

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baculoviral IAP repeat-containing protein 2 [174-256] (Homo sapiens (Human)) | BDBM298445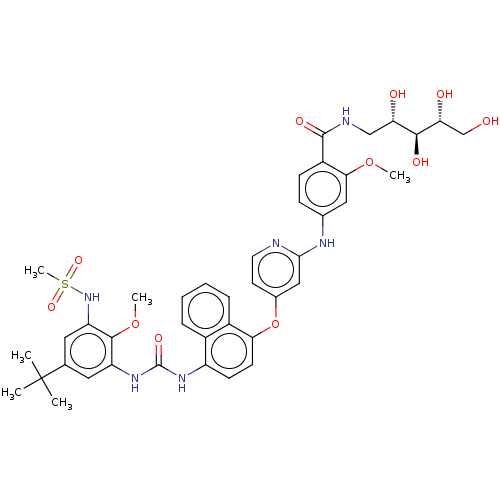 (4-((4-((4-(3-(5-(tert-Butyl)-2-methoxy-3-(methylsu...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description p38 MAPKγ: The inhibitory activities of compounds of the invention against p38MAPKγ (MAPK12: Invitrogen), are evaluated in a similar fashio... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 [174-256] (Homo sapiens (Human)) | BDBM298449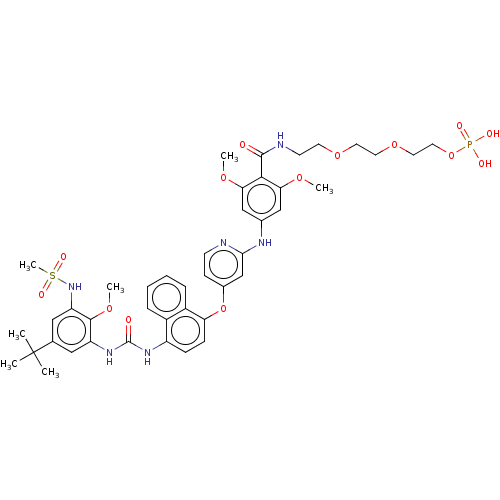 (2-(2-(2-(4-((4-((4-(3-(5-(tert-butyl)-2-methoxy-3-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description p38 MAPKγ: The inhibitory activities of compounds of the invention against p38MAPKγ (MAPK12: Invitrogen), are evaluated in a similar fashio... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 [174-256] (Homo sapiens (Human)) | BDBM337707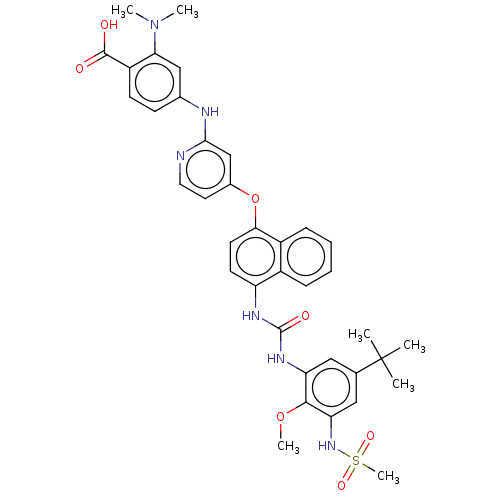 (US10392346, Example 17(am) | US10941115, Example 1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description p38 MAPKγ: The inhibitory activities of compounds of the invention against p38MAPKγ (MAPK12: Invitrogen), are evaluated in a similar fashio... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM255476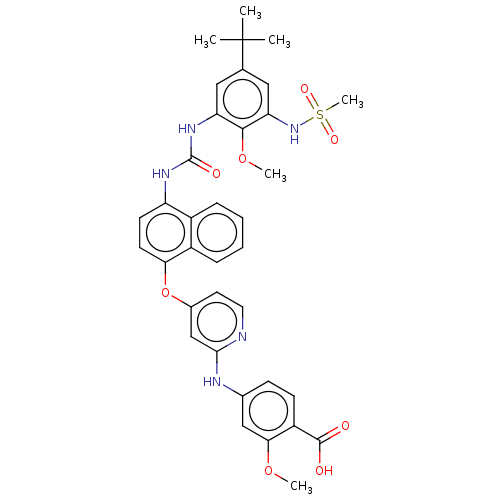 (US10125100, Example 1 | US10392346, Example 1 | US...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description c-Src and Syk: The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM381166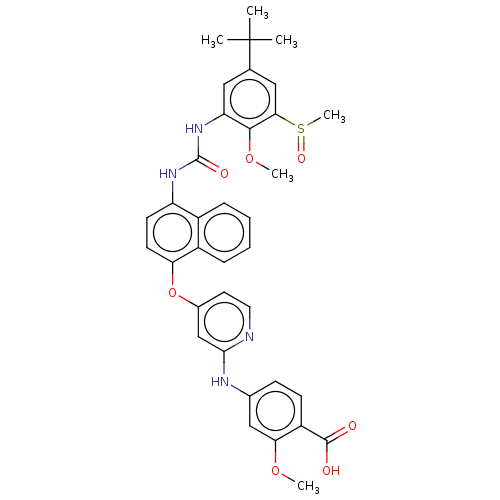 (US9890185, Example 79(g)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description Method 2: The inhibitory activities of compounds of the invention against p38MAPKγ (MAPK12: Invitrogen), are evaluated in a similar fashion to t... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM381082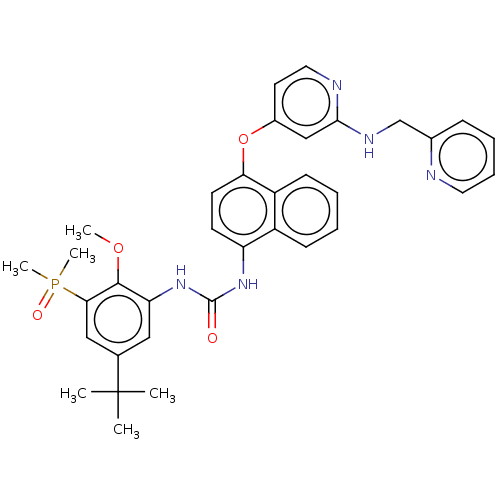 (US9890185, Example 38(e)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description Method 1: The inhibitory activities of test compounds against the p38 MAPKα isoform (MAPK14: Invitrogen), are evaluated indirectly by determinin... