 Found 108 hits with Last Name = 'shibue' and Initial = 't'
Found 108 hits with Last Name = 'shibue' and Initial = 't' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50084125
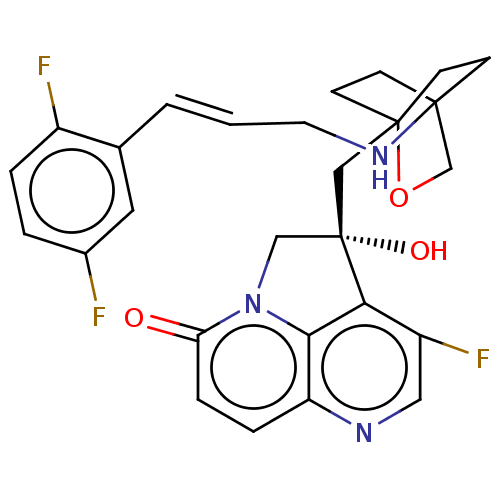
(CHEMBL3425935)Show SMILES O[C@@]1(CC23CCC(CC2)(CO3)NC\C=C\c2cc(F)ccc2F)Cn2c3c1c(F)cnc3ccc2=O |r,wD:1.0,(2.14,2.31,;.92,2.5,;.32,3.91,;-1.21,4.13,;-1.59,2.67,;-3.12,2.49,;-4.05,3.74,;-2.63,3.19,;-2.41,4.71,;-3.43,5.14,;-1.91,5.34,;-5.55,3.5,;-6.11,2.06,;-7.63,1.82,;-8.19,.38,;-9.72,.15,;-10.69,1.35,;-12.21,1.12,;-12.99,2.08,;-12.77,-.32,;-11.81,-1.53,;-10.28,-1.29,;-9.51,-2.26,;-1,2.52,;-1.3,1,;,.24,;1.32,1,;2.63,.24,;3.7,.86,;2.63,-1.26,;1.32,-2.01,;,-1.26,;-1.3,-2.01,;-2.61,-1.26,;-2.61,.24,;-3.68,.86,)| Show InChI InChI=1S/C27H26F3N3O3/c28-18-3-4-19(29)17(12-18)2-1-11-32-25-7-9-26(10-8-25,36-16-25)14-27(35)15-33-22(34)6-5-21-24(33)23(27)20(30)13-31-21/h1-6,12-13,32,35H,7-11,14-16H2/b2-1+/t25?,26?,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of MK499 from human ERG |
Bioorg Med Chem Lett 25: 2473-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.063
BindingDB Entry DOI: 10.7270/Q2NZ89BD |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50084144
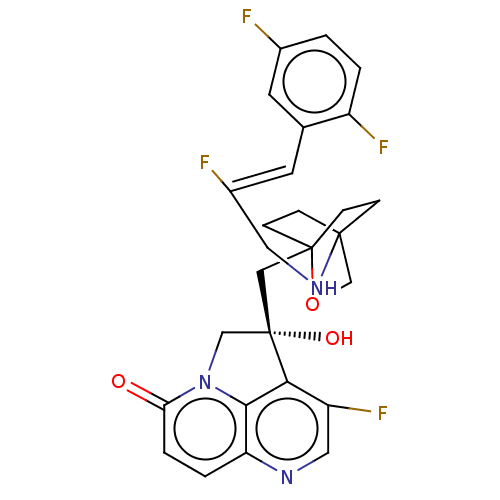
(CHEMBL3425938)Show SMILES O[C@@]1(CC23CCC(CC2)(CO3)NC\C(F)=C\c2cc(F)ccc2F)Cn2c3c1c(F)cnc3ccc2=O |r,wD:1.0,(2.14,2.31,;.92,2.5,;.32,3.91,;-1.21,4.13,;-1.59,2.67,;-3.12,2.49,;-4.05,3.74,;-2.63,3.19,;-2.41,4.71,;-3.43,5.14,;-1.91,5.34,;-5.55,3.5,;-6.11,2.06,;-7.63,1.82,;-8.41,2.79,;-8.19,.38,;-9.72,.15,;-10.69,1.35,;-12.21,1.12,;-12.99,2.08,;-12.77,-.32,;-11.81,-1.53,;-10.28,-1.29,;-9.51,-2.26,;-1,2.52,;-1.3,1,;,.24,;1.32,1,;2.63,.24,;3.7,.86,;2.63,-1.26,;1.32,-2.01,;,-1.26,;-1.3,-2.01,;-2.61,-1.26,;-2.61,.24,;-3.68,.86,)| Show InChI InChI=1S/C27H25F4N3O3/c28-17-1-2-19(30)16(9-17)10-18(29)11-33-25-5-7-26(8-6-25,37-15-25)13-27(36)14-34-22(35)4-3-21-24(34)23(27)20(31)12-32-21/h1-4,9-10,12,33,36H,5-8,11,13-15H2/b18-10-/t25?,26?,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of MK499 from human ERG |
Bioorg Med Chem Lett 25: 2473-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.063
BindingDB Entry DOI: 10.7270/Q2NZ89BD |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50084143

(CHEMBL3425937)Show SMILES O[C@@]1(CC23CCC(CC2)(CO3)NC\C(F)=C/c2cc(F)ccc2F)Cn2c3c1c(F)cnc3ccc2=O |r,wD:1.0,(2.14,2.31,;.92,2.5,;.32,3.91,;-1.21,4.13,;-1.59,2.67,;-3.12,2.49,;-4.05,3.74,;-2.63,3.19,;-2.41,4.71,;-3.43,5.14,;-1.91,5.34,;-5.55,3.5,;-6.11,2.06,;-7.63,1.82,;-8.41,2.79,;-8.19,.38,;-7.23,-.82,;-5.7,-.58,;-4.73,-1.78,;-3.51,-1.59,;-5.29,-3.22,;-6.81,-3.46,;-7.78,-2.26,;-9,-2.45,;-1,2.52,;-1.3,1,;,.24,;1.32,1,;2.63,.24,;3.7,.86,;2.63,-1.26,;1.32,-2.01,;,-1.26,;-1.3,-2.01,;-2.61,-1.26,;-2.61,.24,;-3.68,.86,)| Show InChI InChI=1S/C27H25F4N3O3/c28-17-1-2-19(30)16(9-17)10-18(29)11-33-25-5-7-26(8-6-25,37-15-25)13-27(36)14-34-22(35)4-3-21-24(34)23(27)20(31)12-32-21/h1-4,9-10,12,33,36H,5-8,11,13-15H2/b18-10+/t25?,26?,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of MK499 from human ERG |
Bioorg Med Chem Lett 25: 2473-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.063
BindingDB Entry DOI: 10.7270/Q2NZ89BD |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50084042
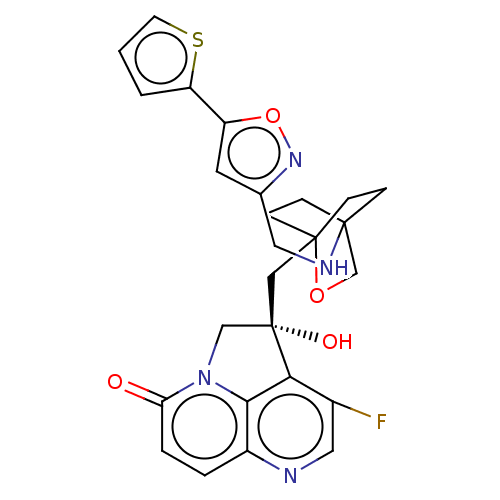
(CHEMBL3425943)Show SMILES O[C@@]1(CC23CCC(CC2)(CO3)NCc2cc(on2)-c2cccs2)Cn2c3c1c(F)cnc3ccc2=O |r,wD:1.0,(2.15,2.33,;.93,2.51,;.33,3.93,;-1.21,4.15,;-1.6,2.69,;-3.13,2.51,;-4.07,3.76,;-2.64,3.2,;-2.42,4.74,;-3.45,5.17,;-1.92,5.37,;-5.58,3.52,;-6.14,2.07,;-7.68,1.83,;-8.37,.46,;-9.9,.71,;-10.14,2.24,;-8.75,2.94,;-11.01,-.39,;-10.87,-1.92,;-12.31,-2.5,;-13.31,-1.32,;-12.5,0,;-1,2.53,;-1.3,1,;,.25,;1.32,1,;2.65,.25,;3.72,.87,;2.65,-1.27,;1.32,-2.02,;,-1.27,;-1.3,-2.02,;-2.63,-1.27,;-2.63,.25,;-3.7,.87,)| Show InChI InChI=1S/C26H25FN4O4S/c27-17-12-28-18-3-4-21(32)31-14-26(33,22(17)23(18)31)13-25-7-5-24(6-8-25,15-34-25)29-11-16-10-19(35-30-16)20-2-1-9-36-20/h1-4,9-10,12,29,33H,5-8,11,13-15H2/t24?,25?,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of MK499 from human ERG |
Bioorg Med Chem Lett 25: 2473-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.063
BindingDB Entry DOI: 10.7270/Q2NZ89BD |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50084124
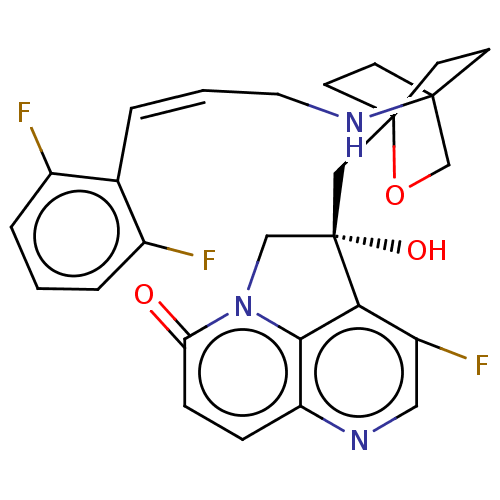
(CHEMBL3425934)Show SMILES O[C@@]1(CC23CCC(CC2)(CO3)NC\C=C\c2c(F)cccc2F)Cn2c3c1c(F)cnc3ccc2=O |r,wD:1.0,(2.14,2.31,;.92,2.5,;.32,3.91,;-1.21,4.13,;-1.59,2.67,;-3.12,2.49,;-4.05,3.74,;-2.63,3.19,;-2.41,4.71,;-3.43,5.14,;-1.91,5.34,;-5.55,3.5,;-6.11,2.06,;-7.63,1.82,;-8.19,.38,;-9.72,.15,;-10.69,1.35,;-10.24,2.5,;-12.21,1.12,;-12.77,-.32,;-11.81,-1.53,;-10.28,-1.29,;-9.51,-2.26,;-1,2.52,;-1.3,1,;,.24,;1.32,1,;2.63,.24,;3.7,.86,;2.63,-1.26,;1.32,-2.01,;,-1.26,;-1.3,-2.01,;-2.61,-1.26,;-2.61,.24,;-3.68,.86,)| Show InChI InChI=1S/C27H26F3N3O3/c28-18-4-1-5-19(29)17(18)3-2-12-32-25-8-10-26(11-9-25,36-16-25)14-27(35)15-33-22(34)7-6-21-24(33)23(27)20(30)13-31-21/h1-7,13,32,35H,8-12,14-16H2/b3-2+/t25?,26?,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of MK499 from human ERG |
Bioorg Med Chem Lett 25: 2473-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.063
BindingDB Entry DOI: 10.7270/Q2NZ89BD |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50084147

