 Found 635 hits with Last Name = 'takeuchi' and Initial = 't'
Found 635 hits with Last Name = 'takeuchi' and Initial = 't' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
DNA polymerase beta
(Rattus norvegicus) | BDBM50448689
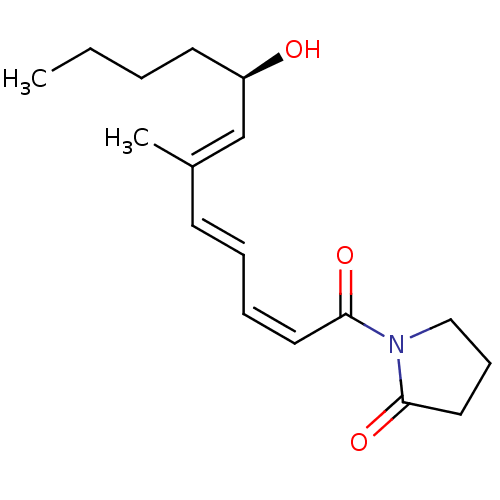
(CHEMBL3127730)Show SMILES CCCC[C@@H](O)\C=C(/C)\C=C\C=C/C(=O)N1CCCC1=O |r| Show InChI InChI=1S/C17H25NO3/c1-3-4-9-15(19)13-14(2)8-5-6-10-16(20)18-12-7-11-17(18)21/h5-6,8,10,13,15,19H,3-4,7,9,11-12H2,1-2H3/b8-5+,10-6-,14-13+/t15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kobe Gakuin University
Curated by ChEMBL
| Assay Description
Competitive inhibition of recombinant rat DNA polymerase beta using poly(dA)/oligo(dT)18 as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem 22: 1070-6 (2014)
Article DOI: 10.1016/j.bmc.2013.12.038
BindingDB Entry DOI: 10.7270/Q22V2HMW |
More data for this
Ligand-Target Pair | |
DNA polymerase kappa
(Homo sapiens (Human)) | BDBM50379292
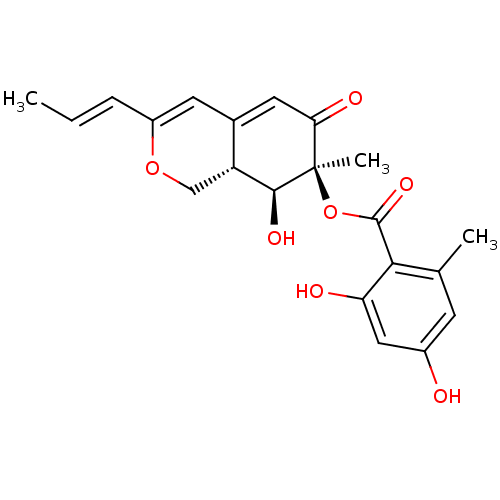
(CHEMBL2011646)Show SMILES C\C=C\C1=CC2=CC(=O)[C@@](C)(OC(=O)c3c(C)cc(O)cc3O)[C@@H](O)[C@@H]2CO1 |r,t:3,5| Show InChI InChI=1S/C21H22O7/c1-4-5-14-7-12-8-17(24)21(3,19(25)15(12)10-27-14)28-20(26)18-11(2)6-13(22)9-16(18)23/h4-9,15,19,22-23,25H,10H2,1-3H3/b5-4+/t15-,19+,21-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Science University of Tokyo
Curated by ChEMBL
| Assay Description
Competitive inhibition of human C-terminal-His6-tagged DNA polymerase kappa expressed in Escherichia coli using dTTP as substrate by Dixon plot analy... |
J Nat Prod 75: 135-41 (2012)
Article DOI: 10.1021/np200523b
BindingDB Entry DOI: 10.7270/Q2T154NG |
More data for this
Ligand-Target Pair | |
DNA polymerase beta
(Rattus norvegicus) | BDBM50448689

(CHEMBL3127730)Show SMILES CCCC[C@@H](O)\C=C(/C)\C=C\C=C/C(=O)N1CCCC1=O |r| Show InChI InChI=1S/C17H25NO3/c1-3-4-9-15(19)13-14(2)8-5-6-10-16(20)18-12-7-11-17(18)21/h5-6,8,10,13,15,19H,3-4,7,9,11-12H2,1-2H3/b8-5+,10-6-,14-13+/t15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kobe Gakuin University
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of recombinant rat DNA polymerase beta using dTTP as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem 22: 1070-6 (2014)
Article DOI: 10.1016/j.bmc.2013.12.038
BindingDB Entry DOI: 10.7270/Q22V2HMW |
More data for this
Ligand-Target Pair | |
DNA polymerase kappa
(Homo sapiens (Human)) | BDBM50379292

