

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuraminidase (Influenza A virus) | BDBM50484774 (CHEMBL1956716) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Chulalongkorn University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus H7N3 N3 neuraminidase expressed in baculovirus using 2'-(4-Methylumbelliferyl)-alpha-D-acetyl neuraminic acid as subs... | Bioorg Med Chem 20: 2152-7 (2012) Article DOI: 10.1016/j.bmc.2012.01.026 BindingDB Entry DOI: 10.7270/Q2KS6VDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50484773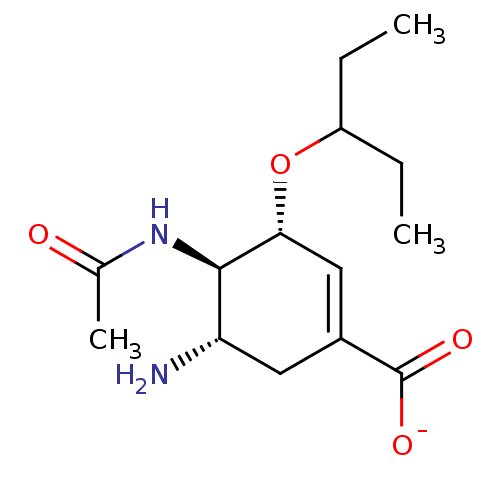 (OSELTAMIVIR POTTASIUM CARBOXYLATE | Oseltamivir Po...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Chulalongkorn University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus H7N3 N3 neuraminidase expressed in baculovirus using 2'-(4-Methylumbelliferyl)-alpha-D-acetyl neuraminic acid as subs... | Bioorg Med Chem 20: 2152-7 (2012) Article DOI: 10.1016/j.bmc.2012.01.026 BindingDB Entry DOI: 10.7270/Q2KS6VDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM5025 (Oseltamivir | US10919856, POSITIVE CONTROL | ethyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Chulalongkorn University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus H7N3 N3 neuraminidase expressed in baculovirus using 2'-(4-Methylumbelliferyl)-alpha-D-acetyl neuraminic acid as subs... | Bioorg Med Chem 20: 2152-7 (2012) Article DOI: 10.1016/j.bmc.2012.01.026 BindingDB Entry DOI: 10.7270/Q2KS6VDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50484775 (CHEMBL1956713) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Chulalongkorn University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus H7N3 N3 neuraminidase expressed in baculovirus using 2'-(4-Methylumbelliferyl)-alpha-D-acetyl neuraminic acid as subs... | Bioorg Med Chem 20: 2152-7 (2012) Article DOI: 10.1016/j.bmc.2012.01.026 BindingDB Entry DOI: 10.7270/Q2KS6VDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM5446 (CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human DYKDDDD-tagged EGFR (669 to 1210 residues) expressed in baculovirus expression system using poly-Glu-Tyr (4:1) as sub... | Citation and Details Article DOI: 10.1039/d0md00436g BindingDB Entry DOI: 10.7270/Q2NS0ZNJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuraminidase (Influenza A virus) | BDBM4994 ((3R,4R,5S)-5-amino-4-acetamido-3-(pentan-3-yloxy)c...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Chulalongkorn University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/duck/Singapore/3/1997(H5N3)) neuraminidase N3 | Bioorg Med Chem 20: 2152-7 (2012) Article DOI: 10.1016/j.bmc.2012.01.026 BindingDB Entry DOI: 10.7270/Q2KS6VDT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuraminidase (Influenza A virus) | BDBM4994 ((3R,4R,5S)-5-amino-4-acetamido-3-(pentan-3-yloxy)c...