(CHEMBL3425941)Show SMILES O[C@@]1(CC23CCC(CC2)(CO3)NCCCc2cc(F)ccc2F)Cn2c3c1c(F)cnc3ccc2=O |r,wD:1.0,(2.14,2.31,;.92,2.5,;.32,3.91,;-1.21,4.13,;-1.59,2.67,;-3.12,2.49,;-4.05,3.74,;-2.63,3.19,;-2.41,4.71,;-3.43,5.14,;-1.91,5.34,;-5.55,3.5,;-6.11,2.06,;-7.63,1.82,;-8.19,.38,;-9.72,.15,;-10.69,1.35,;-12.21,1.12,;-12.99,2.08,;-12.77,-.32,;-11.81,-1.53,;-10.28,-1.29,;-9.51,-2.26,;-1,2.52,;-1.3,1,;,.24,;1.32,1,;2.63,.24,;3.7,.86,;2.63,-1.26,;1.32,-2.01,;,-1.26,;-1.3,-2.01,;-2.61,-1.26,;-2.61,.24,;-3.68,.86,)| Show InChI InChI=1S/C27H28F3N3O3/c28-18-3-4-19(29)17(12-18)2-1-11-32-25-7-9-26(10-8-25,36-16-25)14-27(35)15-33-22(34)6-5-21-24(33)23(27)20(30)13-31-21/h3-6,12-13,32,35H,1-2,7-11,14-16H2/t25?,26?,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of MK499 from human ERG |
Bioorg Med Chem Lett 25: 2473-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.063
BindingDB Entry DOI: 10.7270/Q2NZ89BD |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50084148

(CHEMBL3425942)Show SMILES O[C@@]1(CC23CCC(CC2)(CO3)NCOc2cc(F)ccc2F)Cn2c3c1c(F)cnc3ccc2=O |r,wD:1.0,(2.15,2.32,;.93,2.51,;.33,3.93,;-1.21,4.15,;-1.6,2.69,;-3.13,2.51,;-4.07,3.76,;-2.64,3.2,;-2.42,4.74,;-3.45,5.17,;-1.92,5.37,;-5.58,3.52,;-6.14,2.07,;-7.68,1.83,;-8.24,.39,;-7.26,-.82,;-7.82,-2.27,;-7.04,-3.24,;-9.35,-2.51,;-10.33,-1.3,;-9.77,.14,;-10.55,1.11,;-1,2.53,;-1.3,1,;,.25,;1.32,1,;2.64,.25,;3.72,.87,;2.64,-1.27,;1.32,-2.02,;,-1.27,;-1.3,-2.02,;-2.63,-1.27,;-2.63,.25,;-3.7,.87,)| Show InChI InChI=1S/C25H24F3N3O4/c26-15-1-2-16(27)19(9-15)34-14-30-23-5-7-24(8-6-23,35-13-23)11-25(33)12-31-20(32)4-3-18-22(31)21(25)17(28)10-29-18/h1-4,9-10,30,33H,5-8,11-14H2/t23?,24?,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of MK499 from human ERG |
Bioorg Med Chem Lett 25: 2473-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.063
BindingDB Entry DOI: 10.7270/Q2NZ89BD |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50084145

(CHEMBL3425939)Show SMILES O[C@@]1(CC23CCC(CC2)(CO3)NC\C=C\c2ccccc2)Cn2c3c1c(F)cnc3ccc2=O |r,wD:1.0,(2.14,2.31,;.92,2.5,;.32,3.91,;-1.21,4.13,;-1.59,2.67,;-3.12,2.49,;-4.05,3.74,;-2.63,3.19,;-2.41,4.71,;-3.43,5.14,;-1.91,5.34,;-5.55,3.5,;-6.11,2.06,;-7.63,1.82,;-8.19,.38,;-9.72,.15,;-10.28,-1.29,;-11.81,-1.53,;-12.77,-.32,;-12.21,1.12,;-10.69,1.35,;-1,2.52,;-1.3,1,;,.24,;1.32,1,;2.63,.24,;3.7,.86,;2.63,-1.26,;1.32,-2.01,;,-1.26,;-1.3,-2.01,;-2.61,-1.26,;-2.61,.24,;-3.68,.86,)| Show InChI InChI=1S/C27H28FN3O3/c28-20-15-29-21-8-9-22(32)31-17-27(33,23(20)24(21)31)16-26-12-10-25(11-13-26,18-34-26)30-14-4-7-19-5-2-1-3-6-19/h1-9,15,30,33H,10-14,16-18H2/b7-4+/t25?,26?,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of MK499 from human ERG |
Bioorg Med Chem Lett 25: 2473-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.063
BindingDB Entry DOI: 10.7270/Q2NZ89BD |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50084146
(CHEMBL3425940)Show SMILES O[C@@]1(CC23CCC(CC2)(CO3)NC\C=C\c2ccccn2)Cn2c3c1c(F)cnc3ccc2=O |r,wD:1.0,(2.15,2.32,;.93,2.51,;.33,3.93,;-1.21,4.14,;-1.59,2.68,;-3.13,2.5,;-4.07,3.76,;-2.64,3.2,;-2.42,4.73,;-3.45,5.16,;-1.92,5.36,;-5.58,3.52,;-6.14,2.07,;-7.67,1.83,;-8.23,.39,;-9.76,.15,;-10.74,1.36,;-12.27,1.12,;-12.83,-.33,;-11.86,-1.54,;-10.33,-1.3,;-1,2.53,;-1.3,1,;,.25,;1.32,1,;2.64,.25,;3.72,.87,;2.64,-1.26,;1.32,-2.02,;,-1.26,;-1.3,-2.02,;-2.62,-1.26,;-2.62,.25,;-3.7,.87,)| Show InChI InChI=1S/C26H27FN4O3/c27-19-14-29-20-6-7-21(32)31-16-26(33,22(19)23(20)31)15-25-10-8-24(9-11-25,17-34-25)30-13-3-5-18-4-1-2-12-28-18/h1-7,12,14,30,33H,8-11,13,15-17H2/b5-3+/t24?,25?,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of MK499 from human ERG |
Bioorg Med Chem Lett 25: 2473-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.063
BindingDB Entry DOI: 10.7270/Q2NZ89BD |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50084126
(CHEMBL3425936)Show SMILES O[C@@]1(CC23CCC(CC2)(CO3)NC\C=C\c2ccc(F)cc2)Cn2c3c1c(F)cnc3ccc2=O |r,wD:1.0,(2.14,2.31,;.92,2.5,;.32,3.91,;-1.21,4.13,;-1.59,2.67,;-3.12,2.49,;-4.05,3.74,;-2.63,3.19,;-2.41,4.71,;-3.43,5.14,;-1.91,5.34,;-5.55,3.5,;-6.11,2.06,;-7.63,1.82,;-8.19,.38,;-9.72,.15,;-10.28,-1.29,;-11.81,-1.53,;-12.77,-.32,;-13.99,-.51,;-12.21,1.12,;-10.69,1.35,;-1,2.52,;-1.3,1,;,.24,;1.32,1,;2.63,.24,;3.7,.86,;2.63,-1.26,;1.32,-2.01,;,-1.26,;-1.3,-2.01,;-2.61,-1.26,;-2.61,.24,;-3.68,.86,)| Show InChI InChI=1S/C27H27F2N3O3/c28-19-5-3-18(4-6-19)2-1-13-31-25-9-11-26(12-10-25,35-17-25)15-27(34)16-32-22(33)8-7-21-24(32)23(27)20(29)14-30-21/h1-8,14,31,34H,9-13,15-17H2/b2-1+/t25?,26?,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of MK499 from human ERG |
Bioorg Med Chem Lett 25: 2473-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.063
BindingDB Entry DOI: 10.7270/Q2NZ89BD |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50113633

