

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Platelet-activating factor acetylhydrolase (Homo sapiens (Human)) | BDBM50102161 (1-{2-[4-(3,4-Dichloro-phenyl)-piperazin-1-yl]-2-ox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Laboratory of Lipid Metabolism and Atherosclerosis Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Lp-PLA2 | Bioorg Med Chem Lett 15: 1525-7 (2005) Article DOI: 10.1016/j.bmcl.2004.11.063 BindingDB Entry DOI: 10.7270/Q28915CK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor acetylhydrolase (Homo sapiens (Human)) | BDBM50102161 (1-{2-[4-(3,4-Dichloro-phenyl)-piperazin-1-yl]-2-ox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Laboratory of Lipid Metabolism and Atherosclerosis Curated by ChEMBL | Assay Description In vitro inhibitory activity against human lipoprotein-associated phospholiphase A2 | Bioorg Med Chem Lett 15: 1525-7 (2005) Article DOI: 10.1016/j.bmcl.2004.11.063 BindingDB Entry DOI: 10.7270/Q28915CK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM375523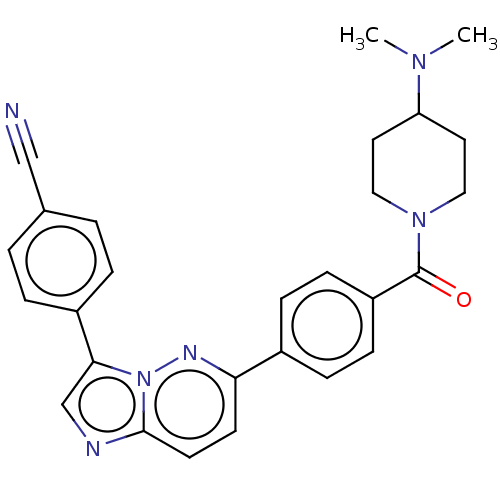 (4-(6-(4-(4-(dimethylamino)piperidine-1-carbonyl)ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of GST-tagged MNK1 (37 to 341 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using JH3 peptide as substrate preincubat... | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM375523 (4-(6-(4-(4-(dimethylamino)piperidine-1-carbonyl)ph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of GST-tagged MNK2 (72 to 385 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using JH3 peptide as substrate preincubat... | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM375525 (4-(6-(4-(piperazine-1-carbonyl)phenyl)imidazo[1,2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of GST-tagged MNK1 (37 to 341 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using JH3 peptide as substrate preincubat... | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM375513 ((4-(3-(4-chlorophenyl)imidazo[1,2-b]pyridazin-6-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of GST-tagged MNK1 (37 to 341 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using JH3 peptide as substrate preincubat... | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50147178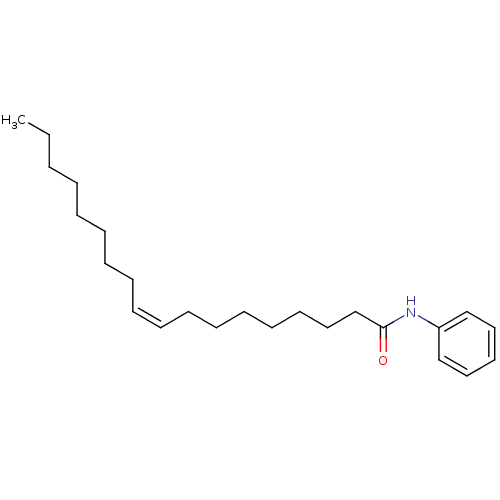 ((Z)-Octadec-9-enoic acid phenylamide | CHEMBL15611...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Inhibitory activity against rat liver microsomal Acyl coenzyme A:cholesterol acyltransferase | Bioorg Med Chem Lett 14: 4277-80 (2004) Article DOI: 10.1016/j.bmcl.2004.05.086 BindingDB Entry DOI: 10.7270/Q261113Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM375522 (4-(6-(4-(morpholine-4-carbonyl)phenyl)imidazo[1,2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of GST-tagged MNK1 (37 to 341 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using JH3 peptide as substrate