

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50537869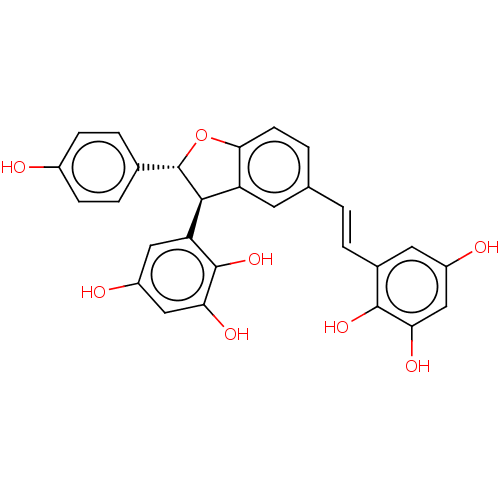 (CHEMBL4638367) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyungpook National University Curated by ChEMBL | Assay Description Inhibition of PTP1B (unknown origin) assessed as decrease in p-nitrophenolate formation using using pNPP as substrate by Dixon plot analysis | J Nat Prod 83: 323-332 (2020) Article DOI: 10.1021/acs.jnatprod.9b00777 BindingDB Entry DOI: 10.7270/Q2GM8BVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50537867 (CHEMBL4649760) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyungpook National University Curated by ChEMBL | Assay Description Inhibition of PTP1B (unknown origin) assessed as decrease in p-nitrophenolate formation using using pNPP as substrate by Dixon plot analysis | J Nat Prod 83: 323-332 (2020) Article DOI: 10.1021/acs.jnatprod.9b00777 BindingDB Entry DOI: 10.7270/Q2GM8BVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50537869 (CHEMBL4638367) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyungpook National University Curated by ChEMBL | Assay Description Inhibition of PTP1B (unknown origin) assessed as decrease in p-nitrophenolate formation using using pNPP as substrate incubated for 15 mins by spectr... | J Nat Prod 83: 323-332 (2020) Article DOI: 10.1021/acs.jnatprod.9b00777 BindingDB Entry DOI: 10.7270/Q2GM8BVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50537867 (CHEMBL4649760) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyungpook National University Curated by ChEMBL | Assay Description Inhibition of PTP1B (unknown origin) assessed as decrease in p-nitrophenolate formation using using pNPP as substrate incubated for 15 mins by spectr... | J Nat Prod 83: 323-332 (2020) Article DOI: 10.1021/acs.jnatprod.9b00777 BindingDB Entry DOI: 10.7270/Q2GM8BVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50148911 ((3beta)-3-hydroxyurs-12-en-28-oic acid | 3beta-hyd...