(CHEMBL2011646)Show SMILES C\C=C\C1=CC2=CC(=O)[C@@](C)(OC(=O)c3c(C)cc(O)cc3O)[C@@H](O)[C@@H]2CO1 |r,t:3,5| Show InChI InChI=1S/C21H22O7/c1-4-5-14-7-12-8-17(24)21(3,19(25)15(12)10-27-14)28-20(26)18-11(2)6-13(22)9-16(18)23/h4-9,15,19,22-23,25H,10H2,1-3H3/b5-4+/t15-,19+,21-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.81E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Science University of Tokyo
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of human C-terminal-His6-tagged DNA polymerase kappa expressed in Escherichia coli using ploy(dA)/oligo(dT)18 as substrate... |
J Nat Prod 75: 135-41 (2012)
Article DOI: 10.1021/np200523b
BindingDB Entry DOI: 10.7270/Q2T154NG |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606383
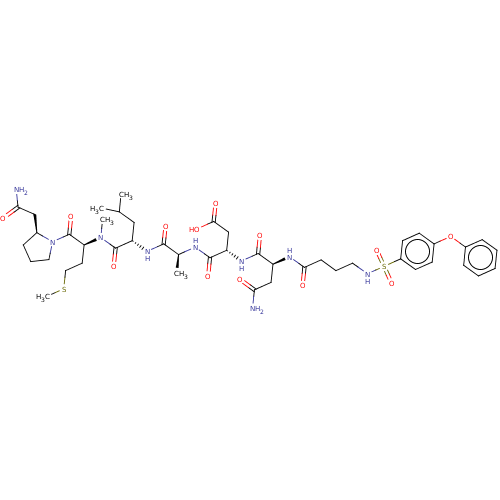
(CHEMBL5209489)Show SMILES CSCC[C@H](N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCNS(=O)(=O)c1ccc(Oc2ccccc2)cc1)C(=O)N1CCC[C@H]1CC(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0440 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606385
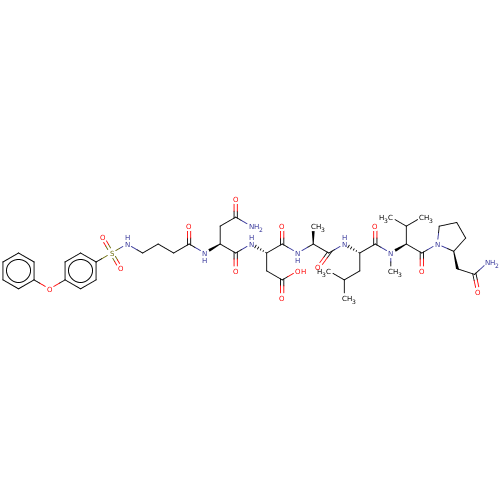
(CHEMBL5203315)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCNS(=O)(=O)c1ccc(Oc2ccccc2)cc1)C(=O)N(C)[C@@H](C(C)C)C(=O)N1CCC[C@H]1CC(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0460 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606395
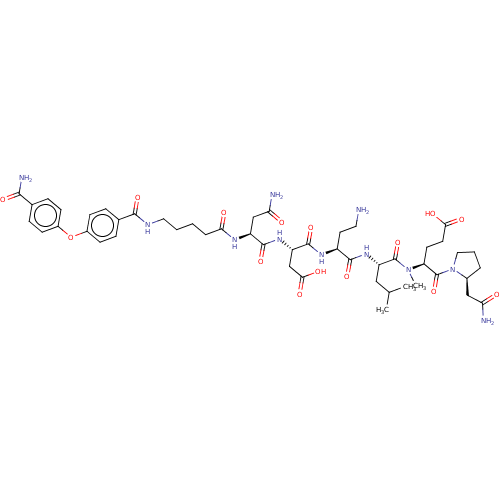
(CHEMBL5207094)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCN)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCCNC(=O)c1ccc(Oc2ccc(cc2)C(N)=O)cc1)C(=O)N(C)[C@@H](CCC(O)=O)C(=O)N1CCC[C@H]1CC(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0510 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606384
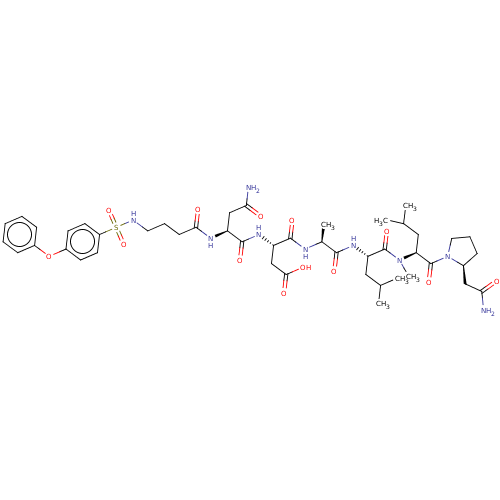
(CHEMBL5183245)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCNS(=O)(=O)c1ccc(Oc2ccccc2)cc1)C(=O)N(C)[C@@H](CC(C)C)C(=O)N1CCC[C@H]1CC(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606386
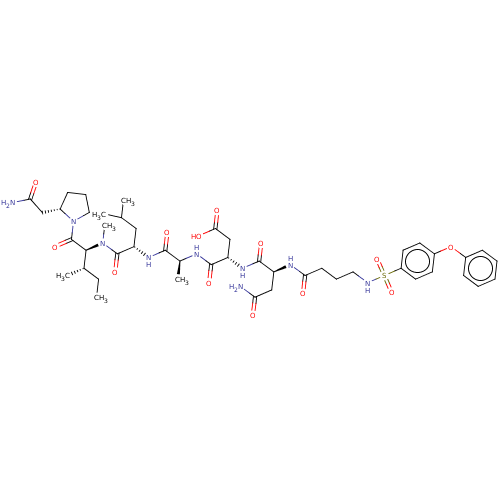
(CHEMBL5182207)Show SMILES CC[C@H](C)[C@H](N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCNS(=O)(=O)c1ccc(Oc2ccccc2)cc1)C(=O)N1CCC[C@H]1CC(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606380
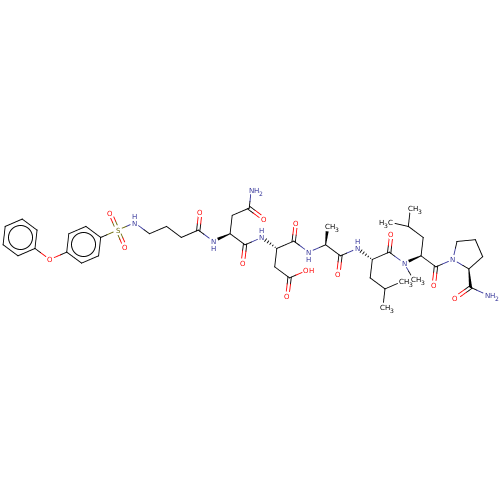
(CHEMBL5191373)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCNS(=O)(=O)c1ccc(Oc2ccccc2)cc1)C(=O)N(C)[C@@H](CC(C)C)C(=O)N1CCC[C@H]1C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0660 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606390
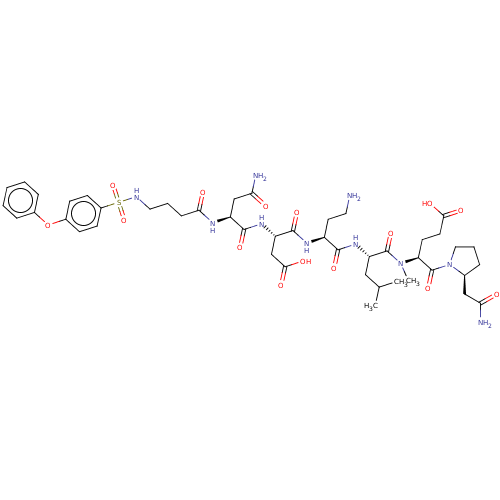