preincubat... | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM375513 ((4-(3-(4-chlorophenyl)imidazo[1,2-b]pyridazin-6-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of GST-tagged MNK2 (72 to 385 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using JH3 peptide as substrate preincubat... | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM375525 (4-(6-(4-(piperazine-1-carbonyl)phenyl)imidazo[1,2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of GST-tagged MNK2 (72 to 385 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using JH3 peptide as substrate preincubat... | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50455538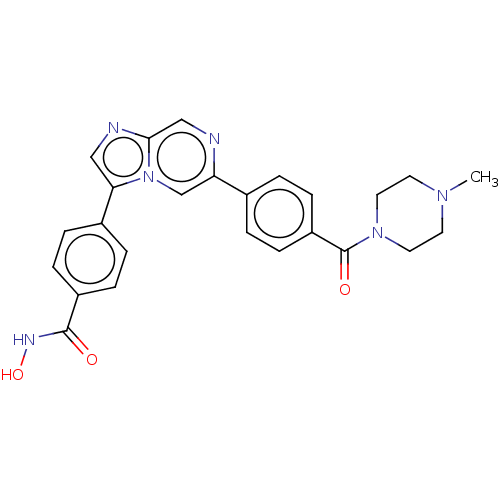 (CHEMBL4214092) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of GST-tagged MNK2 (72 to 385 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using JH3 peptide as substrate preincubat... | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM375522 (4-(6-(4-(morpholine-4-carbonyl)phenyl)imidazo[1,2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of GST-tagged MNK2 (72 to 385 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using JH3 peptide as substrate preincubat... | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50455538 (CHEMBL4214092) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of GST-tagged MNK1 (37 to 341 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using JH3 peptide as substrate preincubat... | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM375544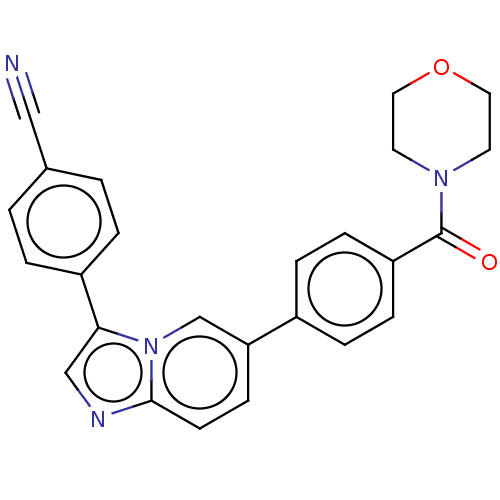 (4-(6-(4-(morpholine-4-carbonyl)phenyl)imidazo[1,2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of GST-tagged MNK1 (37 to 341 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using JH3 peptide as substrate preincubat... | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM375543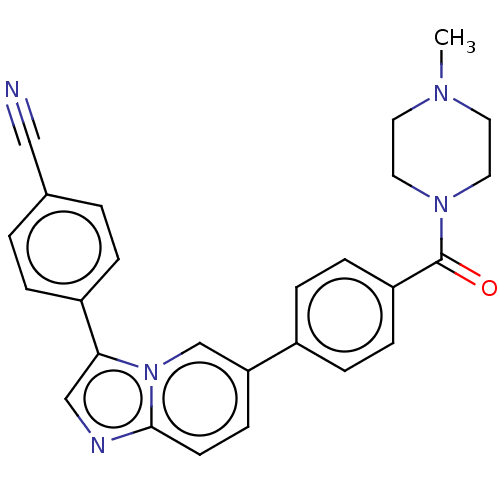 (4-(6-(4-(4-Methylpiperazine-1-carbonyl)phenyl)imid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of GST-tagged MNK2 (72 to 385 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using JH3 peptide as substrate preincubat... | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM375543 (4-(6-(4-(4-Methylpiperazine-1-carbonyl)phenyl)imid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of GST-tagged MNK1 (37 to 341 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using JH3 peptide as substrate preincubat... | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM375544 (4-(6-(4-(morpholine-4-carbonyl)phenyl)imidazo[1,2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of GST-tagged MNK2 (72 to 385 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using JH3 peptide as substrate preincubat... | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM375315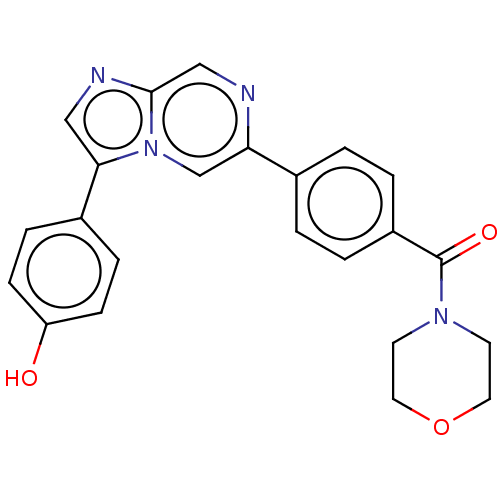 ((4-(3-(4-hydroxyphenyl)imidazo[1,2-a]pyrazin-6-yl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 