(CHEMBL3604691)Show SMILES COc1ccc2ncc(F)c(CCC34CCC(CC3)(CO4)NCc3cc(C)c4OCC(=O)Nc4n3)c2n1 |(-4.02,2.78,;-4.02,1.54,;-2.69,.77,;-2.69,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.67,-.77,;2.67,.77,;3.74,1.39,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.05,;4.42,14.95,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;,.77,;-1.33,1.54,)| Show InChI InChI=1S/C27H30FN5O4/c1-16-11-17(31-25-24(16)36-14-21(34)32-25)12-30-26-7-9-27(10-8-26,37-15-26)6-5-18-19(28)13-29-20-3-4-22(35-2)33-23(18)20/h3-4,11,13,30H,5-10,12,14-15H2,1-2H3,(H,31,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 3636-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.057
BindingDB Entry DOI: 10.7270/Q2VH5QNN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50113638

(CHEMBL3604797)Show SMILES COc1ccc2ncc(F)c(CCC34CCC(CC3)(CO4)NCc3nc4NC(=O)COc4c(C)c3C=C)c2n1 |(-4.02,2.78,;-4.02,1.54,;-2.69,.77,;-2.69,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.67,-.77,;2.67,.77,;3.74,1.39,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.57,;5.24,14.05,;4.19,15.17,;4.63,16.64,;3.78,17.54,;6.13,17,;7.18,15.88,;6.74,14.4,;7.8,13.28,;9,13.57,;7.36,11.79,;8.41,10.66,;8.05,9.48,;,.77,;-1.33,1.54,)| Show InChI InChI=1S/C29H32FN5O4/c1-4-18-17(2)26-27(34-23(36)15-38-26)33-22(18)14-32-28-9-11-29(12-10-28,39-16-28)8-7-19-20(30)13-31-21-5-6-24(37-3)35-25(19)21/h4-6,13,32H,1,7-12,14-16H2,2-3H3,(H,33,34,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 3636-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.057
BindingDB Entry DOI: 10.7270/Q2VH5QNN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50113634

(CHEMBL3604692)Show SMILES COc1ccc2ncc(F)c(CCC34CCC(CC3)(CO4)NCc3nc4NC(=O)COc4c(C)c3C)c2n1 |(-4.02,2.78,;-4.02,1.54,;-2.69,.77,;-2.69,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.67,-.77,;2.67,.77,;3.74,1.39,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.57,;5.24,14.05,;4.19,15.17,;4.63,16.64,;3.78,17.54,;6.13,17,;7.18,15.88,;6.74,14.4,;7.8,13.28,;9,13.57,;7.36,11.79,;8.2,10.89,;,.77,;-1.33,1.54,)| Show InChI InChI=1S/C28H32FN5O4/c1-16-17(2)25-26(33-22(35)14-37-25)32-21(16)13-31-27-8-10-28(11-9-27,38-15-27)7-6-18-19(29)12-30-20-4-5-23(36-3)34-24(18)20/h4-5,12,31H,6-11,13-15H2,1-3H3,(H,32,33,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 3636-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.057
BindingDB Entry DOI: 10.7270/Q2VH5QNN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082303
(CHEMBL3305005)Show SMILES COc1ccc2ncc(F)c(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)c2n1 |(5.32,-5.97,;5.32,-7.51,;6.66,-8.28,;6.65,-9.83,;7.99,-10.6,;9.32,-9.82,;10.66,-10.59,;11.99,-9.81,;11.98,-8.26,;11.97,-6.72,;10.65,-7.5,;10.64,-5.96,;11.97,-5.19,;13.3,-5.95,;13.3,-7.49,;14.63,-8.25,;15.97,-7.48,;15.28,-6.35,;14.06,-7.27,;15.96,-5.94,;14.62,-5.17,;17.29,-8.27,;17.28,-9.81,;18.6,-10.59,;19.93,-9.84,;21.25,-10.62,;21.24,-12.16,;22.57,-12.94,;22.54,-14.49,;21.2,-15.24,;21.18,-16.78,;19.88,-14.45,;19.9,-12.92,;18.57,-12.13,;9.32,-8.28,;7.98,-7.51,)| Show InChI InChI=1S/C26H28FN5O4/c1-34-22-5-3-19-23(32-22)17(18(27)13-28-19)6-7-26-10-8-25(9-11-26,15-36-26)29-12-16-2-4-20-24(30-16)31-21(33)14-35-20/h2-5,13,29H,6-12,14-15H2,1H3,(H,30,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHO cells measured for 5 mins by automated patch clamp assay |
Bioorg Med Chem Lett 25: 3636-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.057
BindingDB Entry DOI: 10.7270/Q2VH5QNN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082303

(CHEMBL3305005)Show SMILES COc1ccc2ncc(F)c(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)c2n1 |(5.32,-5.97,;5.32,-7.51,;6.66,-8.28,;6.65,-9.83,;7.99,-10.6,;9.32,-9.82,;10.66,-10.59,;11.99,-9.81,;11.98,-8.26,;11.97,-6.72,;10.65,-7.5,;10.64,-5.96,;11.97,-5.19,;13.3,-5.95,;13.3,-7.49,;14.63,-8.25,;15.97,-7.48,;15.28,-6.35,;14.06,-7.27,;15.96,-5.94,;14.62,-5.17,;17.29,-8.27,;17.28,-9.81,;18.6,-10.59,;19.93,-9.84,;21.25,-10.62,;21.24,-12.16,;22.57,-12.94,;22.54,-14.49,;21.2,-15.24,;21.18,-16.78,;19.88,-14.45,;19.9,-12.92,;18.57,-12.13,;9.32,-8.28,;7.98,-7.51,)| Show InChI InChI=1S/C26H28FN5O4/c1-34-22-5-3-19-23(32-22)17(18(27)13-28-19)6-7-26-10-8-25(9-11-26,15-36-26)29-12-16-2-4-20-24(30-16)31-21(33)14-35-20/h2-5,13,29H,6-12,14-15H2,1H3,(H,30,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Activity at beta-1 adrenergic receptor |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50113635
(CHEMBL3604693)Show SMILES CCc1c(C)c2OCC(=O)Nc2nc1CNC12CCC(CCc3c(F)cnc4ccc(OC)nc34)(CC1)OC2 |(8.05,9.48,;8.41,10.66,;7.36,11.79,;7.8,13.28,;9,13.57,;6.74,14.4,;7.18,15.88,;6.13,17,;4.63,16.64,;3.78,17.54,;4.19,15.17,;5.24,14.05,;4.8,12.57,;5.86,11.44,;5.41,9.96,;3.9,9.61,;3.46,8.16,;4.56,7.06,;4.17,5.57,;2.68,5.4,;2.67,3.85,;1.34,3.08,;1.33,1.54,;2.67,.77,;3.74,1.39,;2.67,-.77,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.69,-.77,;-2.69,.77,;-4.02,1.54,;-4.02,2.78,;-1.33,1.54,;,.77,;2.27,6.67,;3.81,6.67,;1.58,6.27,;1.98,7.74,)| Show InChI InChI=1S/C29H34FN5O4/c1-4-18-17(2)26-27(34-23(36)15-38-26)33-22(18)14-32-28-9-11-29(12-10-28,39-16-28)8-7-19-20(30)13-31-21-5-6-24(37-3)35-25(19)21/h5-6,13,32H,4,7-12,14-16H2,1-3H3,(H,33,34,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 3636-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.057
BindingDB Entry DOI: 10.7270/Q2VH5QNN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50113640

(CHEMBL3604799)Show SMILES COc1ccc2ncc(F)c(CCC34CCC(CC3)(CO4)NCc3ccc4OC(C)C(=O)Nc4n3)c2n1 |(-4.02,2.78,;-4.02,1.54,;-2.69,.77,;-2.69,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.67,-.77,;2.67,.77,;3.74,1.39,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.05,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.07,17.4,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;,.77,;-1.33,1.54,)| Show InChI InChI=1S/C27H30FN5O4/c1-16-25(34)33-24-21(37-16)5-3-17(31-24)13-30-26-9-11-27(12-10-26,36-15-26)8-7-18-19(28)14-29-20-4-6-22(35-2)32-23(18)20/h3-6,14,16,30H,7-13,15H2,1-2H3,(H,31,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 3636-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.057
BindingDB Entry DOI: 10.7270/Q2VH5QNN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50113640

(CHEMBL3604799)Show SMILES COc1ccc2ncc(F)c(CCC34CCC(CC3)(CO4)NCc3ccc4OC(C)C(=O)Nc4n3)c2n1 |(-4.02,2.78,;-4.02,1.54,;-2.69,.77,;-2.69,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.67,-.77,;2.67,.77,;3.74,1.39,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.05,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.07,17.4,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;,.77,;-1.33,1.54,)| Show InChI InChI=1S/C27H30FN5O4/c1-16-25(34)33-24-21(37-16)5-3-17(31-24)13-30-26-9-11-27(12-10-26,36-15-26)8-7-18-19(28)14-29-20-4-6-22(35-2)32-23(18)20/h3-6,14,16,30H,7-13,15H2,1-2H3,(H,31,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 3636-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.057
BindingDB Entry DOI: 10.7270/Q2VH5QNN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50113643

