 Found 557 hits with Last Name = 'pau' and Initial = 'v'
Found 557 hits with Last Name = 'pau' and Initial = 'v' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50464771

(CHEMBL4279417)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-c1c(oc2c(-[#6@@H]-3-[#6]=[#6](-[#6])-[#6]-[#6@@H](-[#6@H]-3-[#6](=O)-c3ccc(-[#8])cc3-[#8])-c3ccc(-[#8])cc3-[#8])c(-[#8])cc(-[#8])c2c1=O)-c1ccc(-[#8])cc1-[#8] |r,t:11| Show InChI InChI=1S/C40H36O11/c1-18(2)4-8-26-38(50)36-33(48)17-32(47)35(40(36)51-39(26)25-11-7-22(43)16-31(25)46)28-13-19(3)12-27(23-9-5-20(41)14-29(23)44)34(28)37(49)24-10-6-21(42)15-30(24)45/h4-7,9-11,13-17,27-28,34,41-48H,8,12H2,1-3H3/t27-,28-,34-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de Santa Catarina
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of human PTP1B using p-nitrophenyl phosphate as substrate up to 7 uM preincubated for 10 mins followed by substrate additi... |
Eur J Med Chem 144: 277-288 (2018)
Article DOI: 10.1016/j.ejmech.2017.11.087
BindingDB Entry DOI: 10.7270/Q2GX4F79 |
More data for this
Ligand-Target Pair | |
TYR_PHOSPHATASE_2 domain-containing protein
(Mycobacterium tuberculosis) | BDBM50464769
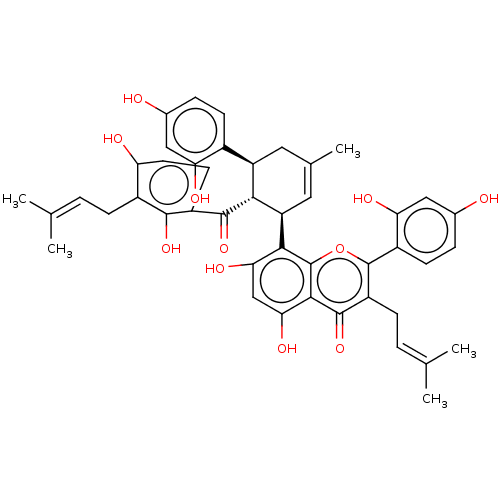
(CHEMBL4290098)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-c1c(-[#8])ccc(-[#6](=O)-[#6@@H]-2-[#6@H](-[#6]-[#6](-[#6])=[#6]-[#6@H]-2-c2c(-[#8])cc(-[#8])c3c2oc(c(-[#6]\[#6]=[#6](\[#6])-[#6])c3=O)-c2ccc(-[#8])cc2-[#8])-c2ccc(-[#8])cc2-[#8])c1-[#8] |r,c:17| Show InChI InChI=1S/C45H44O11/c1-21(2)6-10-27-33(48)15-14-29(41(27)53)42(54)38-31(26-12-8-24(46)18-34(26)49)16-23(5)17-32(38)39-36(51)20-37(52)40-43(55)30(11-7-22(3)4)44(56-45(39)40)28-13-9-25(47)19-35(28)50/h6-9,12-15,17-20,31-32,38,46-53H,10-11,16H2,1-5H3/t31-,32-,38-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de Santa Catarina
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis PtpB using p-nitrophenyl phosphate as substrate preincubated for 10 mins followed by substrate addition meas... |
Eur J Med Chem 144: 277-288 (2018)
Article DOI: 10.1016/j.ejmech.2017.11.087
BindingDB Entry DOI: 10.7270/Q2GX4F79 |
More data for this
Ligand-Target Pair | |
TYR_PHOSPHATASE_2 domain-containing protein
(Mycobacterium tuberculosis) | BDBM50464771

(CHEMBL4279417)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-c1c(oc2c(-[#6@@H]-3-[#6]=[#6](-[#6])-[#6]-[#6@@H](-[#6@H]-3-[#6](=O)-c3ccc(-[#8])cc3-[#8])-c3ccc(-[#8])cc3-[#8])c(-[#8])cc(-[#8])c2c1=O)-c1ccc(-[#8])cc1-[#8] |r,t:11| Show InChI InChI=1S/C40H36O11/c1-18(2)4-8-26-38(50)36-33(48)17-32(47)35(40(36)51-39(26)25-11-7-22(43)16-31(25)46)28-13-19(3)12-27(23-9-5-20(41)14-29(23)44)34(28)37(49)24-10-6-21(42)15-30(24)45/h4-7,9-11,13-17,27-28,34,41-48H,8,12H2,1-3H3/t27-,28-,34-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de Santa Catarina
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis PtpB using p-nitrophenyl phosphate as substrate preincubated for 10 mins followed by substrate addition meas... |
Eur J Med Chem 144: 277-288 (2018)
Article DOI: 10.1016/j.ejmech.2017.11.087
BindingDB Entry DOI: 10.7270/Q2GX4F79 |
More data for this
Ligand-Target Pair | |
TYR_PHOSPHATASE_2 domain-containing protein
(Mycobacterium tuberculosis) | BDBM50464770