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyungpook National University Curated by ChEMBL | Assay Description Inhibition of PTP1B (unknown origin) assessed as decrease in p-nitrophenolate formation using using pNPP as substrate incubated for 15 mins by spectr... | J Nat Prod 83: 323-332 (2020) Article DOI: 10.1021/acs.jnatprod.9b00777 BindingDB Entry DOI: 10.7270/Q2GM8BVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50537874 (CHEMBL4647539) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyungpook National University Curated by ChEMBL | Assay Description Inhibition of PTP1B (unknown origin) assessed as decrease in p-nitrophenolate formation using using pNPP as substrate incubated for 15 mins by spectr... | J Nat Prod 83: 323-332 (2020) Article DOI: 10.1021/acs.jnatprod.9b00777 BindingDB Entry DOI: 10.7270/Q2GM8BVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50391109 (CHEMBL179166 | Sodium orthovanadate (SOV) | Vanada...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyungpook National University Curated by ChEMBL | Assay Description Inhibition of PTP1B (unknown origin) assessed as decrease in p-nitrophenolate formation using using pNPP as substrate incubated for 15 mins by spectr... | J Nat Prod 83: 323-332 (2020) Article DOI: 10.1021/acs.jnatprod.9b00777 BindingDB Entry DOI: 10.7270/Q2GM8BVJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50537871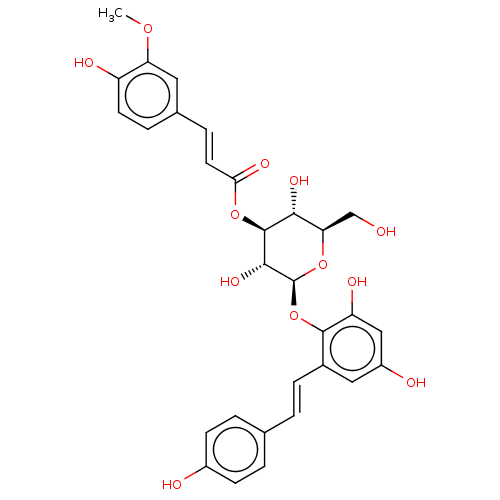 (CHEMBL4639409) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.74E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyungpook National University Curated by ChEMBL | Assay Description Inhibition of PTP1B (unknown origin) assessed as decrease in p-nitrophenolate formation using using pNPP as substrate incubated for 15 mins by spectr... | J Nat Prod 83: 323-332 (2020) Article DOI: 10.1021/acs.jnatprod.9b00777 BindingDB Entry DOI: 10.7270/Q2GM8BVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50537866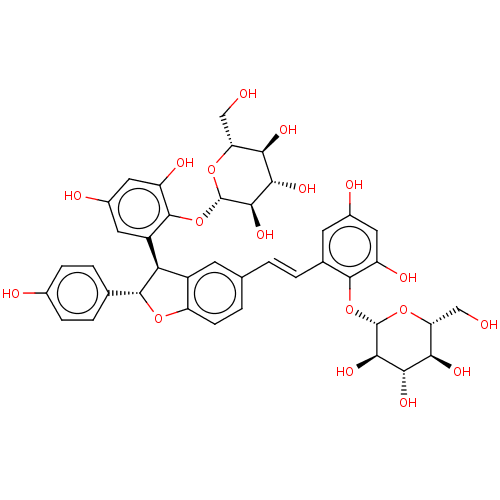 (CHEMBL4636723) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyungpook National University Curated by ChEMBL | Assay Description Inhibition of PTP1B (unknown origin) assessed as decrease in p-nitrophenolate formation using using pNPP as substrate incubated for 15 mins by spectr... | J Nat Prod 83: 323-332 (2020) Article DOI: 10.1021/acs.jnatprod.9b00777 BindingDB Entry DOI: 10.7270/Q2GM8BVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50537868 (CHEMBL4639813) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.49E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyungpook National University Curated by ChEMBL | Assay Description Inhibition of PTP1B (unknown origin) assessed as decrease in p-nitrophenolate formation using using pNPP as substrate incubated for 15 mins by spectr... | J Nat Prod 83: 323-332 (2020) Article DOI: 10.1021/acs.jnatprod.9b00777 BindingDB Entry DOI: 10.7270/Q2GM8BVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50537870 (CHEMBL4647374) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.76E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyungpook National University Curated by ChEMBL | Assay Description Inhibition of PTP1B (unknown origin) assessed as decrease in p-nitrophenolate formation using using pNPP as substrate incubated for 15 mins by spectr... | J Nat Prod 83: 323-332 (2020) Article DOI: 10.1021/acs.jnatprod.9b00777 BindingDB Entry DOI: 10.7270/Q2GM8BVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50537875 (CHEMBL4643421) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyungpook National University Curated by ChEMBL | Assay Description Inhibition of PTP1B (unknown origin) assessed as decrease in p-nitrophenolate formation using using pNPP as substrate incubated for 15 mins by spectr... | J Nat Prod 83: 323-332 (2020) Article DOI: 10.1021/acs.jnatprod.9b00777 BindingDB Entry DOI: 10.7270/Q2GM8BVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50537876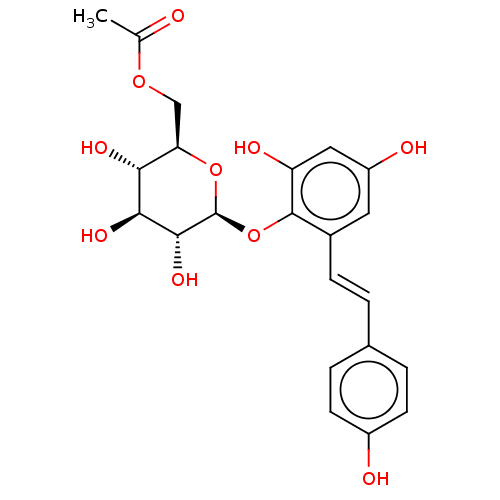 (CHEMBL4647457) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyungpook National University Curated by ChEMBL | Assay Description Inhibition of PTP1B (unknown origin) assessed as decrease in p-nitrophenolate formation using using pNPP as substrate incubated for 15 mins by spectr... | J Nat Prod 83: 323-332 (2020) Article DOI: 10.1021/acs.jnatprod.9b00777 BindingDB Entry DOI: 10.7270/Q2GM8BVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50537877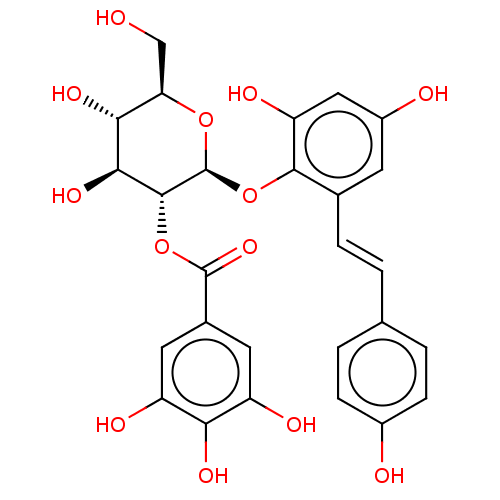 (CHEMBL4648424) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyungpook National University Curated by ChEMBL | Assay Description Inhibition of PTP1B (unknown origin) assessed as decrease in p-nitrophenolate formation using using pNPP as substrate incubated for 15 mins by spectr... | J Nat Prod 83: 323-332 (2020) Article DOI: 10.1021/acs.jnatprod.9b00777 