in viral replication after 3 days by luciferase reporter gene assa... | J Med Chem 61: 4052-4066 (2018) Article DOI: 10.1021/acs.jmedchem.8b00082 BindingDB Entry DOI: 10.7270/Q269764H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50453107 (CHEMBL4215241) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.00700 | n/a | n/a | n/a | n/a |
AbbVie Curated by ChEMBL | Assay Description Inhibition of NS5A in HuH7 cell infected HCV genotype 1b Con1 assessed as decrease in viral replication after 3 days by luciferase reporter gene assa... | J Med Chem 61: 4052-4066 (2018) Article DOI: 10.1021/acs.jmedchem.8b00082 BindingDB Entry DOI: 10.7270/Q269764H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nonstructural protein 5A (Hepatitis C virus) | BDBM50453110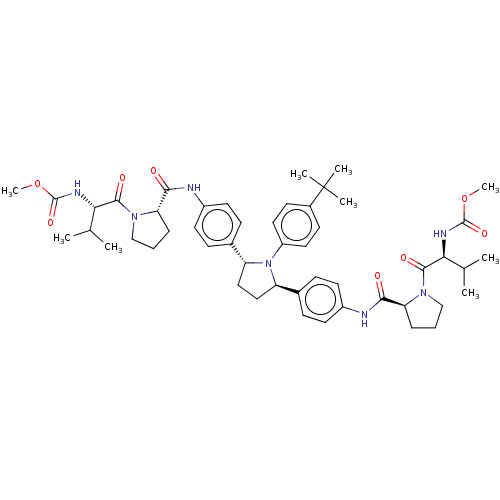 (CHEMBL3127327) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.136 | n/a | n/a | n/a | n/a |
AbbVie Curated by ChEMBL | Assay Description Inhibition of NS5A in HuH7 cell infected HCV genotype 1a H77 assessed as decrease in viral replication after 3 days by luciferase reporter gene assay | J Med Chem 61: 4052-4066 (2018) Article DOI: 10.1021/acs.jmedchem.8b00082 BindingDB Entry DOI: 10.7270/Q269764H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50453111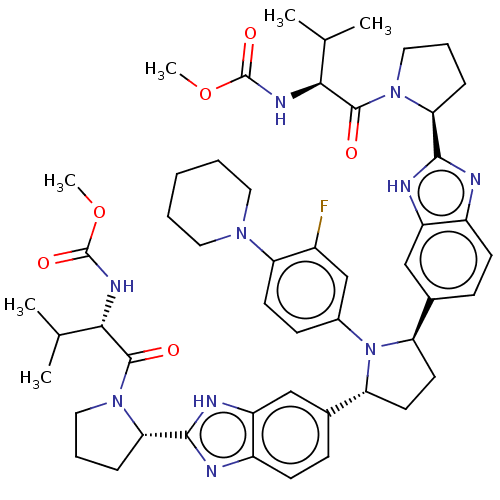 (CHEMBL4208073) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.0480 | n/a | n/a | n/a | n/a |
AbbVie Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1b Con1 NS5A expressed in HuH7 cell infected HCV genotype 2a assessed as decrease in viral replication after 3 days by luc... | J Med Chem 61: 4052-4066 (2018) Article DOI: 10.1021/acs.jmedchem.8b00082 BindingDB Entry DOI: 10.7270/Q269764H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50453112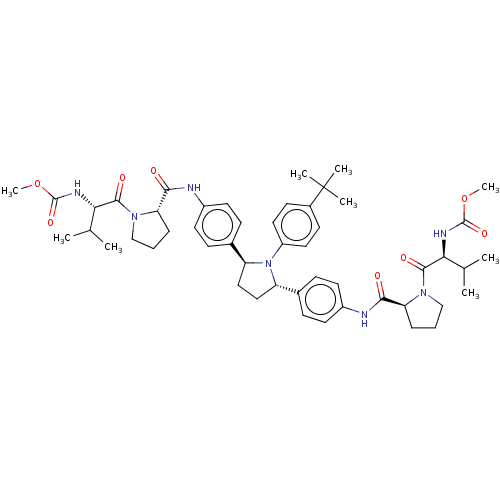 (ABT-267 | CHEBI:85183 | Ombitasvir) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.00430 | n/a | n/a | n/a | n/a |
AbbVie Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1b Con1 NS5A expressed in HuH7 cell infected HCV genotype 2b assessed as decrease in viral replication after 3 days by luc... | J Med Chem 61: 4052-4066 (2018) Article DOI: 10.1021/acs.jmedchem.8b00082 BindingDB Entry DOI: 10.7270/Q269764H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50453113 (CHEMBL4210254) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.0110 | n/a | n/a | n/a | n/a |
AbbVie Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1b Con1 NS5A expressed in HuH7 cell infected HCV genotype 3a assessed as decrease in viral replication after 3 days by luc... | J Med Chem 61: 4052-4066 (2018) Article DOI: 10.1021/acs.jmedchem.8b00082 BindingDB Entry DOI: 10.7270/Q269764H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50453114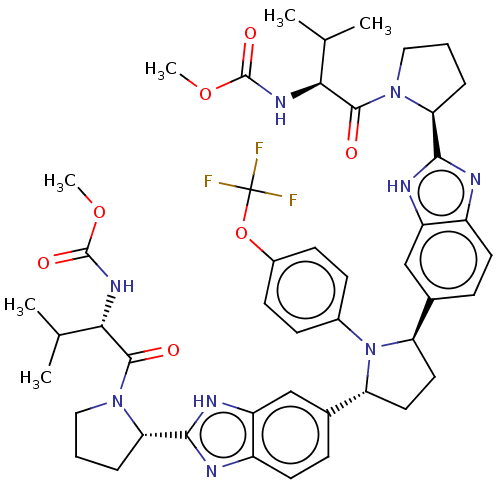 (CHEMBL4211348) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.0130 | n/a | n/a | n/a | n/a |
AbbVie Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1b Con1 NS5A expressed in HuH7 cell infected HCV genotype 4a assessed as decrease in viral replication after 3 days by luc... | J Med Chem 61: 4052-4066 (2018) Article DOI: 10.1021/acs.jmedchem.8b00082 BindingDB Entry DOI: 10.7270/Q269764H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 247 total ) | Next | Last >> |