113 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of GST-tagged MNK2 (72 to 385 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using JH3 peptide as substrate preincubat... | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor acetylhydrolase (Homo sapiens (Human)) | BDBM50193715 ((2Z)-{[(morpholin-4-ylcarbonyl)oxy]imino}(phenyl)a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Laboratory of Lipid Metabolism and Atherosclerosis Curated by ChEMBL | Assay Description Inhibition of human Low density lipoprotein-associated phospholipase A2 | Bioorg Med Chem Lett 16: 5576-9 (2006) Article DOI: 10.1016/j.bmcl.2006.08.031 BindingDB Entry DOI: 10.7270/Q2TM79RH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM375315 ((4-(3-(4-hydroxyphenyl)imidazo[1,2-a]pyrazin-6-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of GST-tagged MNK1 (37 to 341 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using JH3 peptide as substrate preincubat... | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 1/2 (Homo sapiens (Human)) | BDBM375315 ((4-(3-(4-hydroxyphenyl)imidazo[1,2-a]pyrazin-6-yl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of MNK1/2 in human HeLa cells assessed as decrease in eIF4E phosphorylation at Ser209 after 2 hrs by AlphaScreen assay | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50147178 ((Z)-Octadec-9-enoic acid phenylamide | CHEMBL15611...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit Acyl coenzyme A:cholesterol acyltransferase 1 | Bioorg Med Chem Lett 14: 3109-12 (2004) Article DOI: 10.1016/j.bmcl.2004.04.023 BindingDB Entry DOI: 10.7270/Q2X929Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50147178 ((Z)-Octadec-9-enoic acid phenylamide | CHEMBL15611...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Laboratory of Lipid Metabolism and Atherosclerosis Curated by ChEMBL | Assay Description Inhibition of human ACAT1 expressed in Hi5 cells | Bioorg Med Chem Lett 16: 5580-3 (2006) Article DOI: 10.1016/j.bmcl.2006.08.032 BindingDB Entry DOI: 10.7270/Q2PV6K0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM375313 ((4-(3-(4-hydroxyphenyl)imidazo[1,2-a]pyrazin-6-yl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of GST-tagged MNK2 (72 to 385 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using JH3 peptide as substrate preincubat... | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50147178 ((Z)-Octadec-9-enoic acid phenylamide | CHEMBL15611...