BindingDB Entry DOI: 10.7270/Q2GM8BVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50537878 (CHEMBL4649674) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyungpook National University Curated by ChEMBL | Assay Description Inhibition of PTP1B (unknown origin) assessed as decrease in p-nitrophenolate formation using using pNPP as substrate incubated for 15 mins by spectr... | J Nat Prod 83: 323-332 (2020) Article DOI: 10.1021/acs.jnatprod.9b00777 BindingDB Entry DOI: 10.7270/Q2GM8BVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50537873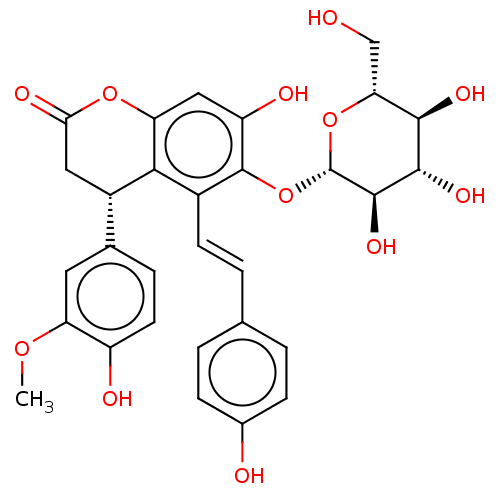 (CHEMBL4633335) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyungpook National University Curated by ChEMBL | Assay Description Inhibition of PTP1B (unknown origin) assessed as decrease in p-nitrophenolate formation using using pNPP as substrate incubated for 15 mins by spectr... | J Nat Prod 83: 323-332 (2020) Article DOI: 10.1021/acs.jnatprod.9b00777 BindingDB Entry DOI: 10.7270/Q2GM8BVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50537879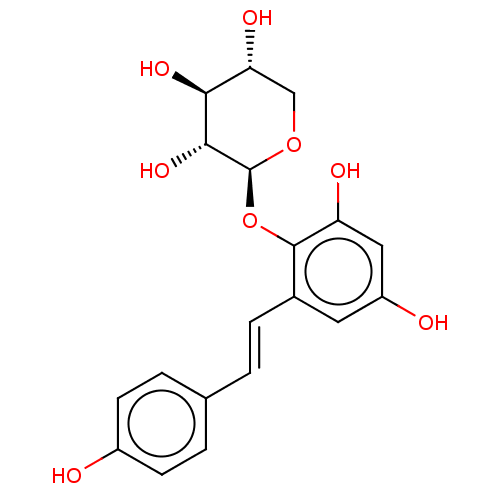 (CHEMBL4641043) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyungpook National University Curated by ChEMBL | Assay Description Inhibition of PTP1B (unknown origin) assessed as decrease in p-nitrophenolate formation using using pNPP as substrate incubated for 15 mins by spectr... | J Nat Prod 83: 323-332 (2020) Article DOI: 10.1021/acs.jnatprod.9b00777 BindingDB Entry DOI: 10.7270/Q2GM8BVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50537880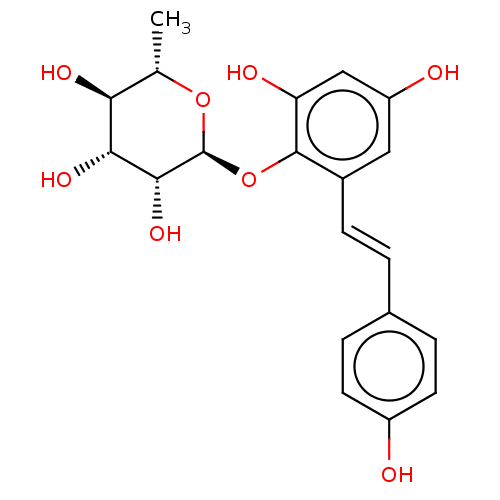 (CHEMBL4647972) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyungpook National University Curated by ChEMBL | Assay Description Inhibition of PTP1B (unknown origin) assessed as decrease in p-nitrophenolate formation using using pNPP as substrate incubated for 15 mins by spectr... | J Nat Prod 83: 323-332 (2020) Article DOI: 10.1021/acs.jnatprod.9b00777 BindingDB Entry DOI: 10.7270/Q2GM8BVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50537881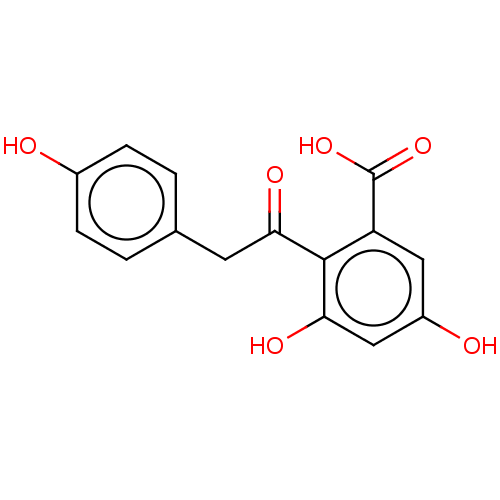 (CHEMBL4210319) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyungpook National University Curated by ChEMBL | Assay Description Inhibition of PTP1B (unknown origin) assessed