(CHEMBL5185612)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCN)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCNS(=O)(=O)c1ccc(Oc2ccccc2)cc1)C(=O)N(C)[C@@H](CCC(O)=O)C(=O)N1CCC[C@H]1CC(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0680 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606381
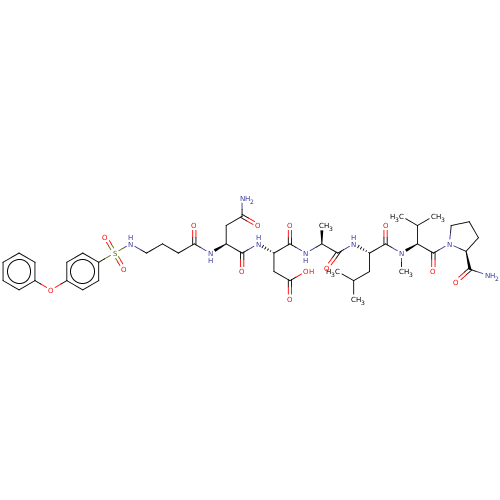
(CHEMBL5198135)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCNS(=O)(=O)c1ccc(Oc2ccccc2)cc1)C(=O)N(C)[C@@H](C(C)C)C(=O)N1CCC[C@H]1C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0780 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606377
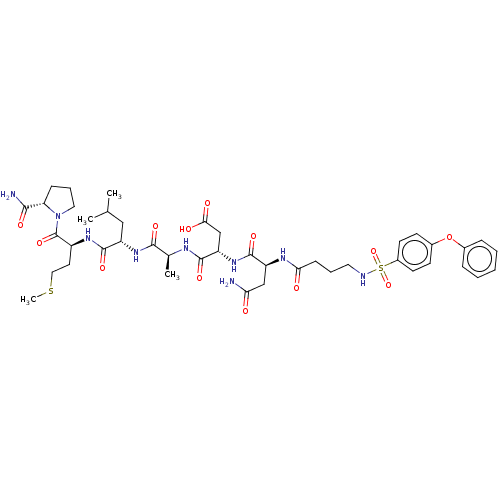
(CHEMBL5186594)Show SMILES CSCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCNS(=O)(=O)c1ccc(Oc2ccccc2)cc1)C(=O)N1CCC[C@H]1C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0830 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606378
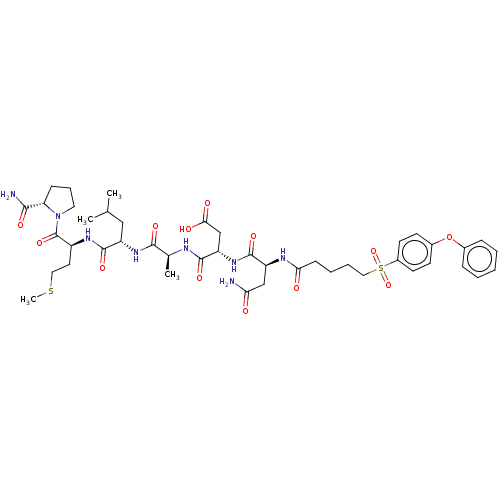
(CHEMBL5170274)Show SMILES CSCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCCS(=O)(=O)c1ccc(Oc2ccccc2)cc1)C(=O)N1CCC[C@H]1C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0890 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606388
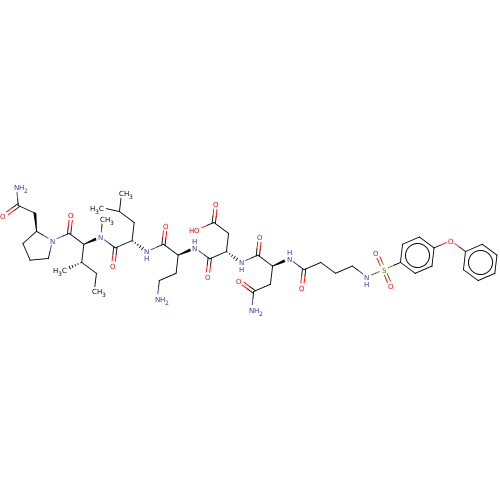
(CHEMBL5203492)Show SMILES CC[C@H](C)[C@H](N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@H](CCN)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCNS(=O)(=O)c1ccc(Oc2ccccc2)cc1)C(=O)N1CCC[C@H]1CC(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50399537
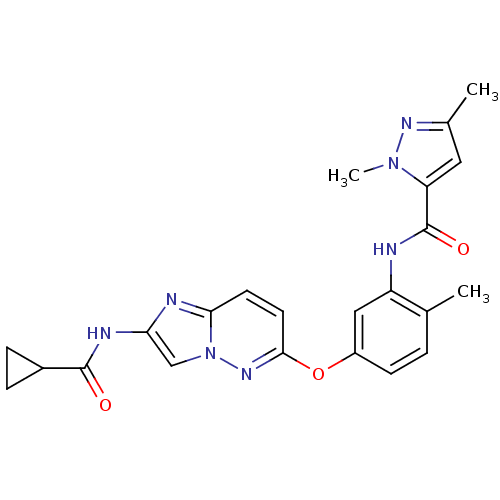
(CHEMBL2180604)Show SMILES Cc1cc(C(=O)Nc2cc(Oc3ccc4nc(NC(=O)C5CC5)cn4n3)ccc2C)n(C)n1 Show InChI InChI=1S/C23H23N7O3/c1-13-4-7-16(11-17(13)24-23(32)18-10-14(2)27-29(18)3)33-21-9-8-20-25-19(12-30(20)28-21)26-22(31)15-5-6-15/h4,7-12,15H,5-6H2,1-3H3,(H,24,32)(H,26,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0930 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 (unknown origin) using biotinylated poly(Glu:Tyr) as substrate after 60 mins by AlphaScreen assay in presence of 1000 uM of ATP |
Bioorg Med Chem 21: 2333-45 (2013)
Article DOI: 10.1016/j.bmc.2013.01.074
BindingDB Entry DOI: 10.7270/Q22B90C1 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606382
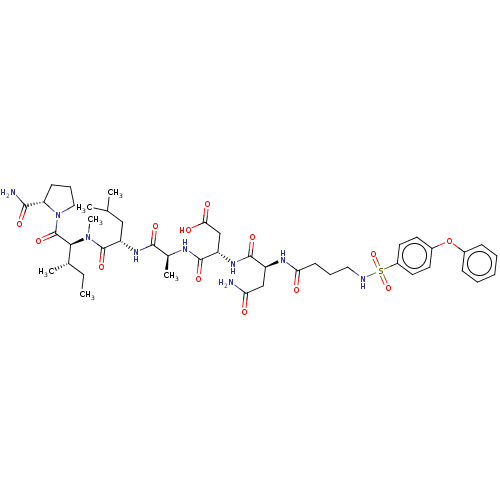
(CHEMBL5191820)Show SMILES CC[C@H](C)[C@H](N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCNS(=O)(=O)c1ccc(Oc2ccccc2)cc1)C(=O)N1CCC[C@H]1C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0970 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606392
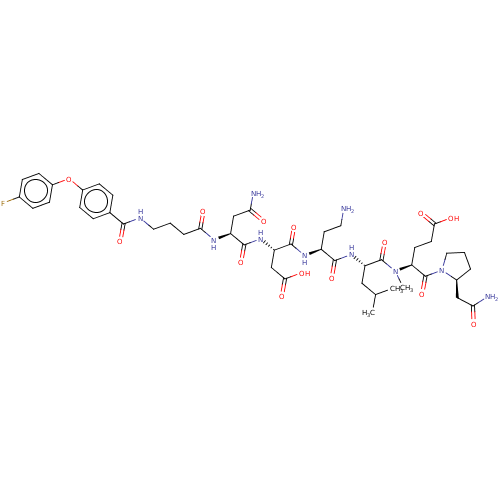
(CHEMBL5178802)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCN)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCNC(=O)c1ccc(Oc2ccc(F)cc2)cc1)C(=O)N(C)[C@@H](CCC(O)=O)C(=O)N1CCC[C@H]1CC(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606379