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Inhibitory activity against human Acyl coenzyme A:cholesterol acyltransferase 1 | Bioorg Med Chem Lett 14: 4277-80 (2004) Article DOI: 10.1016/j.bmcl.2004.05.086 BindingDB Entry DOI: 10.7270/Q261113Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM375353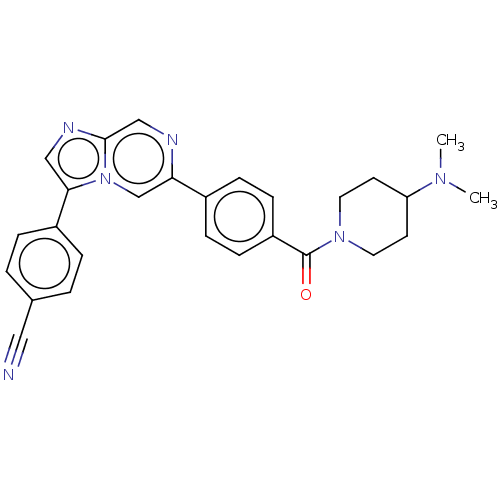 (4-(6-(4-(4-(dimethylamino)piperidine-1-carbonyl)ph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 143 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of GST-tagged MNK2 (72 to 385 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using JH3 peptide as substrate preincubat... | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50455541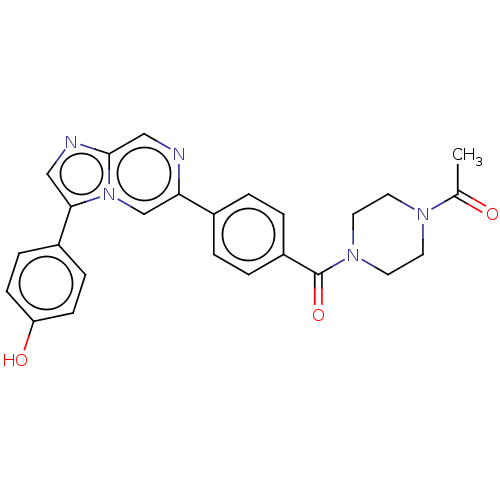 (CHEMBL4208890) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of GST-tagged MNK2 (72 to 385 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using JH3 peptide as substrate preincubat... | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50147178 ((Z)-Octadec-9-enoic acid phenylamide | CHEMBL15611...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Inhibitory activity against human Acyl coenzyme A:cholesterol acyltransferase 2 | Bioorg Med Chem Lett 14: 4277-80 (2004) Article DOI: 10.1016/j.bmcl.2004.05.086 BindingDB Entry DOI: 10.7270/Q261113Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50150482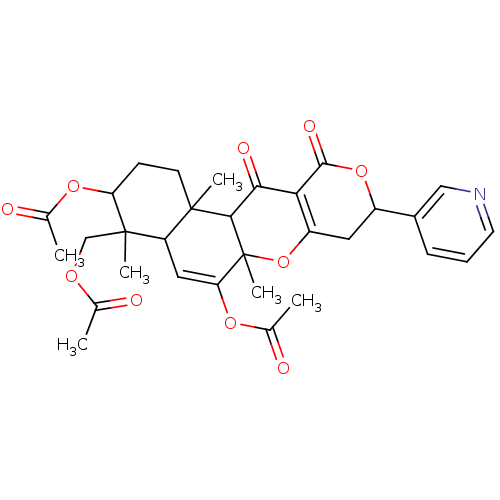 (Acetic acid 3-acetoxy-4-acetoxymethyl-12-hydroxy-4...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Inhibitory activity against rat liver microsomal Acyl coenzyme A:cholesterol acyltransferase | Bioorg Med Chem Lett 14: 4277-80 (2004) Article DOI: 10.1016/j.bmcl.2004.05.086 BindingDB Entry DOI: 10.7270/Q261113Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 2 (Homo sapiens (Human)) | BDBM50147178 ((Z)-Octadec-9-enoic acid phenylamide | CHEMBL15611...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Laboratory of Lipid Metabolism and Atherosclerosis Curated by ChEMBL | Assay Description Inhibition of human ACAT2 expressed in Hi5 cells | Bioorg Med Chem Lett 16: 5580-3 (2006) Article DOI: 10.1016/j.bmcl.2006.08.032 BindingDB Entry DOI: 10.7270/Q2PV6K0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 2 (Homo sapiens (Human)) | BDBM50147178 ((Z)-Octadec-9-enoic acid phenylamide | CHEMBL15611...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit Acyl coenzyme A:cholesterol acyltransferase 2 | Bioorg Med Chem Lett 14: 3109-12 (2004) Article DOI: 10.1016/j.bmcl.2004.04.023 BindingDB Entry DOI: 10.7270/Q2X929Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50455545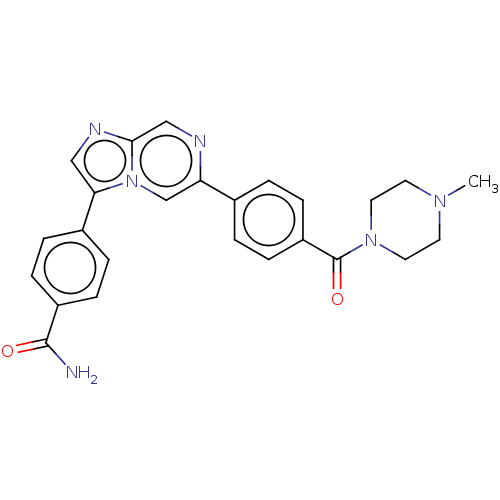 (CHEMBL4204506) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 187 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of GST-tagged MNK2 (72 to 385 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using JH3 peptide as substrate preincubat... | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 1/2 (Homo sapiens (Human)) | BDBM375313 ((4-(3-(4-hydroxyphenyl)imidazo[1,2-a]pyrazin-6-yl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 189 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of MNK1/2 in human HeLa cells assessed as decrease in eIF4E phosphorylation at Ser209 after 2 hrs by AlphaScreen assay | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50455540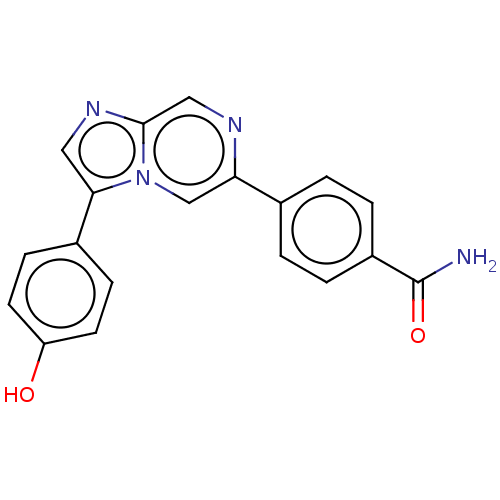 (CHEMBL4212480) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 193 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of GST-tagged MNK2 (72 to 385 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using JH3 peptide as substrate preincubat... | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50455543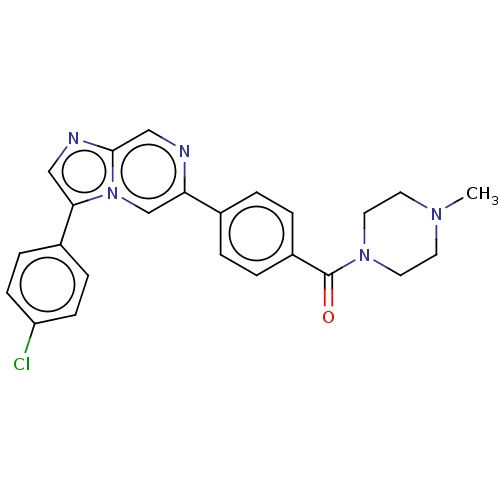 (CHEMBL4211757) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 217 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of GST-tagged MNK2 (72 to 385 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using JH3 peptide as substrate preincubat... | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 1/2 (Homo sapiens (Human)) | BDBM375525 (4-(6-(4-(piperazine-1-carbonyl)phenyl)imidazo[1,2-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of MNK1/2 in human HeLa cells assessed as decrease in eIF4E phosphorylation at Ser209 after 2 hrs by AlphaScreen assay | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50455541 (CHEMBL4208890) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 224 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of GST-tagged MNK1 (37 to 341 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using JH3 peptide as substrate preincubat... | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 1/2 (Homo sapiens (Human)) | BDBM375543 (4-(6-(4-(4-Methylpiperazine-1-carbonyl)phenyl)imid...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 229 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of MNK1/2 in human HeLa cells assessed as decrease in eIF4E phosphorylation at Ser209 after 2 hrs by AlphaScreen assay | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM375353 (4-(6-(4-(4-(dimethylamino)piperidine-1-carbonyl)ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 238 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of GST-tagged MNK1 (37 to 341 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using JH3 peptide as substrate preincubat... | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM375313 ((4-(3-(4-hydroxyphenyl)imidazo[1,2-a]pyrazin-6-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 284 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of GST-tagged MNK1 (37 to 341 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using JH3 peptide as substrate preincubat... | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50455545 (CHEMBL4204506) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 294 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of GST-tagged MNK1 (37 to 341 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using JH3 peptide as substrate preincubat... | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50455540 (CHEMBL4212480) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of GST-tagged MNK1 (37 to 341 