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Chulalongkorn University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/duck/Germany/1215/1973(H2N3)) neuraminidase N3 | Bioorg Med Chem 20: 2152-7 (2012) Article DOI: 10.1016/j.bmc.2012.01.026 BindingDB Entry DOI: 10.7270/Q2KS6VDT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50484776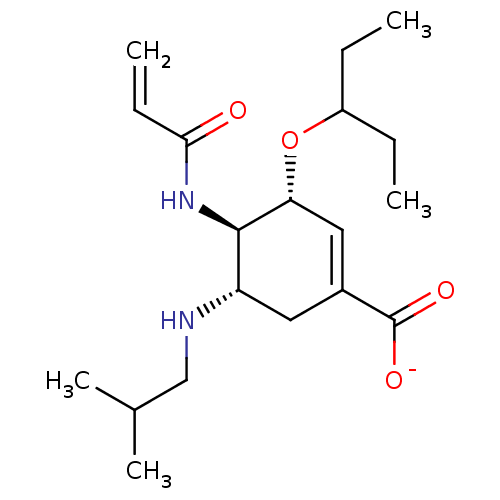 (CHEMBL1956717) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Chulalongkorn University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus H7N1 N1 neuraminidase expressed in baculovirus using 2'-(4-Methylumbelliferyl)-alpha-D-acetyl neuraminic acid as subs... | Bioorg Med Chem 20: 2152-7 (2012) Article DOI: 10.1016/j.bmc.2012.01.026 BindingDB Entry DOI: 10.7270/Q2KS6VDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50562022 (CHEMBL4797515) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human JAK2 JH1 domain expressed in baculovirus infected insect cells using poly-Glu-Tyr (51 to 68 residues) as substrate in... | Citation and Details Article DOI: 10.1039/d0md00436g BindingDB Entry DOI: 10.7270/Q2NS0ZNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50355501 (INCB-018424 | RUXOLITINIB | RUXOLITINIB PHOSPHATE ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human JAK2 JH1 domain expressed in baculovirus infected insect cells using poly-Glu-Tyr (51 to 68 residues) as substrate in... | Citation and Details Article DOI: 10.1039/d0md00436g BindingDB Entry DOI: 10.7270/Q2NS0ZNJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50562021 (CHEMBL4798434) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human JAK2 JH1 domain expressed in baculovirus infected insect cells using poly-Glu-Tyr (51 to 68 residues) as substrate in... | Citation and Details Article DOI: 10.1039/d0md00436g BindingDB Entry DOI: 10.7270/Q2NS0ZNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM5025 (Oseltamivir | US10919856, POSITIVE CONTROL | ethyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Chulalongkorn University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus H7N1 N1 neuraminidase expressed in baculovirus using 2'-(4-Methylumbelliferyl)-alpha-D-acetyl neuraminic acid as subs... | Bioorg Med Chem 20: 2152-7 (2012) Article DOI: 10.1016/j.bmc.2012.01.026 BindingDB Entry DOI: 10.7270/Q2KS6VDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50484776 (CHEMBL1956717) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Chulalongkorn University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus H7N3 N3 neuraminidase expressed in baculovirus using 2'-(4-Methylumbelliferyl)-alpha-D-acetyl neuraminic acid as subs... | Bioorg Med Chem 20: 2152-7 (2012) Article DOI: 10.1016/j.bmc.2012.01.026 BindingDB Entry DOI: 10.7270/Q2KS6VDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM5446 (CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human EGFR TK domain expressed in Escherichia coli BL21 (DE3) pLysS using poly-Glu-Tyr (51 to 68 residues) as substrate incubated for 1... | Citation and Details Article DOI: 10.1039/d0md00436g BindingDB Entry DOI: 10.7270/Q2NS0ZNJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50484774 (CHEMBL1956716) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Chulalongkorn University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus H7N1 N1 neuraminidase expressed in baculovirus using 2'-(4-Methylumbelliferyl)-alpha-D-acetyl neuraminic acid as subs... | Bioorg Med Chem 20: 2152-7 (2012) Article DOI: 10.1016/j.bmc.2012.01.026 BindingDB Entry DOI: 10.7270/Q2KS6VDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50562023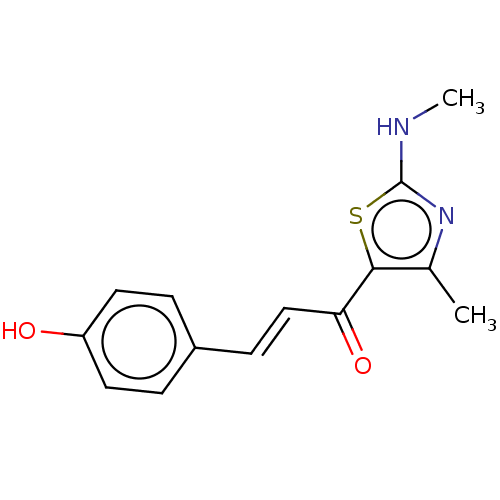 (CHEMBL4750368) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human