(CHEMBL5180149)Show SMILES CSCC[C@H](N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCNS(=O)(=O)c1ccc(Oc2ccccc2)cc1)C(=O)N1CCC[C@H]1C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.128 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606396
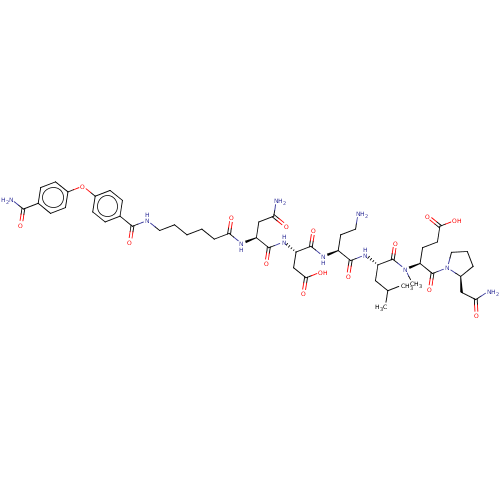
(CHEMBL5206743)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCN)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCCCNC(=O)c1ccc(Oc2ccc(cc2)C(N)=O)cc1)C(=O)N(C)[C@@H](CCC(O)=O)C(=O)N1CCC[C@H]1CC(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606387
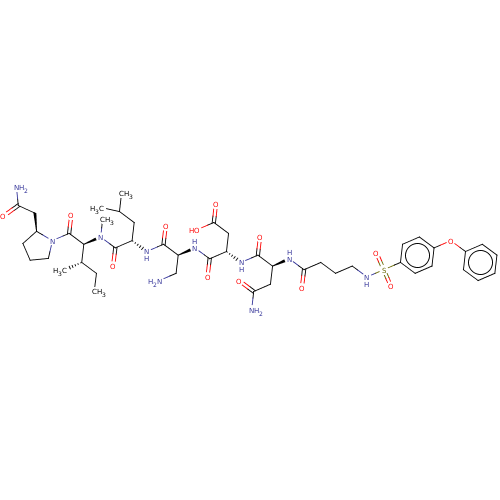
(CHEMBL5206852)Show SMILES CC[C@H](C)[C@H](N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@H](CN)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCNS(=O)(=O)c1ccc(Oc2ccccc2)cc1)C(=O)N1CCC[C@H]1CC(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606393