(CHEMBL3604802)Show SMILES COc1ccc2ncc(F)c(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)N(C)c4n3)c2n1 |(-4.02,2.78,;-4.02,1.54,;-2.69,.77,;-2.69,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.67,-.77,;2.67,.77,;3.74,1.39,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;10.16,12.72,;7.81,13.27,;7.37,11.8,;,.77,;-1.33,1.54,)| Show InChI InChI=1S/C27H30FN5O4/c1-33-23(34)15-36-21-5-3-17(31-25(21)33)13-30-26-9-11-27(12-10-26,37-16-26)8-7-18-19(28)14-29-20-4-6-22(35-2)32-24(18)20/h3-6,14,30H,7-13,15-16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 3636-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.057
BindingDB Entry DOI: 10.7270/Q2VH5QNN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50113639

(CHEMBL3604798)Show SMILES COc1ccc2ncc(F)c(CCC34CCC(CC3)(CO4)NCc3nc4NC(=O)COc4c(C)c3F)c2n1 |(-4.02,2.78,;-4.02,1.54,;-2.69,.77,;-2.69,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.67,-.77,;2.67,.77,;3.74,1.39,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.57,;5.24,14.05,;4.19,15.17,;4.63,16.64,;3.78,17.54,;6.13,17,;7.18,15.88,;6.74,14.4,;7.8,13.28,;9,13.57,;7.36,11.79,;8.2,10.89,;,.77,;-1.33,1.54,)| Show InChI InChI=1S/C27H29F2N5O4/c1-15-22(29)19(32-25-24(15)37-13-20(35)33-25)12-31-26-7-9-27(10-8-26,38-14-26)6-5-16-17(28)11-30-18-3-4-21(36-2)34-23(16)18/h3-4,11,31H,5-10,12-14H2,1-2H3,(H,32,33,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 3636-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.057
BindingDB Entry DOI: 10.7270/Q2VH5QNN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50113632

(CHEMBL3604690)Show SMILES COc1ccc2ncc(F)c(CCC34CCC(CC3)(CO4)NCc3nc4NC(=O)COc4cc3C)c2n1 |(-4.02,2.78,;-4.02,1.54,;-2.69,.77,;-2.69,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.67,-.77,;2.67,.77,;3.74,1.39,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.57,;5.24,14.05,;4.19,15.17,;4.63,16.64,;3.78,17.54,;6.13,17,;7.18,15.88,;6.74,14.4,;7.8,13.28,;7.36,11.79,;8.2,10.89,;,.77,;-1.33,1.54,)| Show InChI InChI=1S/C27H30FN5O4/c1-16-11-21-25(32-22(34)14-36-21)31-20(16)13-30-26-7-9-27(10-8-26,37-15-26)6-5-17-18(28)12-29-19-3-4-23(35-2)33-24(17)19/h3-4,11-12,30H,5-10,13-15H2,1-2H3,(H,31,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 3636-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.057
BindingDB Entry DOI: 10.7270/Q2VH5QNN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50113637

(CHEMBL3604694)Show SMILES COc1ccc2ncc(F)c(CCC34CCC(CC3)(CO4)NCc3nc4NC(=O)COc4c(C)c3C(C)C)c2n1 |(-4.02,2.78,;-4.02,1.54,;-2.69,.77,;-2.69,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.67,-.77,;2.67,.77,;3.74,1.39,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.57,;5.24,14.05,;4.19,15.17,;4.63,16.64,;3.78,17.54,;6.13,17,;7.18,15.88,;6.74,14.4,;7.8,13.28,;9,13.57,;7.36,11.79,;8.41,10.66,;8.05,9.48,;9.61,10.94,;,.77,;-1.33,1.54,)| Show InChI InChI=1S/C30H36FN5O4/c1-17(2)25-18(3)27-28(35-23(37)15-39-27)34-22(25)14-33-29-9-11-30(12-10-29,40-16-29)8-7-19-20(31)13-32-21-5-6-24(38-4)36-26(19)21/h5-6,13,17,33H,7-12,14-16H2,1-4H3,(H,34,35,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 3636-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.057
BindingDB Entry DOI: 10.7270/Q2VH5QNN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50113644
(CHEMBL3604803)Show SMILES COc1ccc2ncc(F)c(CCC34CCC(CC3)(CO4)N(C)Cc3ccc4OCC(=O)Nc4n3)c2n1 |(-4.02,2.78,;-4.02,1.54,;-2.69,.77,;-2.69,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.67,-.77,;2.67,.77,;3.74,1.39,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;3.06,10.51,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;,.77,;-1.33,1.54,)| Show InChI InChI=1S/C27H30FN5O4/c1-33(14-17-3-5-21-25(30-17)31-22(34)15-36-21)26-9-11-27(12-10-26,37-16-26)8-7-18-19(28)13-29-20-4-6-23(35-2)32-24(18)20/h3-6,13H,7-12,14-16H2,1-2H3,(H,30,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 3636-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.057
BindingDB Entry DOI: 10.7270/Q2VH5QNN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50113631
(CHEMBL3604688)Show SMILES COc1ccc2ncc(F)c(CCC34CCC(CC3)(CO4)NCc3nc4NC(=O)COc4cc3F)c2n1 |(-4.02,2.78,;-4.02,1.54,;-2.69,.77,;-2.69,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.67,-.77,;2.67,.77,;3.74,1.39,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.57,;5.24,14.05,;4.19,15.17,;4.63,16.64,;3.78,17.54,;6.13,17,;7.18,15.88,;6.74,14.4,;7.79,13.28,;7.36,11.79,;8.2,10.89,;,.77,;-1.33,1.54,)| Show InChI InChI=1S/C26H27F2N5O4/c1-35-22-3-2-18-23(33-22)15(17(28)11-29-18)4-5-26-8-6-25(7-9-26,14-37-26)30-12-19-16(27)10-20-24(31-19)32-21(34)13-36-20/h2-3,10-11,30H,4-9,12-14H2,1H3,(H,31,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 3636-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.057
BindingDB Entry DOI: 10.7270/Q2VH5QNN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50050549

(GSK2140944 | Gepotidacin)Show SMILES O=c1ccc2ncc(=O)n3[C@H](CN4CCC(CC4)NCc4cc5CCCOc5cn4)Cn1c23 |r| Show InChI InChI=1S/C24H28N6O3/c31-22-4-3-20-24-29(22)15-19(30(24)23(32)13-27-20)14-28-7-5-17(6-8-28)25-11-18-10-16-2-1-9-33-21(16)12-26-18/h3-4,10,12-13,17,19,25H,1-2,5-9,11,14-15H2/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Compound was tested for the biological activity at the Beta-1 adrenergic receptor |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50483304
(11-Epi-Tubulysin D)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H]1CCCCN1C)C(=O)N[C@H](C[C@H](OC(C)=O)c1nc(cs1)C(=O)N[C@H](C[C@H](C)C(O)=O)Cc1ccccc1)C(C)C |r| Show InChI InChI=1S/C37H55N5O7S/c1-8-23(4)32(41-34(45)30-16-12-13-17-42(30)7)35(46)39-28(22(2)3)20-31(49-25(6)43)36-40-29(21-50-36)33(44)38-27(18-24(5)37(47)48)19-26-14-10-9-11-15-26/h9-11,14-15,21-24,27-28,30-32H,8,12-13,16-20H2,1-7H3,(H,38,44)(H,39,46)(H,41,45)(H,47,48)/t23-,24-,27+,28+,30+,31-,32-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co Ltd
Curated by ChEMBL
| Assay Description
Inhibition of porcine brain tubulin polymerization by microtubule assembly assay |
Bioorg Med Chem Lett 21: 431-4 (2011)
Article DOI: 10.1016/j.bmcl.2010.10.118
BindingDB Entry DOI: 10.7270/Q21J9DMW |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50012278
((2ALPHA,2''BETA,3BETA,4ALPHA,5BETA)-VINCALEUKOBLAS...)Show SMILES CC[C@]1(O)C[C@@H]2CN(C1)CCc1c([nH]c3ccccc13)[C@@](C2)(C(=O)OC)c1cc2c(cc1OC)N(C)[C@@H]1[C@]22CCN3CC=C[C@](CC)([C@@H]23)[C@@H](OC(C)=O)[C@]1(O)C(=O)OC |r,c:48| Show InChI InChI=1S/C46H58N4O9/c1-8-42(54)23-28-24-45(40(52)57-6,36-30(15-19-49(25-28)26-42)29-13-10-11-14-33(29)47-36)32-21-31-34(22-35(32)56-5)48(4)38-44(31)17-20-50-18-12-16-43(9-2,37(44)50)39(59-27(3)51)46(38,55)41(53)58-7/h10-14,16,21-22,28,37-39,47,54-55H,8-9,15,17-20,23-26H2,1-7H3/t28-,37-,38+,39+,42-,43+,44+,45-,46-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co Ltd
Curated by ChEMBL
| Assay Description
Inhibition of porcine brain tubulin polymerization by microtubule assembly assay |
Bioorg Med Chem Lett 21: 431-4 (2011)
Article DOI: 10.1016/j.bmcl.2010.10.118
BindingDB Entry DOI: 10.7270/Q21J9DMW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tubulin beta chain
(Sus scrofa) | BDBM50483305
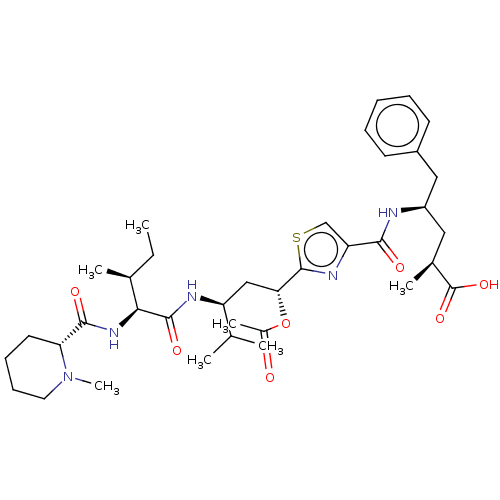
(13-Epi-Tubulysin D)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H]1CCCCN1C)C(=O)N[C@@H](C[C@@H](OC(C)=O)c1nc(cs1)C(=O)N[C@H](C[C@H](C)C(O)=O)Cc1ccccc1)C(C)C |r| Show InChI InChI=1S/C37H55N5O7S/c1-8-23(4)32(41-34(45)30-16-12-13-17-42(30)7)35(46)39-28(22(2)3)20-31(49-25(6)43)36-40-29(21-50-36)33(44)38-27(18-24(5)37(47)48)19-26-14-10-9-11-15-26/h9-11,14-15,21-24,27-28,30-32H,8,12-13,16-20H2,1-7H3,(H,38,44)(H,39,46)(H,41,45)(H,47,48)/t23-,24-,27+,28-,30+,31+,32-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co Ltd
Curated by ChEMBL
| Assay Description
Inhibition of porcine brain tubulin polymerization by microtubule assembly assay |
Bioorg Med Chem Lett 21: 431-4 (2011)
Article DOI: 10.1016/j.bmcl.2010.10.118
BindingDB Entry DOI: 10.7270/Q21J9DMW |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50483306
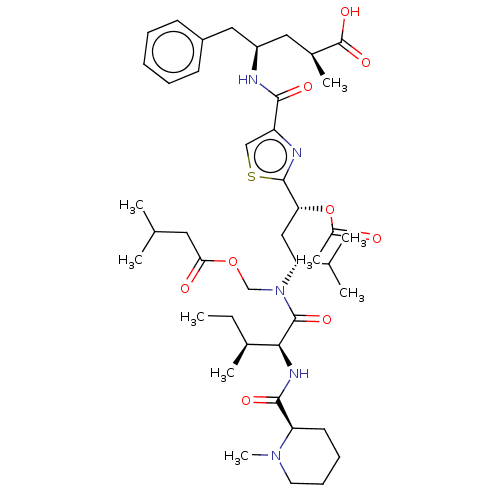
(CHEBI:80036 | Tubulysin D)Show SMILES [H][C@](NC(=O)[C@@]1([H])CCCCN1C)([C@@H](C)CC)C(=O)N(COC(=O)CC(C)C)[C@H](C[C@@H](OC(C)=O)c1nc(cs1)C(=O)N[C@H](C[C@H](C)C(O)=O)Cc1ccccc1)C(C)C |r| Show InChI InChI=1S/C43H65N5O9S/c1-10-28(6)38(46-40(52)34-18-14-15-19-47(34)9)42(53)48(25-56-37(50)20-26(2)3)35(27(4)5)23-36(57-30(8)49)41-45-33(24-58-41)39(51)44-32(21-29(7)43(54)55)22-31-16-12-11-13-17-31/h11-13,16-17,24,26-29,32,34-36,38H,10,14-15,18-23,25H2,1-9H3,(H,44,51)(H,46,52)(H,54,55)/t28-,29-,32+,34+,35+,36+,38-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co Ltd
Curated by ChEMBL
| Assay Description
Inhibition of porcine brain tubulin polymerization by microtubule assembly assay |
Bioorg Med Chem Lett 21: 431-4 (2011)
Article DOI: 10.1016/j.bmcl.2010.10.118
BindingDB Entry DOI: 10.7270/Q21J9DMW |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082303