EGFR TK domain expressed in Escherichia coli BL21 (DE3) pLysS using poly-Glu-Tyr (51 to 68 residues) as substrate incubated for 1... | Citation and Details Article DOI: 10.1039/d0md00436g BindingDB Entry DOI: 10.7270/Q2NS0ZNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50562023 (CHEMBL4750368) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human JAK2 JH1 domain expressed in baculovirus infected insect cells using poly-Glu-Tyr (51 to 68 residues) as substrate in... | Citation and Details Article DOI: 10.1039/d0md00436g BindingDB Entry DOI: 10.7270/Q2NS0ZNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM4994 ((3R,4R,5S)-5-amino-4-acetamido-3-(pentan-3-yloxy)c...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Chulalongkorn University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/Teal/Hong Kong/W312/97(H6N1)) neuraminidase N1 | Bioorg Med Chem 20: 2152-7 (2012) Article DOI: 10.1016/j.bmc.2012.01.026 BindingDB Entry DOI: 10.7270/Q2KS6VDT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50484773 (OSELTAMIVIR POTTASIUM CARBOXYLATE | Oseltamivir Po...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Chulalongkorn University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus H7N1 N1 neuraminidase expressed in baculovirus using 2'-(4-Methylumbelliferyl)-alpha-D-acetyl neuraminic acid as subs... | Bioorg Med Chem 20: 2152-7 (2012) Article DOI: 10.1016/j.bmc.2012.01.026 BindingDB Entry DOI: 10.7270/Q2KS6VDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM4994 ((3R,4R,5S)-5-amino-4-acetamido-3-(pentan-3-yloxy)c...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Chulalongkorn University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/duck/Alberta/35/1976(H1N1)) neuraminidase N1 | Bioorg Med Chem 20: 2152-7 (2012) Article DOI: 10.1016/j.bmc.2012.01.026 BindingDB Entry DOI: 10.7270/Q2KS6VDT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50484775 (CHEMBL1956713) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
Chulalongkorn University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus H7N1 N1 neuraminidase expressed in baculovirus using 2'-(4-Methylumbelliferyl)-alpha-D-acetyl neuraminic acid as subs... | Bioorg Med Chem 20: 2152-7 (2012) Article DOI: 10.1016/j.bmc.2012.01.026 BindingDB Entry DOI: 10.7270/Q2KS6VDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50377954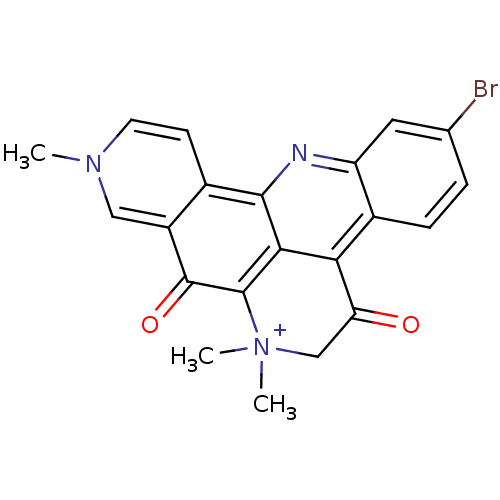 (CHEMBL557422) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
Mahidol University Curated by ChEMBL | Assay Description Inhibition of Torpedo californica AchE activity by Ellman's assay | Bioorg Med Chem 16: 6560-7 (2008) Article DOI: 10.1016/j.bmc.2008.05.027 BindingDB Entry DOI: 10.7270/Q29Z95T9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50562022 (CHEMBL4797515) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 144 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human EGFR TK domain expressed in Escherichia coli BL21 (DE3) pLysS using poly-Glu-Tyr (51 to 68 residues) as substrate incubated for 1... | Citation and Details Article DOI: 10.1039/d0md00436g BindingDB Entry DOI: 10.7270/Q2NS0ZNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50562021 (CHEMBL4798434) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 256 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human EGFR TK domain expressed in Escherichia coli BL21 (DE3) pLysS using poly-Glu-Tyr (51 to 68 residues) as substrate incubated for 1... | Citation and Details Article DOI: 10.1039/d0md00436g BindingDB Entry DOI: 10.7270/Q2NS0ZNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