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 [174-256] (Homo sapiens (Human)) | BDBM298426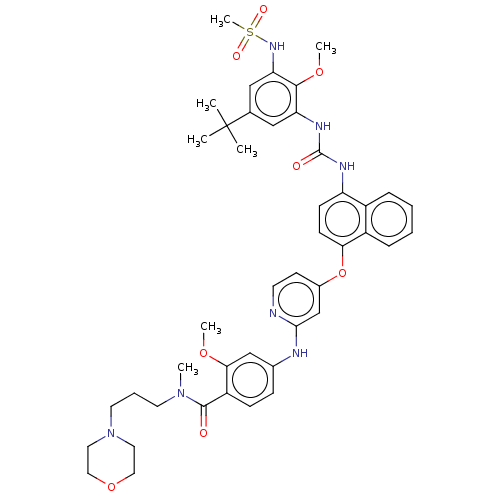 (4-[[4-[[4-[[5-tert-butyl-3-(methanesulfonamido)-2-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description p38 MAPKγ: The inhibitory activities of compounds of the invention against p38MAPKγ (MAPK12: Invitrogen), are evaluated in a similar fashio... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 [174-256] (Homo sapiens (Human)) | BDBM298439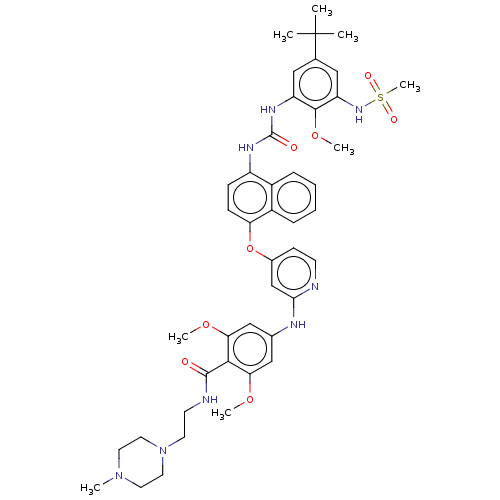 (4-((4-((4-(3-(5-(tert-Butyl)-2-methoxy-3-(methylsu...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description p38 MAPKγ: The inhibitory activities of compounds of the invention against p38MAPKγ (MAPK12: Invitrogen), are evaluated in a similar fashio... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 [174-256] (Homo sapiens (Human)) | BDBM298442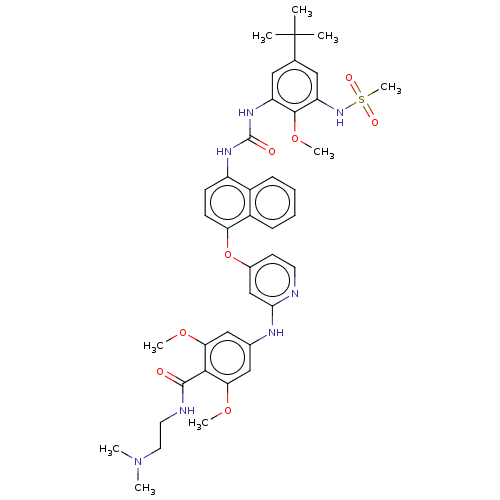 (4-((4-((4-(3-(5-(tert-Butyl)-2-methoxy-3-(methylsu...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description p38 MAPKγ: The inhibitory activities of compounds of the invention against p38MAPKγ (MAPK12: Invitrogen), are evaluated in a similar fashio... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM256521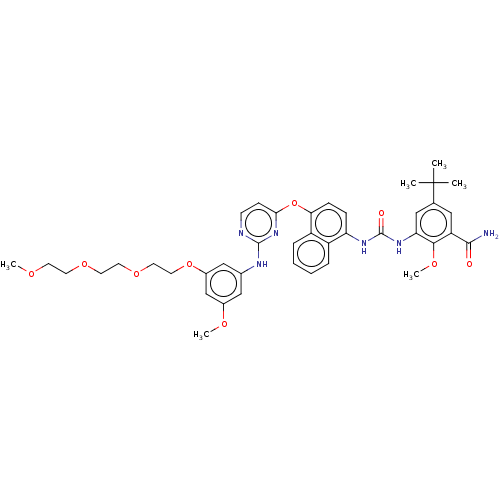 (US10435361, Example 20 | US9481648, 20 | US9790174...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert Limited; Topivert Pharma Limited US Patent | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9481648 (2016) BindingDB Entry DOI: 10.7270/Q24F1PNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM381094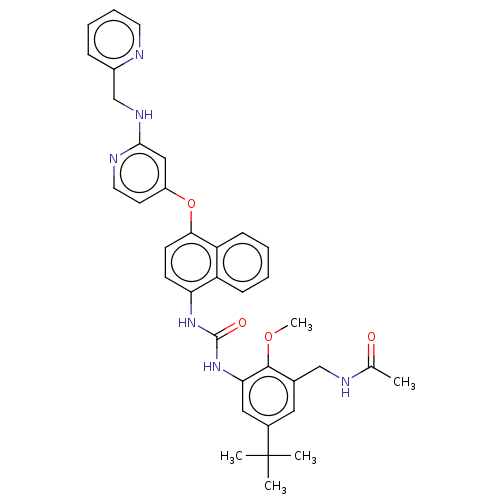 (US9890185, Example 38(q)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description Method 1: The inhibitory activities of test compounds against the p38 MAPKα isoform (MAPK14: Invitrogen), are evaluated indirectly by determinin... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM381167 (US9890185, Example 79(m)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description Method 1: The inhibitory activities of test compounds against the p38 MAPKα isoform (MAPK14: Invitrogen), are evaluated indirectly by determinin... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM381071 (1-(5-(tert-Butyl)-3-(dimethylphosphoryl)-2-methoxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description Method 1: The inhibitory activities of test compounds against the p38 MAPKα isoform (MAPK14: Invitrogen), are evaluated indirectly by determinin... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM381167 (US9890185, Example 79(m)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description Method 2: The inhibitory activities of compounds of the invention against p38MAPKγ (MAPK12: Invitrogen), are evaluated in a similar fashion to t... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 [174-256] (Homo sapiens (Human)) | BDBM298436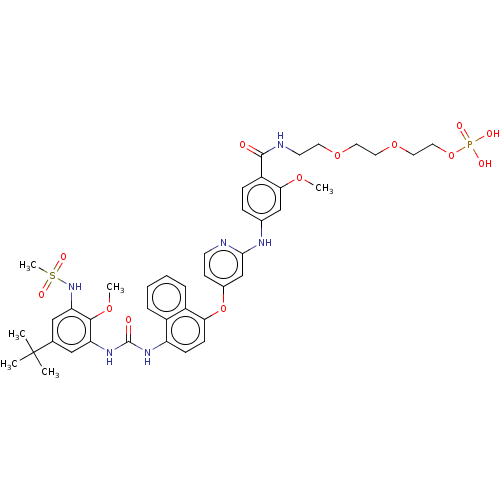 (2-(2-(2-(4-((4-((4-(3-(5-(tert-Butyl)-2-methoxy-3-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description p38 MAPKγ: The inhibitory activities of compounds of the invention against p38MAPKγ (MAPK12: Invitrogen), are evaluated in a similar fashio... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM298436 (2-(2-(2-(4-((4-((4-(3-(5-(tert-Butyl)-2-methoxy-3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description c-Src and Syk: The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM298449 (2-(2-(2-(4-((4-((4-(3-(5-(tert-butyl)-2-methoxy-3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description c-Src and Syk: The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM256511 (US10435361, Example 9 | US9481648, 9 | US9790174, ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert Limited; Topivert Pharma Limited US Patent | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9481648 (2016) BindingDB Entry DOI: 10.7270/Q24F1PNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM337710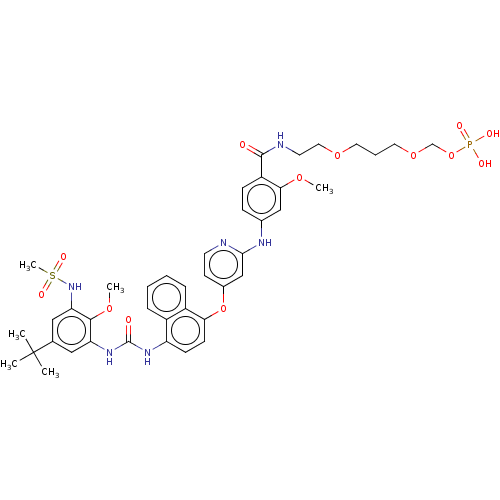 (2-(2-(2-(4-((4-((4-(3-(5-(tert-Butyl)-2-methoxy-3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
RESPIVERT LIMITED; TOPIVERT PHARMA LIMITED US Patent | Assay Description The inhibitory activities of compounds of the invention against p38MAPKγ (MAPK12: Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9751837 (2017) BindingDB Entry DOI: 10.7270/Q2028TP7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM337710 (2-(2-(2-(4-((4-((4-(3-(5-(tert-Butyl)-2-methoxy-3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