(CHEMBL3305005)Show SMILES COc1ccc2ncc(F)c(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)c2n1 |(5.32,-5.97,;5.32,-7.51,;6.66,-8.28,;6.65,-9.83,;7.99,-10.6,;9.32,-9.82,;10.66,-10.59,;11.99,-9.81,;11.98,-8.26,;11.97,-6.72,;10.65,-7.5,;10.64,-5.96,;11.97,-5.19,;13.3,-5.95,;13.3,-7.49,;14.63,-8.25,;15.97,-7.48,;15.28,-6.35,;14.06,-7.27,;15.96,-5.94,;14.62,-5.17,;17.29,-8.27,;17.28,-9.81,;18.6,-10.59,;19.93,-9.84,;21.25,-10.62,;21.24,-12.16,;22.57,-12.94,;22.54,-14.49,;21.2,-15.24,;21.18,-16.78,;19.88,-14.45,;19.9,-12.92,;18.57,-12.13,;9.32,-8.28,;7.98,-7.51,)| Show InChI InChI=1S/C26H28FN5O4/c1-34-22-5-3-19-23(32-22)17(18(27)13-28-19)6-7-26-10-8-25(9-11-26,15-36-26)29-12-16-2-4-20-24(30-16)31-21(33)14-35-20/h2-5,13,29H,6-12,14-15H2,1H3,(H,30,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 3636-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.057
BindingDB Entry DOI: 10.7270/Q2VH5QNN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50113633

(CHEMBL3604691)Show SMILES COc1ccc2ncc(F)c(CCC34CCC(CC3)(CO4)NCc3cc(C)c4OCC(=O)Nc4n3)c2n1 |(-4.02,2.78,;-4.02,1.54,;-2.69,.77,;-2.69,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.67,-.77,;2.67,.77,;3.74,1.39,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.05,;4.42,14.95,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;,.77,;-1.33,1.54,)| Show InChI InChI=1S/C27H30FN5O4/c1-16-11-17(31-25-24(16)36-14-21(34)32-25)12-30-26-7-9-27(10-8-26,37-15-26)6-5-18-19(28)13-29-20-3-4-22(35-2)33-23(18)20/h3-4,11,13,30H,5-10,12,14-15H2,1-2H3,(H,31,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHO cells measured for 5 mins by automated patch clamp assay |
Bioorg Med Chem Lett 25: 3636-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.057
BindingDB Entry DOI: 10.7270/Q2VH5QNN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50113651

(CHEMBL3604807)Show SMILES COc1ccc2ncc(F)c(C[C@H](O)C3CCC(CO3)NCc3nc4NC(=O)COc4cc3F)c2n1 |r| Show InChI InChI=1S/C24H25F2N5O5/c1-34-22-5-3-16-23(31-22)13(15(26)8-28-16)6-18(32)19-4-2-12(10-35-19)27-9-17-14(25)7-20-24(29-17)30-21(33)11-36-20/h3,5,7-8,12,18-19,27,32H,2,4,6,9-11H2,1H3,(H,29,30,33)/t12?,18-,19?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 3636-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.057
BindingDB Entry DOI: 10.7270/Q2VH5QNN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50113642
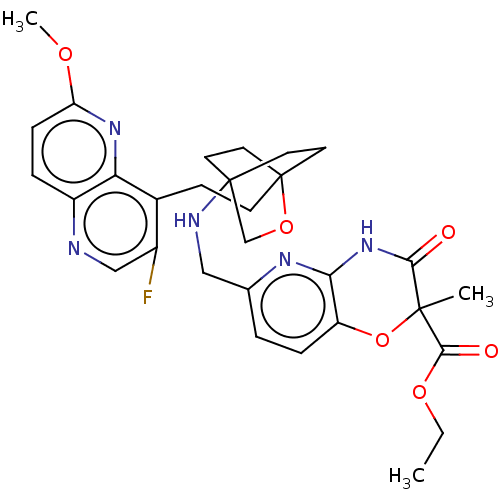
(CHEMBL3604801)Show SMILES CCOC(=O)C1(C)Oc2ccc(CNC34CCC(CCc5c(F)cnc6ccc(OC)nc56)(CC3)OC4)nc2NC1=O |(3.88,17.8,;5.08,18.08,;6.14,16.95,;7.64,17.3,;8,18.48,;8.71,16.22,;9.92,16.47,;7.21,15.87,;6.76,14.39,;5.26,14.05,;4.8,12.56,;5.86,11.44,;5.41,9.96,;3.9,9.61,;3.46,8.16,;4.56,7.06,;4.17,5.57,;2.68,5.4,;2.67,3.85,;1.34,3.08,;1.33,1.54,;2.67,.77,;3.74,1.39,;2.67,-.77,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.69,-.77,;-2.69,.77,;-4.02,1.54,;-4.02,2.78,;-1.33,1.54,;,.77,;2.27,6.67,;3.81,6.67,;1.58,6.27,;1.98,7.74,;7.37,11.8,;7.81,13.27,;9.31,13.62,;9.76,15.09,;10.96,15.37,)| Show InChI InChI=1S/C30H34FN5O6/c1-4-40-27(38)28(2)26(37)36-25-22(42-28)7-5-18(34-25)15-33-29-11-13-30(14-12-29,41-17-29)10-9-19-20(31)16-32-21-6-8-23(39-3)35-24(19)21/h5-8,16,33H,4,9-15,17H2,1-3H3,(H,34,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 3636-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.057
BindingDB Entry DOI: 10.7270/Q2VH5QNN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50113642