(CHEMBL5190160)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCN)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCNC(=O)c1ccc(Oc2ccc(cc2)C(N)=O)cc1)C(=O)N(C)[C@@H](CCC(O)=O)C(=O)N1CCC[C@H]1CC(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606391
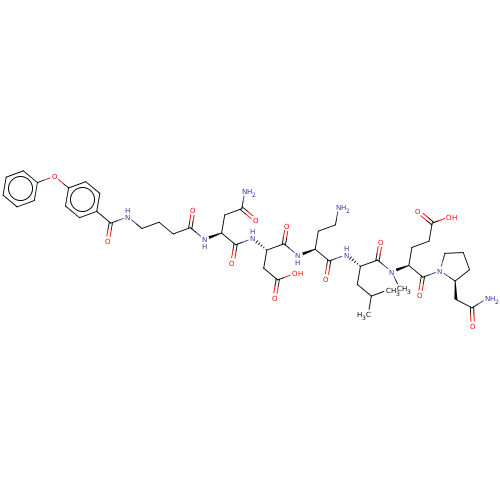
(CHEMBL5207603)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCN)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCNC(=O)c1ccc(Oc2ccccc2)cc1)C(=O)N(C)[C@@H](CCC(O)=O)C(=O)N1CCC[C@H]1CC(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50432398
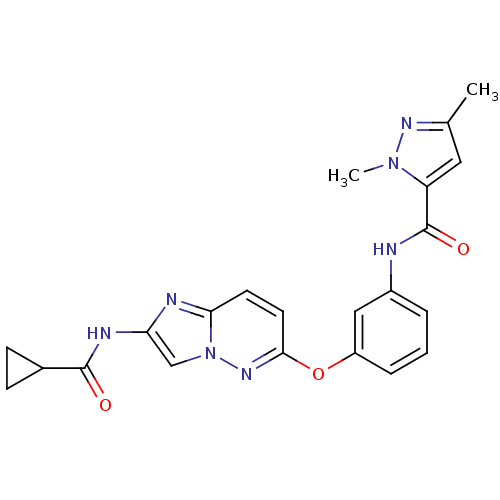
(CHEMBL2348996)Show SMILES Cc1cc(C(=O)Nc2cccc(Oc3ccc4nc(NC(=O)C5CC5)cn4n3)c2)n(C)n1 Show InChI InChI=1S/C22H21N7O3/c1-13-10-17(28(2)26-13)22(31)23-15-4-3-5-16(11-15)32-20-9-8-19-24-18(12-29(19)27-20)25-21(30)14-6-7-14/h3-5,8-12,14H,6-7H2,1-2H3,(H,23,31)(H,25,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 (unknown origin) using biotinylated poly(Glu:Tyr) as substrate after 60 mins by AlphaScreen assay in presence of 1000 uM of ATP |
Bioorg Med Chem 21: 2333-45 (2013)
Article DOI: 10.1016/j.bmc.2013.01.074
BindingDB Entry DOI: 10.7270/Q22B90C1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606397