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using JH3 peptide as substrate preincubat... | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 1/2 (Homo sapiens (Human)) | BDBM375544 (4-(6-(4-(morpholine-4-carbonyl)phenyl)imidazo[1,2-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 321 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of MNK1/2 in human HeLa cells assessed as decrease in eIF4E phosphorylation at Ser209 after 2 hrs by AlphaScreen assay | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 1/2 (Homo sapiens (Human)) | BDBM375513 ((4-(3-(4-chlorophenyl)imidazo[1,2-b]pyridazin-6-yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 326 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of MNK1/2 in human HeLa cells assessed as decrease in eIF4E phosphorylation at Ser209 after 2 hrs by AlphaScreen assay | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50455543 (CHEMBL4211757) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 331 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of GST-tagged MNK1 (37 to 341 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using JH3 peptide as substrate preincubat... | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50455548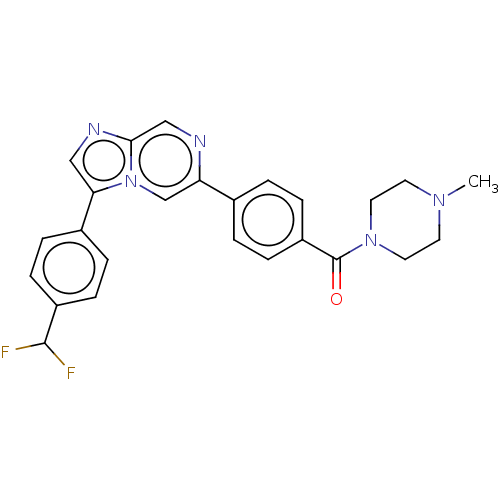 (CHEMBL4202593) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 336 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of GST-tagged MNK2 (72 to 385 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using JH3 peptide as substrate preincubat... | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50455539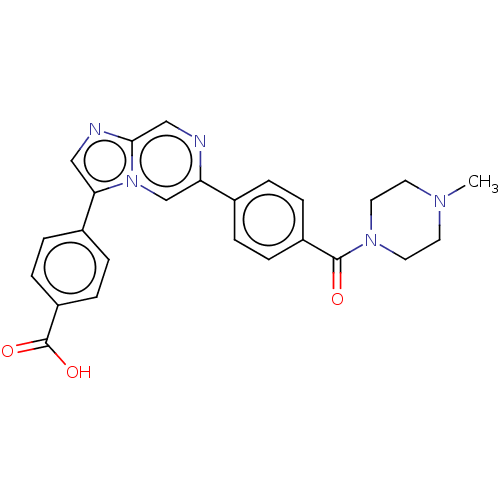 (CHEMBL4217366) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 355 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of GST-tagged MNK2 (72 to 385 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using JH3 peptide as substrate preincubat... | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50455548 (CHEMBL4202593) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 369 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of GST-tagged MNK1 (37 to 341 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using JH3 peptide as substrate preincubat... | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50455551 (CHEMBL4212200) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 389 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of GST-tagged MNK2 (72 to 385 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using JH3 peptide as substrate preincubat... | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50455544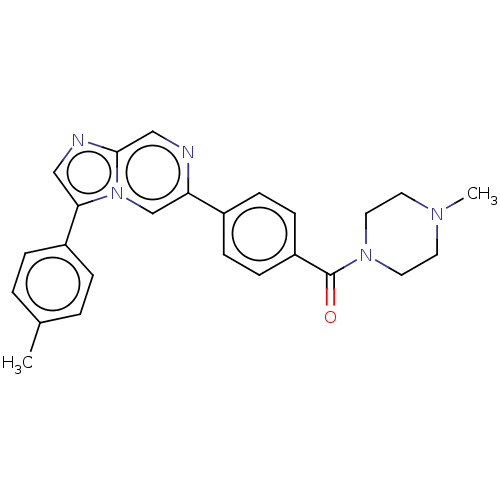 (CHEMBL4212307) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of GST-tagged MNK2 (72 to 385 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using JH3 peptide as substrate preincubat... | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 204 total ) | Next | Last >> |