(CHEMBL3604801)Show SMILES CCOC(=O)C1(C)Oc2ccc(CNC34CCC(CCc5c(F)cnc6ccc(OC)nc56)(CC3)OC4)nc2NC1=O |(3.88,17.8,;5.08,18.08,;6.14,16.95,;7.64,17.3,;8,18.48,;8.71,16.22,;9.92,16.47,;7.21,15.87,;6.76,14.39,;5.26,14.05,;4.8,12.56,;5.86,11.44,;5.41,9.96,;3.9,9.61,;3.46,8.16,;4.56,7.06,;4.17,5.57,;2.68,5.4,;2.67,3.85,;1.34,3.08,;1.33,1.54,;2.67,.77,;3.74,1.39,;2.67,-.77,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.69,-.77,;-2.69,.77,;-4.02,1.54,;-4.02,2.78,;-1.33,1.54,;,.77,;2.27,6.67,;3.81,6.67,;1.58,6.27,;1.98,7.74,;7.37,11.8,;7.81,13.27,;9.31,13.62,;9.76,15.09,;10.96,15.37,)| Show InChI InChI=1S/C30H34FN5O6/c1-4-40-27(38)28(2)26(37)36-25-22(42-28)7-5-18(34-25)15-33-29-11-13-30(14-12-29,41-17-29)10-9-19-20(31)16-32-21-6-8-23(39-3)35-24(19)21/h5-8,16,33H,4,9-15,17H2,1-3H3,(H,34,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 3636-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.057
BindingDB Entry DOI: 10.7270/Q2VH5QNN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50084056

(CHEMBL3425810)Show SMILES O[C@@]1(CC23CCC(CC2)(CO3)NCc2cc3OCCOc3cn2)Cn2c3c1c(F)cnc3ccc2=O |r,wD:1.0,(2.15,2.32,;.93,2.51,;.33,3.93,;-1.21,4.14,;-1.59,2.68,;-3.13,2.5,;-4.07,3.76,;-2.64,3.2,;-2.42,4.73,;-3.45,5.16,;-1.92,5.36,;-5.58,3.52,;-6.14,2.07,;-7.67,1.83,;-8.24,.38,;-9.77,.14,;-10.33,-1.3,;-11.86,-1.54,;-12.83,-.33,;-12.27,1.11,;-10.74,1.35,;-10.18,2.79,;-8.64,3.04,;-1,2.53,;-1.3,1,;,.25,;1.32,1,;2.64,.25,;3.72,.87,;2.64,-1.26,;1.32,-2.02,;,-1.26,;-1.3,-2.02,;-2.62,-1.26,;-2.62,.25,;-3.7,.87,)| Show InChI InChI=1S/C26H27FN4O5/c27-17-11-29-18-1-2-21(32)31-14-26(33,22(17)23(18)31)13-25-5-3-24(4-6-25,15-36-25)30-10-16-9-19-20(12-28-16)35-8-7-34-19/h1-2,9,11-12,30,33H,3-8,10,13-15H2/t24?,25?,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of MK499 from human ERG |
Bioorg Med Chem Lett 25: 2473-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.063
BindingDB Entry DOI: 10.7270/Q2NZ89BD |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50113641
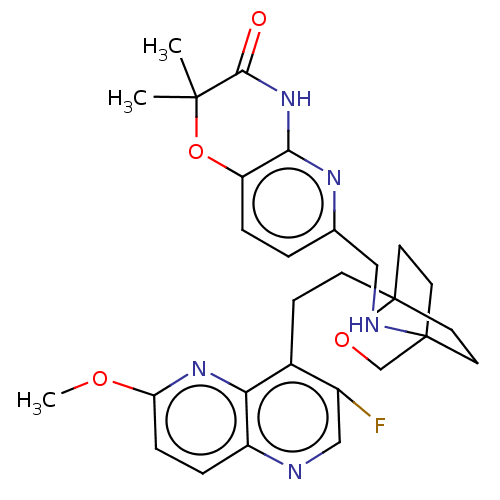
(CHEMBL3604800)Show SMILES COc1ccc2ncc(F)c(CCC34CCC(CC3)(CO4)NCc3ccc4OC(C)(C)C(=O)Nc4n3)c2n1 |(-4.02,2.78,;-4.02,1.54,;-2.69,.77,;-2.69,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.67,-.77,;2.67,.77,;3.74,1.39,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.05,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.92,16.47,;7.84,17.1,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;,.77,;-1.33,1.54,)| Show InChI InChI=1S/C28H32FN5O4/c1-26(2)25(35)34-24-21(38-26)6-4-17(32-24)14-31-27-10-12-28(13-11-27,37-16-27)9-8-18-19(29)15-30-20-5-7-22(36-3)33-23(18)20/h4-7,15,31H,8-14,16H2,1-3H3,(H,32,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 3636-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.057
BindingDB Entry DOI: 10.7270/Q2VH5QNN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
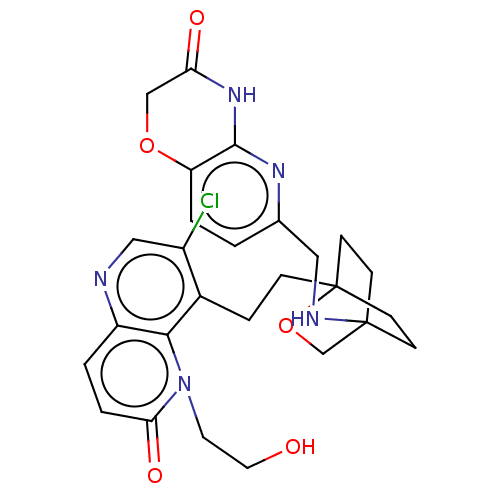
(Homo sapiens (Human)) | BDBM50404171
(CHEMBL5285209)Show SMILES COc1ccc(CN(CCN(C)CCNc2ccc([N+]([O-])=O)c3n[o+][n-]c23)c2ccccn2)cc1 Show InChI InChI=1S/C24H27N7O4/c1-29(14-13-25-20-10-11-21(31(32)33)24-23(20)27-35-28-24)15-16-30(22-5-3-4-12-26-22)17-18-6-8-19(34-2)9-7-18/h3-12,25H,13-17H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Compound was evaluated for the inhibition of microsomal 2,3-oxidosqualene cyclase in Saccharomyces cerevisiae microsomes |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50113632

(CHEMBL3604690)Show SMILES COc1ccc2ncc(F)c(CCC34CCC(CC3)(CO4)NCc3nc4NC(=O)COc4cc3C)c2n1 |(-4.02,2.78,;-4.02,1.54,;-2.69,.77,;-2.69,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.67,-.77,;2.67,.77,;3.74,1.39,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.57,;5.24,14.05,;4.19,15.17,;4.63,16.64,;3.78,17.54,;6.13,17,;7.18,15.88,;6.74,14.4,;7.8,13.28,;7.36,11.79,;8.2,10.89,;,.77,;-1.33,1.54,)| Show InChI InChI=1S/C27H30FN5O4/c1-16-11-21-25(32-22(34)14-36-21)31-20(16)13-30-26-7-9-27(10-8-26,37-15-26)6-5-17-18(28)12-29-19-3-4-23(35-2)33-24(17)19/h3-4,11-12,30H,5-10,13-15H2,1-2H3,(H,31,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHO cells measured for 5 mins by automated patch clamp assay |
Bioorg Med Chem Lett 25: 3636-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.057
BindingDB Entry DOI: 10.7270/Q2VH5QNN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50113634