as decrease in p-nitrophenolate formation using using pNPP as substrate incubated for 15 mins by spectr... | J Nat Prod 83: 323-332 (2020) Article DOI: 10.1021/acs.jnatprod.9b00777 BindingDB Entry DOI: 10.7270/Q2GM8BVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50537865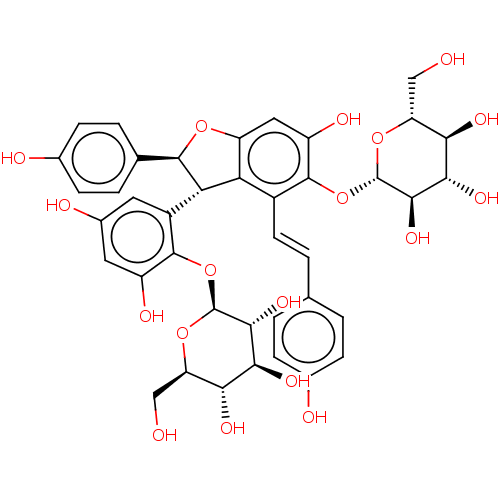 (CHEMBL4639639) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyungpook National University Curated by ChEMBL | Assay Description Inhibition of PTP1B (unknown origin) assessed as decrease in p-nitrophenolate formation using using pNPP as substrate incubated for 15 mins by spectr... | J Nat Prod 83: 323-332 (2020) Article DOI: 10.1021/acs.jnatprod.9b00777 BindingDB Entry DOI: 10.7270/Q2GM8BVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50537872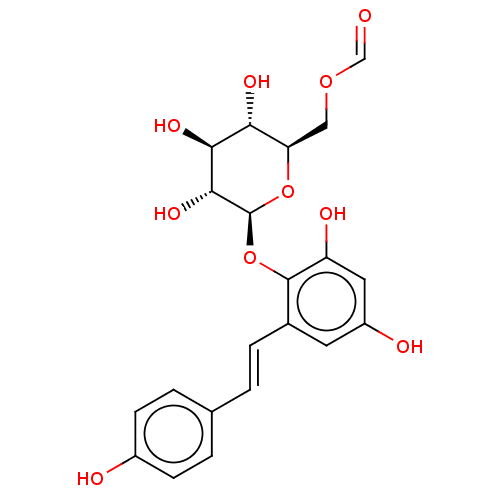 (CHEMBL4635601) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyungpook National University Curated by ChEMBL | Assay Description Inhibition of PTP1B (unknown origin) assessed as decrease in p-nitrophenolate formation using using pNPP as substrate incubated for 15 mins by spectr... | J Nat Prod 83: 323-332 (2020) Article DOI: 10.1021/acs.jnatprod.9b00777 BindingDB Entry DOI: 10.7270/Q2GM8BVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50020713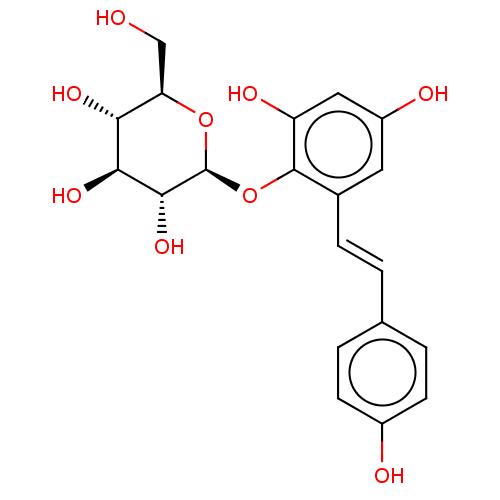 (CHEMBL460860) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyungpook National University Curated by ChEMBL | Assay Description Inhibition of PTP1B (unknown origin) assessed as decrease in p-nitrophenolate formation using using pNPP as substrate incubated for 15 mins by spectr... | J Nat Prod 83: 323-332 (2020) Article DOI: 10.1021/acs.jnatprod.9b00777 BindingDB Entry DOI: 10.7270/Q2GM8BVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM23926 ((E)-resveratrol | 5-[(E)-2-(4-hydroxyphenyl)etheny...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyungpook National University Curated by ChEMBL | Assay Description Inhibition of PTP1B (unknown origin) assessed as decrease in p-nitrophenolate formation using using pNPP as substrate incubated for 15 mins by spectr... | J Nat Prod 83: 323-332 (2020) Article DOI: 10.1021/acs.jnatprod.9b00777 BindingDB Entry DOI: 10.7270/Q2GM8BVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||