(CHEMBL5169565)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCN)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)CCCCNC(=O)c1ccc(Oc2ccc(cc2)C(N)=O)cc1)C(=O)N(C)[C@@H](CCC(O)=O)C(=O)N1CCC[C@H]1CC(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM50554205

(CHEMBL4757242)Show SMILES CCCCCN(C)Cc1cc(ccc1NC(=O)c1cc2cc(F)cc(NC)c2[nH]c1=O)C(O)=O | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Escherichia coli DNA gyrase by measuring supercoiling activity incubated for 60 mins by fluorescence based assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115776
BindingDB Entry DOI: 10.7270/Q2W66QFJ |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM50554208

(CHEMBL4784687)Show SMILES OC(=O)C(F)(F)F.CNc1cc(F)cc2cc(C(=O)Nc3ccc(cc3CNC3CCCCC3)C(O)=O)c(=O)[nH]c12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Escherichia coli DNA gyrase by measuring supercoiling activity incubated for 60 mins by fluorescence based assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115776
BindingDB Entry DOI: 10.7270/Q2W66QFJ |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM50554207

(CHEMBL4758336)Show SMILES Cl.CNc1cc(F)cc2cc(C(=O)Nc3ccc(cc3CN(CCO)C3CCCCC3)C(O)=O)c(=O)[nH]c12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Escherichia coli DNA gyrase by measuring supercoiling activity incubated for 60 mins by fluorescence based assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115776
BindingDB Entry DOI: 10.7270/Q2W66QFJ |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM50554204
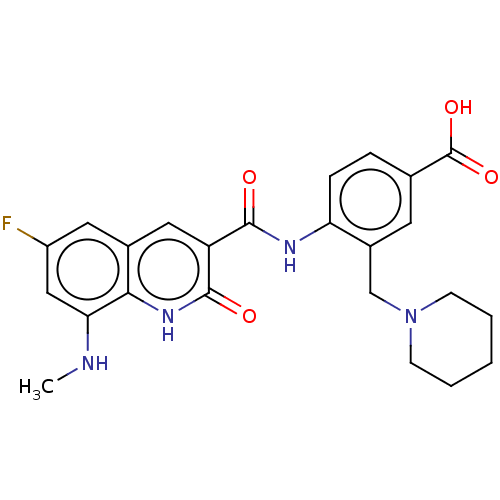
(CHEMBL4743844)Show SMILES CNc1cc(F)cc2cc(C(=O)Nc3ccc(cc3CN3CCCCC3)C(O)=O)c(=O)[nH]c12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Escherichia coli DNA gyrase by measuring supercoiling activity incubated for 60 mins by fluorescence based assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115776
BindingDB Entry DOI: 10.7270/Q2W66QFJ |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM50554200

(CHEMBL4764566)Show SMILES CNc1cc(F)cc2cc(C(=O)Nc3ccc(cc3)C(O)=O)c(=O)[nH]c12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Escherichia coli DNA gyrase by measuring supercoiling activity incubated for 60 mins by fluorescence based assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115776
BindingDB Entry DOI: 10.7270/Q2W66QFJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082445
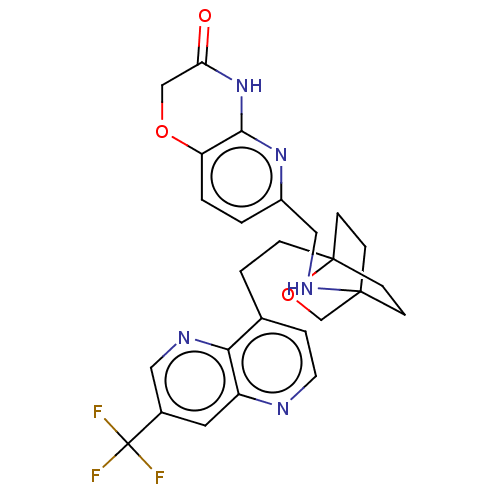
(CHEMBL3422978)Show SMILES FC(F)(F)c1cnc2c(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)ccnc2c1 |(-5.09,-.93,;-4.02,-1.54,;-4.02,-2.78,;-5.09,-2.16,;-2.69,-.77,;-2.69,.77,;-1.33,1.54,;,.77,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;2.67,.77,;2.67,-.77,;1.33,-1.54,;,-.77,;-1.33,-1.54,)| Show InChI InChI=1S/C26H26F3N5O3/c27-26(28,29)17-11-19-22(31-12-17)16(4-10-30-19)3-5-25-8-6-24(7-9-25,15-37-25)32-13-18-1-2-20-23(33-18)34-21(35)14-36-20/h1-2,4,10-12,32H,3,5-9,13-15H2,(H,33,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM50554197
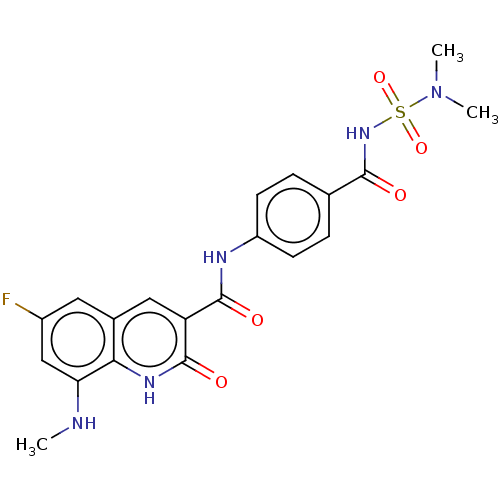
(CHEMBL4776319)Show SMILES CNc1cc(F)cc2cc(C(=O)Nc3ccc(cc3)C(=O)NS(=O)(=O)N(C)C)c(=O)[nH]c12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Escherichia coli DNA gyrase by measuring supercoiling activity incubated for 60 mins by fluorescence based assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115776
BindingDB Entry DOI: 10.7270/Q2W66QFJ |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606376
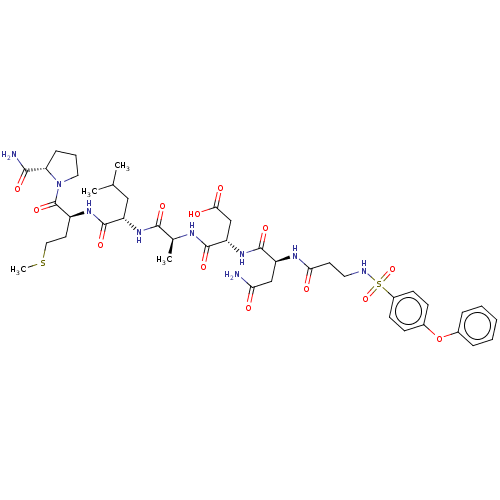
(CHEMBL5202634)Show SMILES CSCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCNS(=O)(=O)c1ccc(Oc2ccccc2)cc1)C(=O)N1CCC[C@H]1C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM50554219