(CHEMBL3604692)Show SMILES COc1ccc2ncc(F)c(CCC34CCC(CC3)(CO4)NCc3nc4NC(=O)COc4c(C)c3C)c2n1 |(-4.02,2.78,;-4.02,1.54,;-2.69,.77,;-2.69,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.67,-.77,;2.67,.77,;3.74,1.39,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.57,;5.24,14.05,;4.19,15.17,;4.63,16.64,;3.78,17.54,;6.13,17,;7.18,15.88,;6.74,14.4,;7.8,13.28,;9,13.57,;7.36,11.79,;8.2,10.89,;,.77,;-1.33,1.54,)| Show InChI InChI=1S/C28H32FN5O4/c1-16-17(2)25-26(33-22(35)14-37-25)32-21(16)13-31-27-8-10-28(11-9-27,38-15-27)7-6-18-19(29)12-30-20-4-5-23(36-3)34-24(18)20/h4-5,12,31H,6-11,13-15H2,1-3H3,(H,32,33,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHO cells measured for 5 mins by automated patch clamp assay |
Bioorg Med Chem Lett 25: 3636-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.057
BindingDB Entry DOI: 10.7270/Q2VH5QNN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
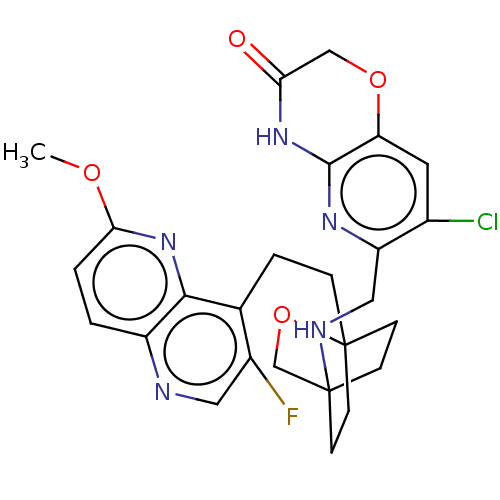
(Homo sapiens (Human)) | BDBM50113630
(CHEMBL3604687)Show SMILES COc1ccc2ncc(F)c(CCC34CCC(CC3)(CO4)NCc3nc4NC(=O)COc4cc3Cl)c2n1 |(-4.02,2.78,;-4.02,1.54,;-2.69,.77,;-2.69,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.67,-.77,;2.67,.77,;3.74,1.39,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.57,;5.24,14.05,;4.19,15.17,;4.63,16.64,;3.78,17.54,;6.13,17,;7.18,15.88,;6.74,14.4,;7.79,13.28,;7.36,11.79,;8.2,10.89,;,.77,;-1.33,1.54,)| Show InChI InChI=1S/C26H27ClFN5O4/c1-35-22-3-2-18-23(33-22)15(17(28)11-29-18)4-5-26-8-6-25(7-9-26,14-37-26)30-12-19-16(27)10-20-24(31-19)32-21(34)13-36-20/h2-3,10-11,30H,4-9,12-14H2,1H3,(H,31,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 3636-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.057
BindingDB Entry DOI: 10.7270/Q2VH5QNN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
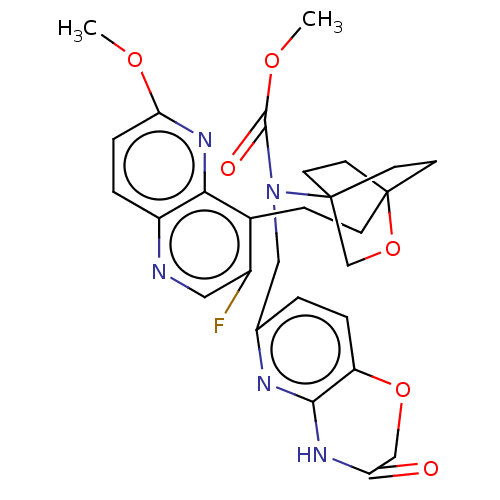
(Homo sapiens (Human)) | BDBM50113647
(CHEMBL3604804)Show SMILES COC(=O)N(Cc1ccc2OCC(=O)Nc2n1)C12CCC(CCc3c(F)cnc4ccc(OC)nc34)(CC1)OC2 |(2.46,13.12,;3.3,12.21,;2.85,10.74,;1.65,10.46,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;3.46,8.16,;4.56,7.06,;4.17,5.57,;2.68,5.4,;2.67,3.85,;1.34,3.08,;1.33,1.54,;2.67,.77,;3.74,1.39,;2.67,-.77,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.69,-.77,;-2.69,.77,;-4.02,1.54,;-4.02,2.78,;-1.33,1.54,;,.77,;2.27,6.67,;3.81,6.67,;1.58,6.27,;1.98,7.74,)| Show InChI InChI=1S/C28H30FN5O6/c1-37-23-6-4-20-24(33-23)18(19(29)13-30-20)7-8-28-11-9-27(10-12-28,16-40-28)34(26(36)38-2)14-17-3-5-21-25(31-17)32-22(35)15-39-21/h3-6,13H,7-12,14-16H2,1-2H3,(H,31,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 3636-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.057
BindingDB Entry DOI: 10.7270/Q2VH5QNN |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
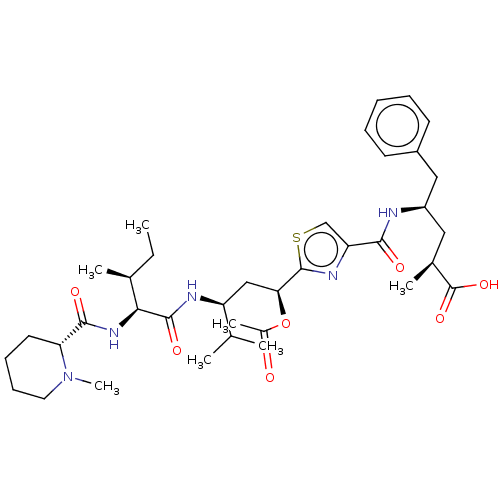
(Sus scrofa) | BDBM50483307
(CHEMBL1643767)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H]1CCCCN1C)C(=O)N[C@@H](C[C@H](OC(C)=O)c1nc(cs1)C(=O)N[C@H](C[C@H](C)C(O)=O)Cc1ccccc1)C(C)C |r| Show InChI InChI=1S/C37H55N5O7S/c1-8-23(4)32(41-34(45)30-16-12-13-17-42(30)7)35(46)39-28(22(2)3)20-31(49-25(6)43)36-40-29(21-50-36)33(44)38-27(18-24(5)37(47)48)19-26-14-10-9-11-15-26/h9-11,14-15,21-24,27-28,30-32H,8,12-13,16-20H2,1-7H3,(H,38,44)(H,39,46)(H,41,45)(H,47,48)/t23-,24-,27+,28-,30+,31-,32-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co Ltd
Curated by ChEMBL
| Assay Description
Inhibition of porcine brain tubulin polymerization by microtubule assembly assay |
Bioorg Med Chem Lett 21: 431-4 (2011)
Article DOI: 10.1016/j.bmcl.2010.10.118
BindingDB Entry DOI: 10.7270/Q21J9DMW |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50084055

(CHEMBL3425808)Show SMILES O[C@@]1(CC23CCC(CC2)(CO3)NCc2ccc3OCC(=O)Nc3c2)Cn2c3c1c(F)cnc3ccc2=O |r,wD:1.0,(2.14,2.31,;.92,2.5,;.32,3.91,;-1.21,4.13,;-1.59,2.67,;-3.12,2.49,;-4.05,3.74,;-2.63,3.19,;-2.41,4.71,;-3.43,5.14,;-1.91,5.34,;-5.55,3.5,;-6.11,2.06,;-7.63,1.82,;-8.6,3.03,;-10.14,2.78,;-10.69,1.34,;-12.22,1.11,;-12.78,-.33,;-11.81,-1.53,;-12.26,-2.68,;-10.29,-1.3,;-9.73,.14,;-8.21,.38,;-1,2.52,;-1.3,1,;,.24,;1.32,1,;2.63,.24,;3.7,.86,;2.63,-1.26,;1.32,-2.01,;,-1.26,;-1.3,-2.01,;-2.61,-1.26,;-2.61,.24,;-3.68,.86,)| Show InChI InChI=1S/C27H27FN4O5/c28-17-11-29-18-2-4-22(34)32-14-27(35,23(17)24(18)32)13-26-7-5-25(6-8-26,15-37-26)30-10-16-1-3-20-19(9-16)31-21(33)12-36-20/h1-4,9,11,30,35H,5-8,10,12-15H2,(H,31,33)/t25?,26?,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHO cells measured over 5 mins by patch clamp assay |
Bioorg Med Chem Lett 25: 2473-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.063
BindingDB Entry DOI: 10.7270/Q2NZ89BD |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50084049
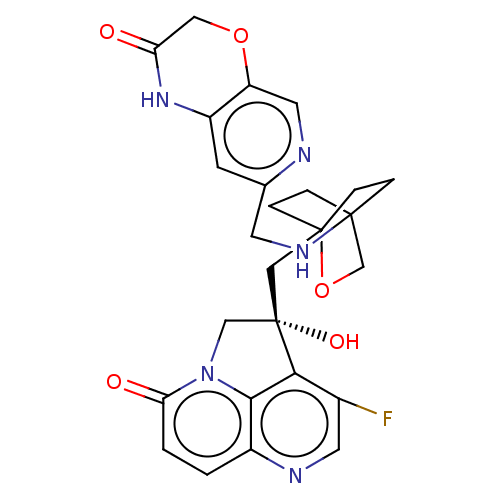
(CHEMBL3425806)Show SMILES O[C@@]1(CC23CCC(CC2)(CO3)NCc2cc3NC(=O)COc3cn2)Cn2c3c1c(F)cnc3ccc2=O |r,wD:1.0,(2.15,2.32,;.93,2.51,;.33,3.93,;-1.21,4.14,;-1.59,2.68,;-3.13,2.5,;-4.07,3.76,;-2.64,3.2,;-2.42,4.73,;-3.45,5.16,;-1.92,5.36,;-5.58,3.52,;-6.14,2.07,;-7.67,1.83,;-8.24,.38,;-9.77,.14,;-10.33,-1.3,;-11.86,-1.54,;-12.31,-2.7,;-12.83,-.33,;-12.27,1.11,;-10.74,1.35,;-10.18,2.79,;-8.64,3.04,;-1,2.53,;-1.3,1,;,.25,;1.32,1,;2.64,.25,;3.72,.87,;2.64,-1.26,;1.32,-2.02,;,-1.26,;-1.3,-2.02,;-2.62,-1.26,;-2.62,.25,;-3.7,.87,)| Show InChI InChI=1S/C26H26FN5O5/c27-16-9-29-17-1-2-21(34)32-13-26(35,22(16)23(17)32)12-25-5-3-24(4-6-25,14-37-25)30-8-15-7-18-19(10-28-15)36-11-20(33)31-18/h1-2,7,9-10,30,35H,3-6,8,11-14H2,(H,31,33)/t24?,25?,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of MK499 from human ERG |
Bioorg Med Chem Lett 25: 2473-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.063
BindingDB Entry DOI: 10.7270/Q2NZ89BD |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50084110
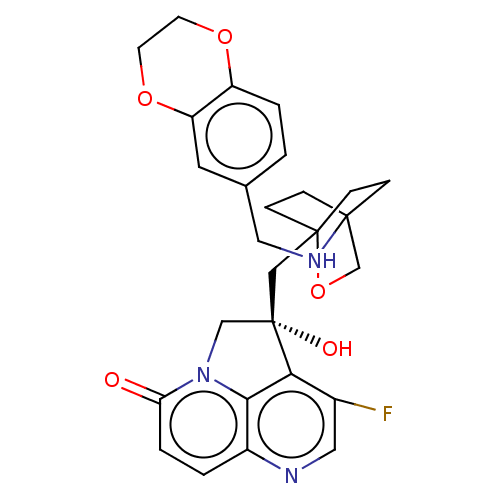
(CHEMBL3425822)Show SMILES O[C@@]1(CC23CCC(CC2)(CO3)NCc2ccc3OCCOc3c2)Cn2c3c1c(F)cnc3ccc2=O |r,wD:1.0,(2.14,2.31,;.92,2.5,;.32,3.91,;-1.21,4.13,;-1.59,2.67,;-3.12,2.49,;-4.05,3.74,;-2.63,3.19,;-2.41,4.71,;-3.43,5.14,;-1.91,5.34,;-5.55,3.5,;-6.11,2.06,;-7.63,1.82,;-8.6,3.03,;-10.14,2.78,;-10.69,1.34,;-12.22,1.11,;-12.78,-.33,;-11.81,-1.53,;-10.29,-1.3,;-9.73,.14,;-8.21,.38,;-1,2.52,;-1.3,1,;,.24,;1.32,1,;2.63,.24,;3.7,.86,;2.63,-1.26,;1.32,-2.01,;,-1.26,;-1.3,-2.01,;-2.61,-1.26,;-2.61,.24,;-3.68,.86,)| Show InChI InChI=1S/C27H28FN3O5/c28-18-13-29-19-2-4-22(32)31-15-27(33,23(18)24(19)31)14-26-7-5-25(6-8-26,16-36-26)30-12-17-1-3-20-21(11-17)35-10-9-34-20/h1-4,11,13,30,33H,5-10,12,14-16H2/t25?,26?,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHO cells measured over 5 mins by patch clamp assay |
Bioorg Med Chem Lett 25: 2473-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.063
BindingDB Entry DOI: 10.7270/Q2NZ89BD |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50068000