RESPIVERT LIMITED; TOPIVERT PHARMA LIMITED US Patent | Assay Description Method 1The inhibitory activities of compounds of the invention against the GSK 3α enzyme isoform (Invitrogen), are evaluated by determining the... | US Patent US9751837 (2017) BindingDB Entry DOI: 10.7270/Q2028TP7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM381123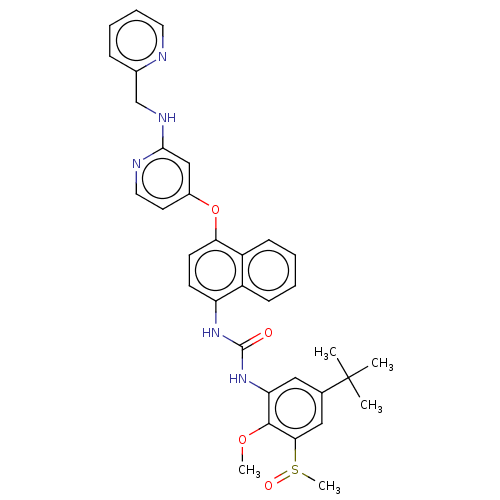 (1-(5-(tert-Butyl)-2-methoxy-3-(methylsulfinyl)phen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description Method 1: The inhibitory activities of test compounds against the p38 MAPKα isoform (MAPK14: Invitrogen), are evaluated indirectly by determinin... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM347466 (3-((4-((4-(3-(5-(tert-Butyl)-2-methoxy-3-(methylsu...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description TBD | US Patent US10435361 (2019) BindingDB Entry DOI: 10.7270/Q25M6839 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM381084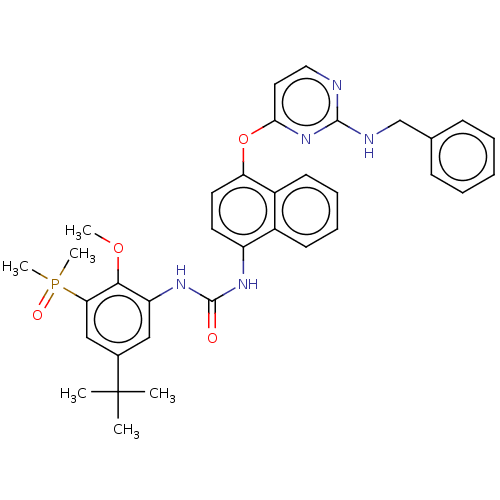 (US9890185, Example 38(g)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description Method 1: The inhibitory activities of test compounds against the p38 MAPKα isoform (MAPK14: Invitrogen), are evaluated indirectly by determinin... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM256515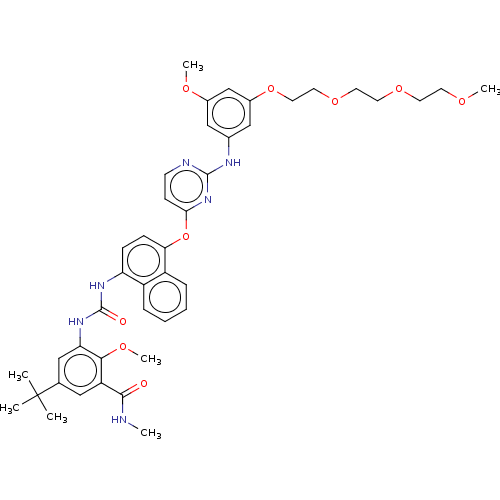 (US10435361, Example 13 | US9481648, 13 | US9790174...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert Limited; Topivert Pharma Limited US Patent | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9481648 (2016) BindingDB Entry DOI: 10.7270/Q24F1PNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM380997 (US9890185, Example 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM256509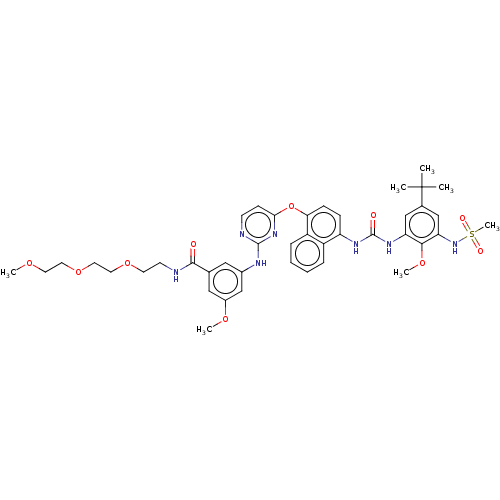 (US9481648, 7) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert Limited; Topivert Pharma Limited US Patent | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9481648 (2016) BindingDB Entry DOI: 10.7270/Q24F1PNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM381092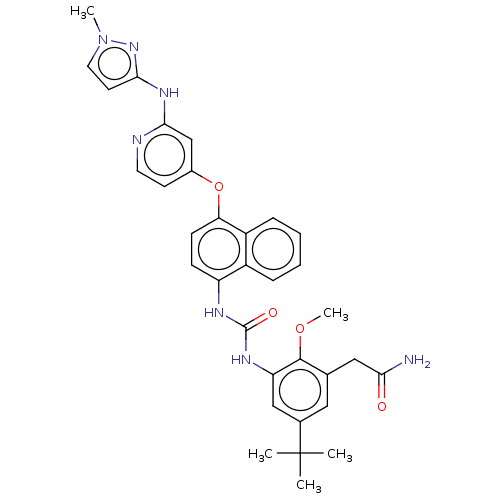 (US9890185, Example 38(o)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description Method 1: The inhibitory activities of test compounds against the p38 MAPKα isoform (MAPK14: Invitrogen), are evaluated indirectly by determinin... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM598412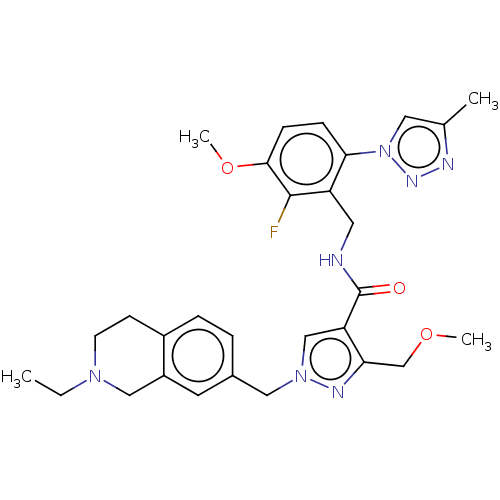 (1-[(2-ethyl-3,4-dihydro-1H-isoquinolin-7-yl)methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Plasma kallikrein inhibitory activity in vitro was determined using standard published methods (see e.g. Johansen et al., Int. J. Tiss. Reac. 1986, 8... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RF5ZZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM598413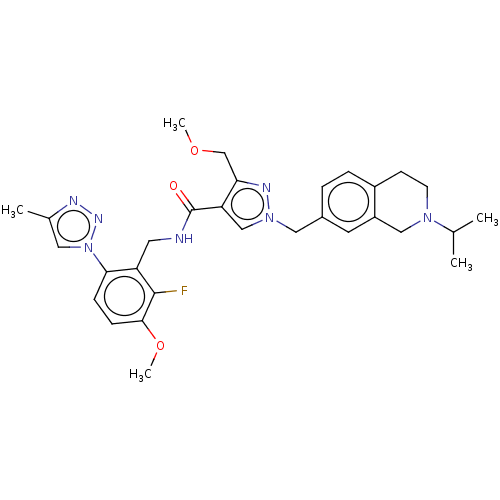 (N-{[2-fluoro-3-methoxy-6-(4-methyl-1,2,3-triazol-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Plasma kallikrein inhibitory activity in vitro was determined using standard published methods (see e.g. Johansen et al., Int. J. Tiss. Reac. 1986, 8... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RF5ZZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM598414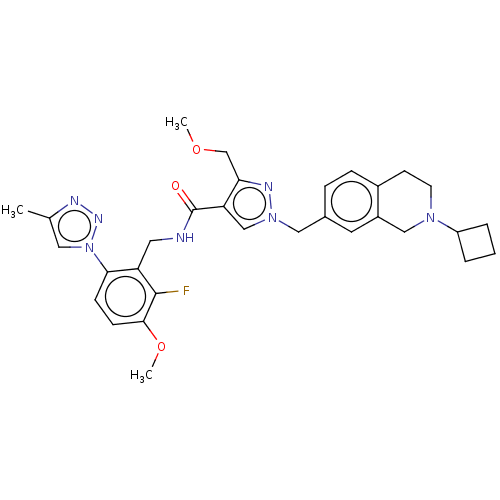 (1-((2-cyclobutyl-1,2,3,4-tetrahydroisoquinolin-7-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Plasma kallikrein inhibitory activity in vitro was determined using standard published methods (see e.g. Johansen et al., Int. J. Tiss. Reac. 1986, 8... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RF5ZZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM598415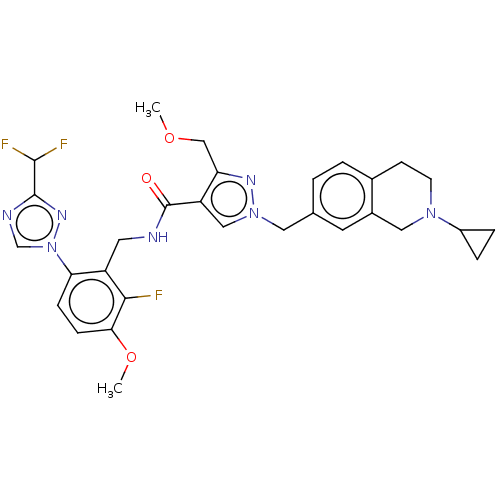 (1-[(2-cyclopropyl-3,4-dihydro-1H-isoquinolin-7-yl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Plasma kallikrein inhibitory activity in vitro was determined using standard published methods (see e.g. Johansen et al., Int. J. Tiss. Reac. 1986, 8... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RF5ZZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM598417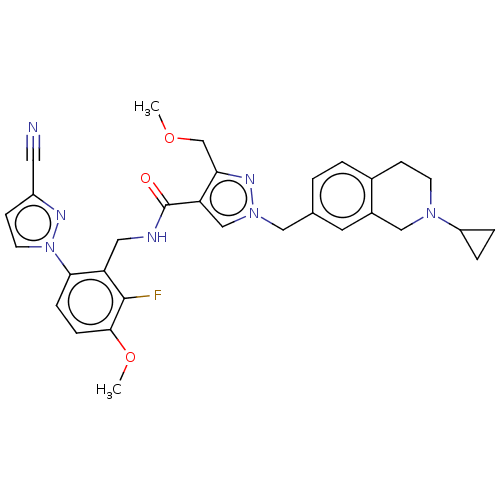 (N-{[6-(3-cyanopyrazol-1-yl)-2-fluoro-3-methoxyphen...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Plasma kallikrein inhibitory activity in vitro was determined using standard published methods (see e.g. Johansen et al., Int. J. Tiss. Reac. 1986, 8... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RF5ZZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM598418 (N-({2-fluoro-3-methoxy-6-[3-(trifluoromethyl)-1,2,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Plasma kallikrein inhibitory activity in vitro was determined using standard published methods (see e.g. Johansen et al., Int. J. Tiss. Reac. 1986, 8... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RF5ZZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM598419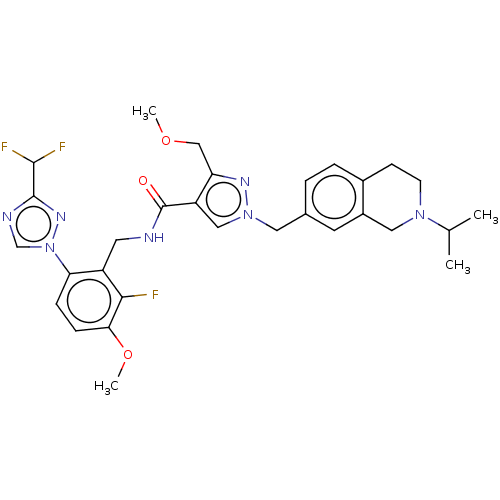 (N-({6-[3-(difluoromethyl)-1,2,4-triazol-1-yl]-2-fl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Plasma kallikrein inhibitory activity in vitro was determined using standard published methods (see e.g. Johansen et al., Int. J. Tiss. Reac. 1986, 8... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RF5ZZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM598420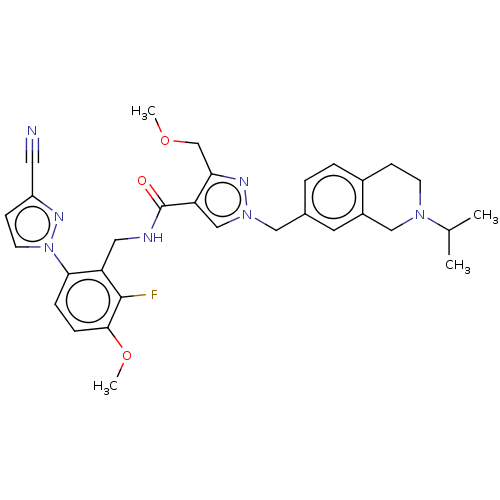 (N-{[6-(3-cyanopyrazol-1-yl)-2-fluoro-3-methoxyphen...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Plasma kallikrein inhibitory activity in vitro was determined using standard published methods (see e.g. Johansen et al., Int. J. Tiss. Reac. 1986, 8... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RF5ZZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM598421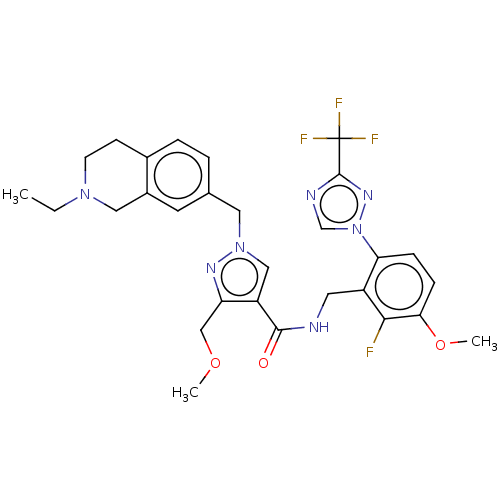 (1-[(2-ethyl-3,4-dihydro-1H-isoquinolin-7-yl)methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Plasma kallikrein inhibitory activity in vitro was determined using standard published methods (see e.g. Johansen et al., Int. J. Tiss. Reac. 1986, 8... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RF5ZZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM598422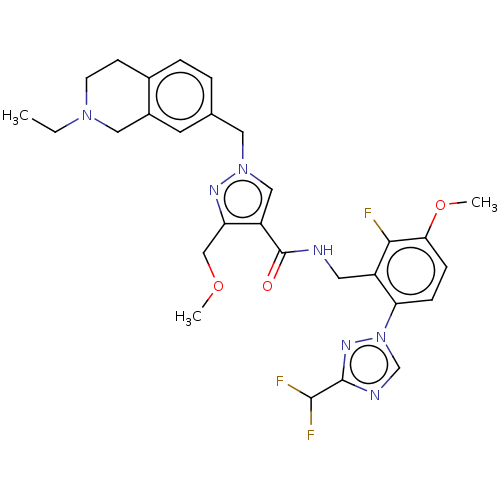 (N-({6-[3-(difluoromethyl)-1,2,4-triazol-1-yl]-2-fl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Plasma kallikrein inhibitory activity in vitro was determined using standard published methods (see e.g. Johansen et al., Int. J. Tiss. Reac. 1986, 8... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RF5ZZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM598423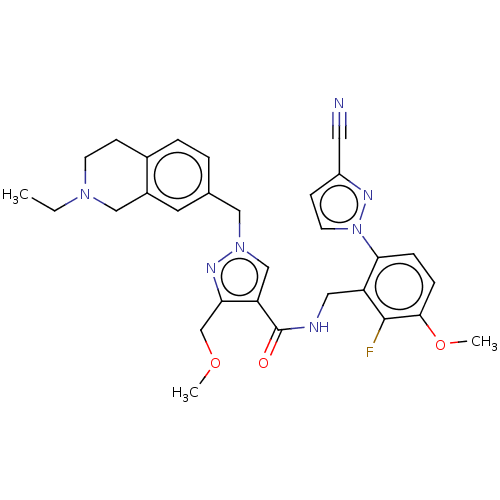 (N-{[6-(3-cyanopyrazol-1-yl)-2-fluoro-3-methoxyphen...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Plasma kallikrein inhibitory activity in vitro was determined using standard published methods (see e.g. Johansen et al., Int. J. Tiss. Reac. 1986, 8... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RF5ZZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM598424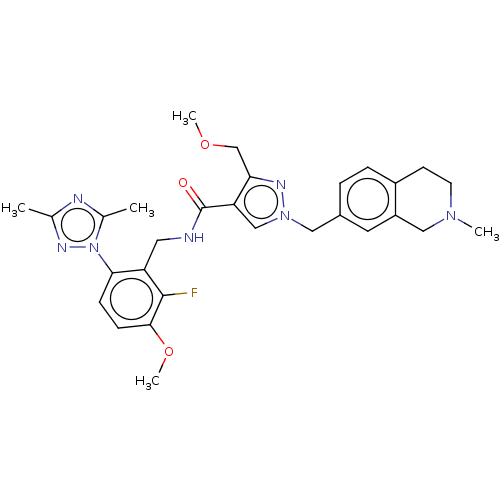 (N-{[6-(dimethyl-1,2,4-triazol-1-yl)-2-fluoro-3-met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Plasma kallikrein inhibitory activity in vitro was determined using standard published methods (see e.g. Johansen et al., Int. J. Tiss. Reac. 1986, 8... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RF5ZZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 [174-256] (Homo sapiens (Human)) | BDBM298392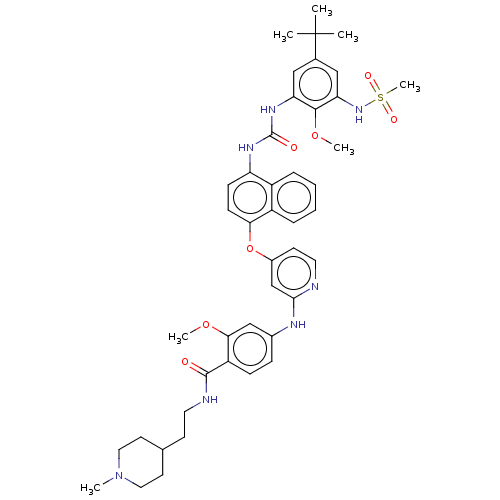 (4-[[4-[[4-[[5-tert-Butyl-3-(methanesulfonamido)-2-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description p38 MAPKγ: The inhibitory activities of compounds of the invention against p38MAPKγ (MAPK12: Invitrogen), are evaluated in a similar fashio... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 [174-256] (Homo sapiens (Human)) | BDBM298425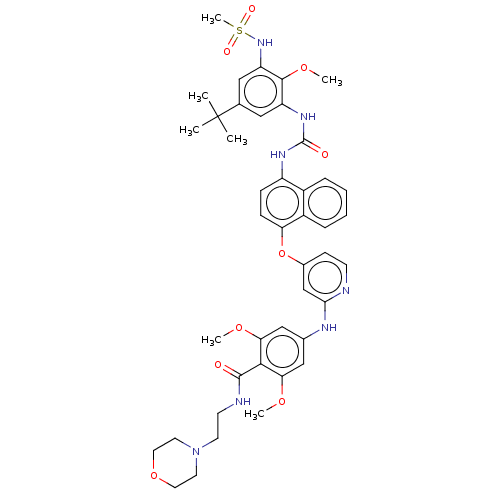 (4-[[4-[[4-[[5-tert-butyl-3-(methanesulfonamido)-2-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description p38 MAPKγ: The inhibitory activities of compounds of the invention against p38MAPKγ (MAPK12: Invitrogen), are evaluated in a similar fashio... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM256519 (US10435361, Example 18 | US9481648, 18 | US9790174...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert Limited; Topivert Pharma Limited US Patent | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9481648 (2016) BindingDB Entry DOI: 10.7270/Q24F1PNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM598493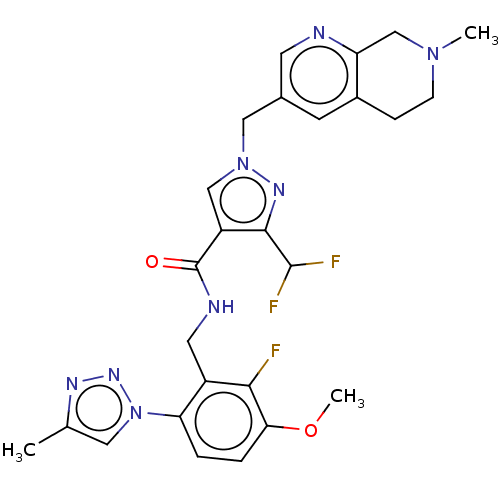 (3-(difluoromethyl)-N-{[2-fluoro-3-methoxy-6-(4-met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Plasma kallikrein inhibitory activity in vitro was determined using standard published methods (see e.g. Johansen et al., Int. J. Tiss. Reac. 1986, 8... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RF5ZZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM598500 (N-{[2-fluoro-3-methoxy-6-(4-methyl-1,2,3-triazol-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Plasma kallikrein inhibitory activity in vitro was determined using standard published methods (see e.g. Johansen et al., Int. J. Tiss. Reac. 1986, 8... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RF5ZZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM598501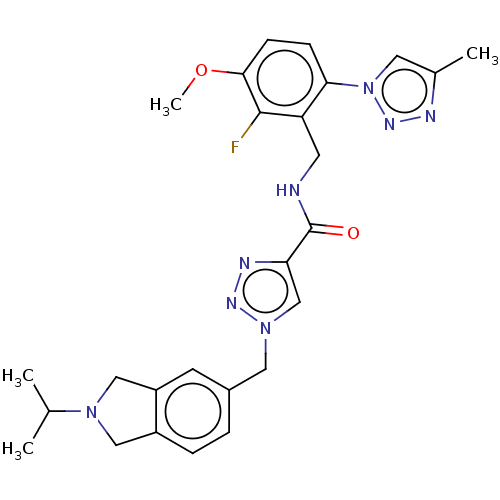 (N-{[2-fluoro-3-methoxy-6-(4-methyl-1,2,3-triazol-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Plasma kallikrein inhibitory activity in vitro was determined using standard published methods (see e.g. Johansen et al., Int. J. Tiss. Reac. 1986, 8... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RF5ZZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM598503 (N-({2-chloro-6-[3-(difluoromethyl)-1,2,4-triazol-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Plasma kallikrein inhibitory activity in vitro was determined using standard published methods (see e.g. Johansen et al., Int. J. Tiss. Reac. 1986, 8... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RF5ZZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM598505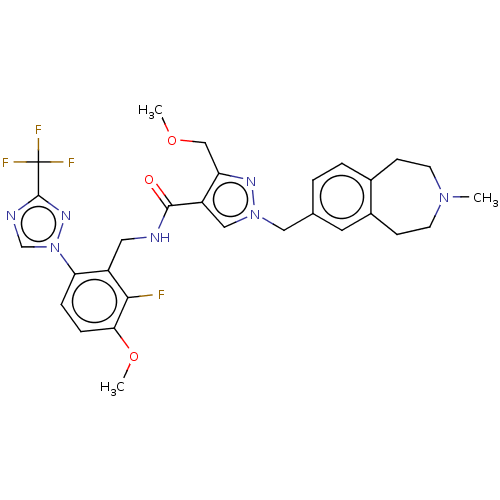 (N-({2-fluoro-3-methoxy-6-[3-(trifluoromethyl)-1,2,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Plasma kallikrein inhibitory activity in vitro was determined using standard published methods (see e.g. Johansen et al., Int. J. Tiss. Reac. 1986, 8... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RF5ZZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM598507 (N-{[2-fluoro-3-methoxy-6-(4-methyl-1,2,3-triazol-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Plasma kallikrein inhibitory activity in vitro was determined using standard published methods (see e.g. Johansen et al., Int. J. Tiss. Reac. 1986, 8... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RF5ZZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM598508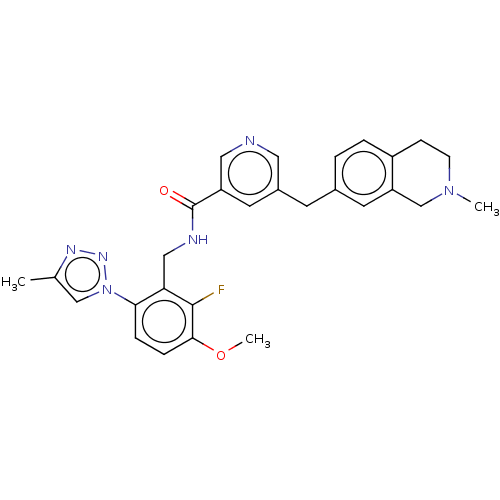 (N-{[2-fluoro-3-methoxy-6-(4-methyl-1,2,3-triazol-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Plasma kallikrein inhibitory activity in vitro was determined using standard published methods (see e.g. Johansen et al., Int. J. Tiss. Reac. 1986, 8... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RF5ZZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM598510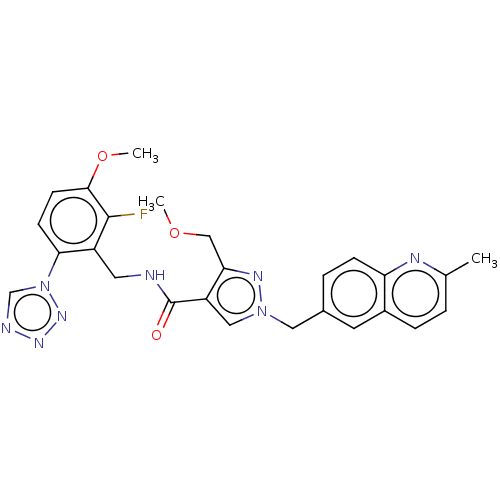 (N-{[2-fluoro-3-methoxy-6-(1,2,3,4-tetrazol-1-yl)ph...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Plasma kallikrein inhibitory activity in vitro was determined using standard published methods (see e.g. Johansen et al., Int. J. Tiss. Reac. 1986, 8... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RF5ZZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1404 total ) | Next | Last >> |