Mahidol University Curated by ChEMBL | Assay Description Inhibition of Torpedo californica AchE activity by Ellman's assay | Bioorg Med Chem 16: 6560-7 (2008) Article DOI: 10.1016/j.bmc.2008.05.027 BindingDB Entry DOI: 10.7270/Q29Z95T9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50484772 (CHEMBL1956714) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chulalongkorn University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus H7N3 N3 neuraminidase expressed in baculovirus using 2'-(4-Methylumbelliferyl)-alpha-D-acetyl neuraminic acid as subs... | Bioorg Med Chem 20: 2152-7 (2012) Article DOI: 10.1016/j.bmc.2012.01.026 BindingDB Entry DOI: 10.7270/Q2KS6VDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50484771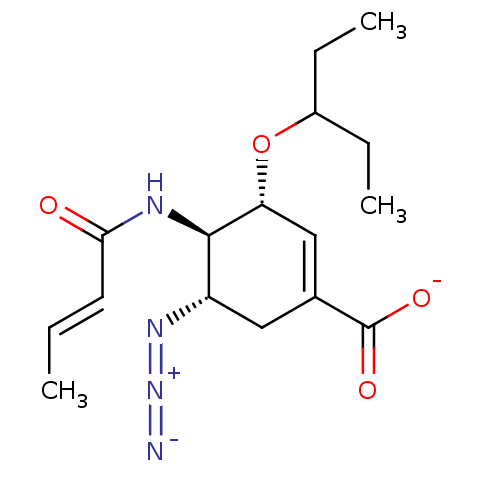 (CHEMBL1956712) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chulalongkorn University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus H7N1 N1 neuraminidase expressed in baculovirus using 2'-(4-Methylumbelliferyl)-alpha-D-acetyl neuraminic acid as subs... | Bioorg Med Chem 20: 2152-7 (2012) Article DOI: 10.1016/j.bmc.2012.01.026 BindingDB Entry DOI: 10.7270/Q2KS6VDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50484771 (CHEMBL1956712) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.93E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chulalongkorn University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus H7N3 N3 neuraminidase expressed in baculovirus using 2'-(4-Methylumbelliferyl)-alpha-D-acetyl neuraminic acid as subs... | Bioorg Med Chem 20: 2152-7 (2012) Article DOI: 10.1016/j.bmc.2012.01.026 BindingDB Entry DOI: 10.7270/Q2KS6VDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50484772 (CHEMBL1956714) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.39E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chulalongkorn University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus H7N1 N1 neuraminidase expressed in baculovirus using 2'-(4-Methylumbelliferyl)-alpha-D-acetyl neuraminic acid as subs... | Bioorg Med Chem 20: 2152-7 (2012) Article DOI: 10.1016/j.bmc.2012.01.026 BindingDB Entry DOI: 10.7270/Q2KS6VDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50243985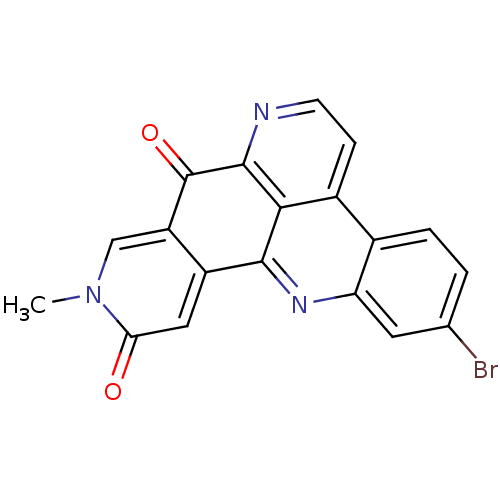 (2-Bromoamphimedine | CHEMBL453326) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Mahidol University Curated by ChEMBL | Assay Description Inhibition of Torpedo californica AchE activity by Ellman's assay | Bioorg Med Chem 16: 6560-7 (2008) Article DOI: 10.1016/j.bmc.2008.05.027 BindingDB Entry DOI: 10.7270/Q29Z95T9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50562020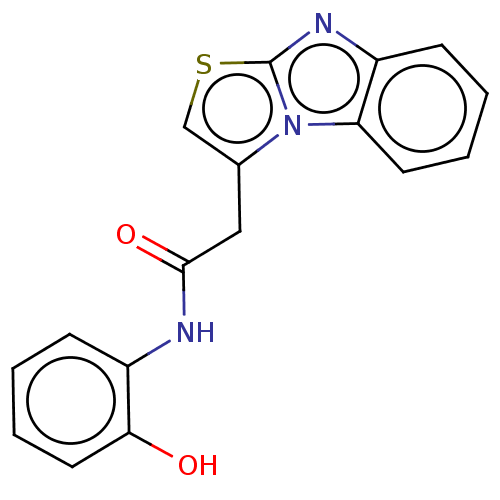 (CHEMBL4798627) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.50E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of EGFR (unknown origin) | Citation and Details Article DOI: 10.1039/d0md00436g BindingDB Entry DOI: 10.7270/Q2NS0ZNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||