(CHEMBL4798551)Show SMILES OC=O.CNc1cc(F)cc2c(NCCCN)c(C(=O)Nc3ccc(cc3)C(O)=O)c(=O)[nH]c12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Escherichia coli DNA gyrase by measuring supercoiling activity incubated for 60 mins by fluorescence based assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115776
BindingDB Entry DOI: 10.7270/Q2W66QFJ |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606394

(CHEMBL5177058)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCN)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCNC(=O)c1ccc(Oc2ccc(cc2)C(N)=O)cc1)C(=O)N(C)[C@@H](CCC(O)=O)C(=O)N1CCC[C@H]1CC(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM50554203
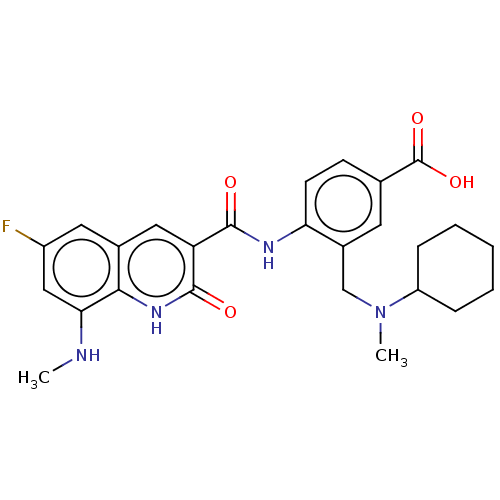
(CHEMBL4779288)Show SMILES Cl.CNc1cc(F)cc2cc(C(=O)Nc3ccc(cc3CN(C)C3CCCCC3)C(O)=O)c(=O)[nH]c12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Escherichia coli DNA gyrase by measuring supercoiling activity incubated for 60 mins by fluorescence based assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115776
BindingDB Entry DOI: 10.7270/Q2W66QFJ |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM50554210

(CHEMBL4757030)Show SMILES CNc1cc(F)cc2cc(C(=O)Nc3ccc(cc3CN(CC(O)=O)C3CCCCC3)C(O)=O)c(=O)[nH]c12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Escherichia coli DNA gyrase by measuring supercoiling activity incubated for 60 mins by fluorescence based assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115776
BindingDB Entry DOI: 10.7270/Q2W66QFJ |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM50554202

(CHEMBL4762067)Show SMILES CNc1cc(F)cc2cc(C(=O)Nc3ccc(cc3CN(C)C3CC3)C(O)=O)c(=O)[nH]c12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Escherichia coli DNA gyrase by measuring supercoiling activity incubated for 60 mins by fluorescence based assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115776
BindingDB Entry DOI: 10.7270/Q2W66QFJ |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606389

(CHEMBL5209121)Show SMILES CC[C@H](C)[C@H](N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCN)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCNS(=O)(=O)c1ccc(Oc2ccccc2)cc1)C(=O)N1CCC[C@H]1CC(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM50554220
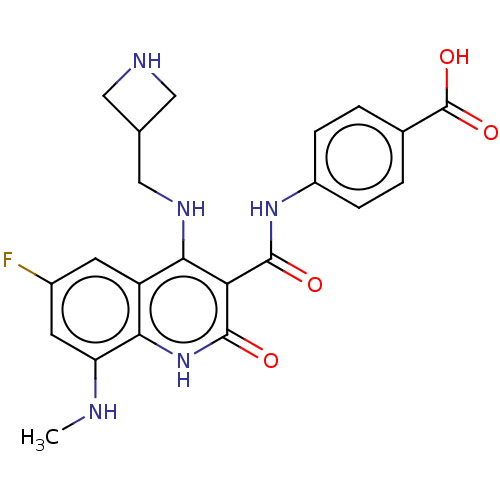
(CHEMBL4764515)Show SMILES OC=O.CNc1cc(F)cc2c(NCC3CNC3)c(C(=O)Nc3ccc(cc3)C(O)=O)c(=O)[nH]c12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Escherichia coli DNA gyrase by measuring supercoiling activity incubated for 60 mins by fluorescence based assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115776
BindingDB Entry DOI: 10.7270/Q2W66QFJ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082380
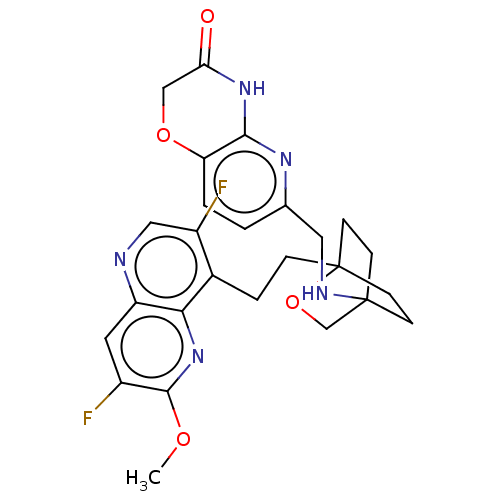
(CHEMBL3422952)Show SMILES COc1nc2c(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)c(F)cnc2cc1F |(-4.02,2.78,;-4.02,1.54,;-2.69,.77,;-1.33,1.54,;,.77,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;2.67,.77,;3.74,1.39,;2.67,-.77,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.69,-.77,;-3.75,-1.39,)| Show InChI InChI=1S/C26H27F2N5O4/c1-35-24-17(27)10-19-22(33-24)16(18(28)12-29-19)4-5-26-8-6-25(7-9-26,14-37-26)30-11-15-2-3-20-23(31-15)32-21(34)13-36-20/h2-3,10,12,30H,4-9,11,13-14H2,1H3,(H,31,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM50554206
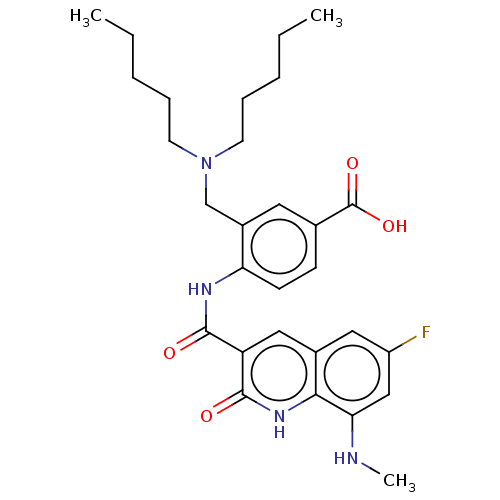
(CHEMBL4798088)Show SMILES CCCCCN(CCCCC)Cc1cc(ccc1NC(=O)c1cc2cc(F)cc(NC)c2[nH]c1=O)C(O)=O | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Escherichia coli DNA gyrase by measuring supercoiling activity incubated for 60 mins by fluorescence based assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115776
BindingDB Entry DOI: 10.7270/Q2W66QFJ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082429