(CHEMBL3305167)Show SMILES COc1ccc2ncc(F)c(C[C@H](O)C34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)c2n1 |r| Show InChI InChI=1S/C26H28FN5O5/c1-35-22-5-3-18-23(32-22)16(17(27)12-28-18)10-20(33)26-8-6-25(7-9-26,14-37-26)29-11-15-2-4-19-24(30-15)31-21(34)13-36-19/h2-5,12,20,29,33H,6-11,13-14H2,1H3,(H,30,31,34)/t20-,25?,26?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Activity at beta-1 adrenergic receptor |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50084043
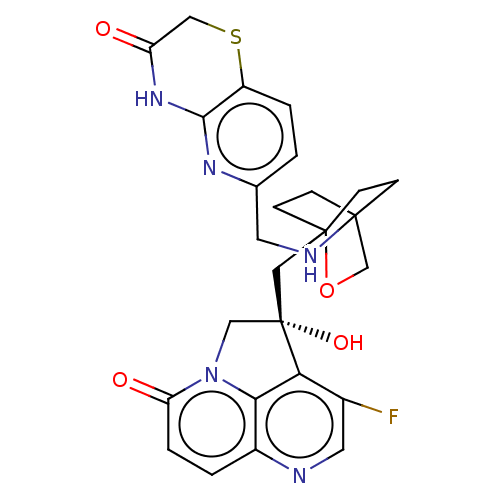
(CHEMBL3425798)Show SMILES O[C@@]1(CC23CCC(CC2)(CO3)NCc2ccc3SCC(=O)Nc3n2)Cn2c3c1c(F)cnc3ccc2=O |r,wD:1.0,(2.15,2.32,;.93,2.51,;.33,3.93,;-1.21,4.14,;-1.59,2.68,;-3.13,2.5,;-4.07,3.76,;-2.64,3.2,;-2.42,4.73,;-3.45,5.16,;-1.92,5.36,;-5.58,3.52,;-6.14,2.07,;-7.67,1.83,;-8.64,3.04,;-10.18,2.79,;-10.74,1.35,;-12.27,1.11,;-12.83,-.33,;-11.86,-1.54,;-12.31,-2.7,;-10.33,-1.3,;-9.77,.14,;-8.24,.38,;-1,2.53,;-1.3,1,;,.25,;1.32,1,;2.64,.25,;3.72,.87,;2.64,-1.26,;1.32,-2.02,;,-1.26,;-1.3,-2.02,;-2.62,-1.26,;-2.62,.25,;-3.7,.87,)| Show InChI InChI=1S/C26H26FN5O4S/c27-16-10-28-17-2-4-20(34)32-13-26(35,21(16)22(17)32)12-25-7-5-24(6-8-25,14-36-25)29-9-15-1-3-18-23(30-15)31-19(33)11-37-18/h1-4,10,29,35H,5-9,11-14H2,(H,30,31,33)/t24?,25?,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHO cells measured over 5 mins by patch clamp assay |
Bioorg Med Chem Lett 25: 2473-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.063
BindingDB Entry DOI: 10.7270/Q2NZ89BD |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50084045

(CHEMBL3425800)Show SMILES O[C@@]1(CC23CCC(CC2)(CO3)NCc2nc3NC(=O)COc3cc2Cl)Cn2c3c1c(F)cnc3ccc2=O |r,wD:1.0,(2.15,2.32,;.93,2.51,;.33,3.93,;-1.21,4.14,;-1.59,2.68,;-3.13,2.5,;-4.07,3.76,;-2.64,3.2,;-2.42,4.73,;-3.45,5.16,;-1.92,5.36,;-5.58,3.52,;-6.14,2.07,;-7.67,1.83,;-8.65,3.05,;-10.18,2.81,;-11.15,4.02,;-12.68,3.79,;-13.46,4.76,;-13.25,2.35,;-12.28,1.14,;-10.75,1.37,;-9.78,.16,;-8.23,.39,;-7.46,-.58,;-1,2.53,;-1.3,1,;,.25,;1.32,1,;2.64,.25,;3.72,.87,;2.64,-1.26,;1.32,-2.02,;,-1.26,;-1.3,-2.02,;-2.62,-1.26,;-2.62,.25,;-3.7,.87,)| Show InChI InChI=1S/C26H25ClFN5O5/c27-14-7-18-23(32-19(34)10-37-18)31-17(14)9-30-24-3-5-25(6-4-24,38-13-24)11-26(36)12-33-20(35)2-1-16-22(33)21(26)15(28)8-29-16/h1-2,7-8,30,36H,3-6,9-13H2,(H,31,32,34)/t24?,25?,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.77E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of MK499 from human ERG |
Bioorg Med Chem Lett 25: 2473-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.063
BindingDB Entry DOI: 10.7270/Q2NZ89BD |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50084122

(CHEMBL3425931)Show SMILES O[C@@]1(CC23CCC(CC2)(CO3)NCc2ccc3OC(F)(F)Oc3c2)Cn2c3c1c(F)cnc3ccc2=O |r,wD:1.0,(2.15,2.32,;.93,2.51,;.33,3.93,;-1.21,4.14,;-1.59,2.68,;-3.13,2.5,;-4.07,3.76,;-2.64,3.2,;-2.42,4.73,;-3.45,5.16,;-1.92,5.36,;-5.58,3.52,;-6.14,2.07,;-7.67,1.83,;-8.63,3.04,;-10.19,2.82,;-10.74,1.37,;-12.19,.82,;-12.11,-.73,;-13.26,-.26,;-11.9,-1.95,;-10.64,-1.13,;-9.78,.16,;-8.24,.37,;-1,2.53,;-1.3,1,;,.25,;1.32,1,;2.64,.25,;3.72,.87,;2.64,-1.26,;1.32,-2.02,;,-1.26,;-1.3,-2.02,;-2.62,-1.26,;-2.62,.25,;-3.7,.87,)| Show InChI InChI=1S/C26H24F3N3O5/c27-16-11-30-17-2-4-20(33)32-13-25(34,21(16)22(17)32)12-24-7-5-23(6-8-24,14-35-24)31-10-15-1-3-18-19(9-15)37-26(28,29)36-18/h1-4,9,11,31,34H,5-8,10,12-14H2/t23?,24?,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.82E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHO cells measured over 5 mins by patch clamp assay |
Bioorg Med Chem Lett 25: 2473-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.063
BindingDB Entry DOI: 10.7270/Q2NZ89BD |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50113649
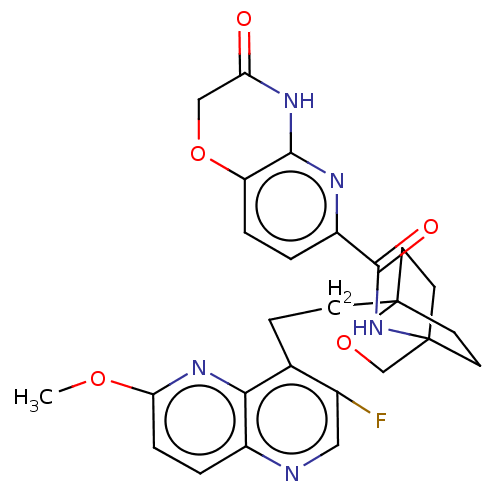
(CHEMBL3604805)Show SMILES COc1ccc2ncc(F)c(CCC34CCC(CC3)(CO4)NC(=O)c3ccc4OCC(=O)Nc4n3)c2n1 |(-4.02,2.78,;-4.02,1.54,;-2.69,.77,;-2.69,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.67,-.77,;2.67,.77,;3.74,1.39,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;6.25,9.06,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;,.77,;-1.33,1.54,)| Show InChI InChI=1S/C26H26FN5O5/c1-35-21-5-3-17-22(31-21)15(16(27)12-28-17)6-7-26-10-8-25(9-11-26,14-37-26)32-24(34)18-2-4-19-23(29-18)30-20(33)13-36-19/h2-5,12H,6-11,13-14H2,1H3,(H,32,34)(H,29,30,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHO cells measured for 5 mins by automated patch clamp assay |
Bioorg Med Chem Lett 25: 3636-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.057
BindingDB Entry DOI: 10.7270/Q2VH5QNN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data