(CHEMBL3422970)Show SMILES Cc1nc2c(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)ccnc2cc1C#N |(-3.75,1.39,;-2.69,.77,;-1.33,1.54,;,.77,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;2.67,.77,;2.67,-.77,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.69,-.77,;-4.02,-1.54,;-5.09,-2.16,)| Show InChI InChI=1S/C27H28N6O3/c1-17-19(13-28)12-21-24(31-17)18(5-11-29-21)4-6-27-9-7-26(8-10-27,16-36-27)30-14-20-2-3-22-25(32-20)33-23(34)15-35-22/h2-3,5,11-12,30H,4,6-10,14-16H2,1H3,(H,32,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082385
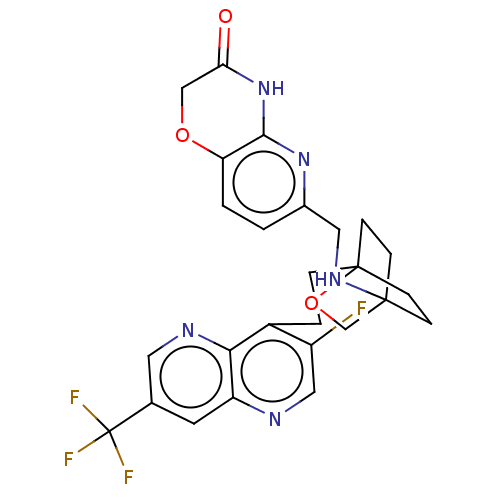
(CHEMBL3422959)Show SMILES Fc1cnc2cc(cnc2c1CCC12CCC(CC1)(CO2)NCc1ccc2OCC(=O)Nc2n1)C(F)(F)F Show InChI InChI=1S/C26H25F4N5O3/c27-18-12-31-19-9-15(26(28,29)30)10-32-22(19)17(18)3-4-25-7-5-24(6-8-25,14-38-25)33-11-16-1-2-20-23(34-16)35-21(36)13-37-20/h1-2,9-10,12,33H,3-8,11,13-14H2,(H,34,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM50554217

(CHEMBL4776836)Show SMILES OC=O.CNc1cc(F)cc2c(NCCN)c(C(=O)Nc3ccc(cc3)C(O)=O)c(=O)[nH]c12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Escherichia coli DNA gyrase by measuring supercoiling activity incubated for 60 mins by fluorescence based assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115776
BindingDB Entry DOI: 10.7270/Q2W66QFJ |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM50554199
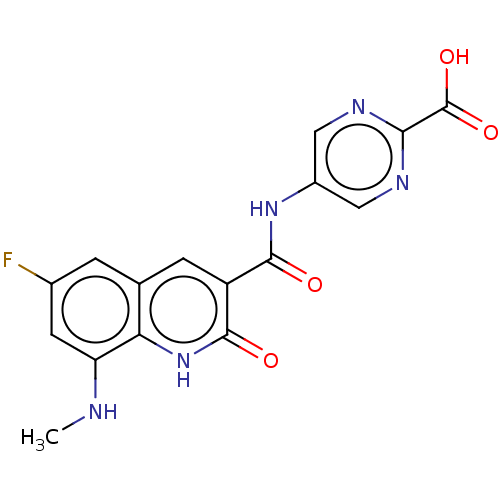
(CHEMBL4763869)Show SMILES CNc1cc(F)cc2cc(C(=O)Nc3cnc(nc3)C(O)=O)c(=O)[nH]c12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Escherichia coli DNA gyrase by measuring supercoiling activity incubated for 60 mins by fluorescence based assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115776
BindingDB Entry DOI: 10.7270/Q2W66QFJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data