(CHEMBL4280952)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-c1c(-[#8])ccc(-[#6](=O)-[#6@@H]-2-[#6@H](-[#6]-[#6](-[#6])=[#6]-[#6@@H]-2-c2c(-[#8])cc(\[#6]=[#6]\c3ccc(-[#8])cc3-[#8])cc2-[#8])-c2ccc(-[#8])cc2-[#8])c1-[#8] |r,c:17| Show InChI InChI=1S/C39H38O9/c1-20(2)4-10-27-31(42)13-12-28(38(27)47)39(48)36-29(26-11-9-25(41)19-33(26)44)14-21(3)15-30(36)37-34(45)16-22(17-35(37)46)5-6-23-7-8-24(40)18-32(23)43/h4-9,11-13,15-19,29-30,36,40-47H,10,14H2,1-3H3/b6-5+/t29-,30+,36-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de Santa Catarina
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis PtpB using p-nitrophenyl phosphate as substrate preincubated for 10 mins followed by substrate addition meas... |
Eur J Med Chem 144: 277-288 (2018)
Article DOI: 10.1016/j.ejmech.2017.11.087
BindingDB Entry DOI: 10.7270/Q2GX4F79 |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591018
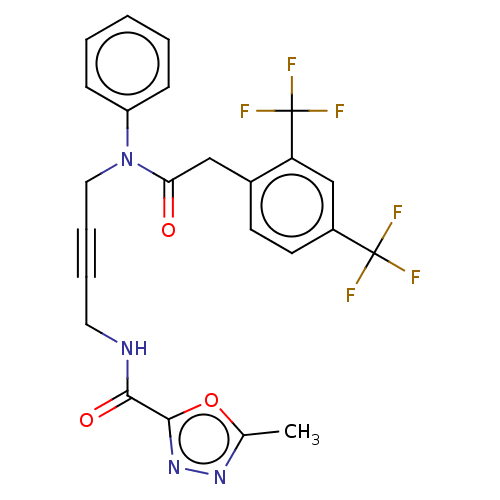
(CHEMBL5204203)Show SMILES Cc1nnc(o1)C(=O)NCC#CCN(C(=O)Cc1ccc(cc1C(F)(F)F)C(F)(F)F)c1ccccc1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0680 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00998
BindingDB Entry DOI: 10.7270/Q2C53QVS |
More data for this
Ligand-Target Pair | |
Cathepsin E
(Homo sapiens (Human)) | BDBM912

((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CC(C)C)C(C)C)C(C)C)[C@@H](O)CC(O)=O |r| Show InChI InChI=1S/C34H63N5O9/c1-17(2)12-23(37-33(47)31(21(9)10)39-34(48)30(20(7)8)38-27(42)14-19(5)6)25(40)15-28(43)35-22(11)32(46)36-24(13-18(3)4)26(41)16-29(44)45/h17-26,30-31,40-41H,12-16H2,1-11H3,(H,35,43)(H,36,46)(H,37,47)(H,38,42)(H,39,48)(H,44,45)/t22-,23-,24-,25-,26-,30-,31-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin E using Mca-Gly-Lys-Pro-Ile-Leu-Phe-Phe-Arg-Leu-Lys-(Dnp)-D-Arg-NH2 as substrate preincubated for 15 mins f... |
Bioorg Med Chem 24: 3276-82 (2016)
Article DOI: 10.1016/j.bmc.2016.04.062
BindingDB Entry DOI: 10.7270/Q2QZ2CWR |
More data for this
Ligand-Target Pair | |
Cathepsin E
(Homo sapiens (Human)) | BDBM912

((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CC(C)C)C(C)C)C(C)C)[C@@H](O)CC(O)=O |r| Show InChI InChI=1S/C34H63N5O9/c1-17(2)12-23(37-33(47)31(21(9)10)39-34(48)30(20(7)8)38-27(42)14-19(5)6)25(40)15-28(43)35-22(11)32(46)36-24(13-18(3)4)26(41)16-29(44)45/h17-26,30-31,40-41H,12-16H2,1-11H3,(H,35,43)(H,36,46)(H,37,47)(H,38,42)(H,39,48)(H,44,45)/t22-,23-,24-,25-,26-,30-,31-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of secreted cathepsin E in human MDA-MB-231 cells using Mca-Gly-Lys-Pro-Ile-Leu-PhePhe-Arg-Leu-Lys-(Dnp)-D-Arg-NH2 as substrate pretreated... |
J Nat Prod 80: 2969-2986 (2017)
Article DOI: 10.1021/acs.jnatprod.7b00551
BindingDB Entry DOI: 10.7270/Q2JS9SXJ |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM912

((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CC(C)C)C(C)C)C(C)C)[C@@H](O)CC(O)=O |r| Show InChI InChI=1S/C34H63N5O9/c1-17(2)12-23(37-33(47)31(21(9)10)39-34(48)30(20(7)8)38-27(42)14-19(5)6)25(40)15-28(43)35-22(11)32(46)36-24(13-18(3)4)26(41)16-29(44)45/h17-26,30-31,40-41H,12-16H2,1-11H3,(H,35,43)(H,36,46)(H,37,47)(H,38,42)(H,39,48)(H,44,45)/t22-,23-,24-,25-,26-,30-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of secreted cathepsin D in human MDA-MB-231 cells using Mca-Gly-Lys-Pro-Ile-Leu-PhePhe-Arg-Leu-Lys-(Dnp)-D-Arg-NH2 as substrate pretreated... |
J Nat Prod 80: 2969-2986 (2017)
Article DOI: 10.1021/acs.jnatprod.7b00551
BindingDB Entry DOI: 10.7270/Q2JS9SXJ |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM912

((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CC(C)C)C(C)C)C(C)C)[C@@H](O)CC(O)=O |r| Show InChI InChI=1S/C34H63N5O9/c1-17(2)12-23(37-33(47)31(21(9)10)39-34(48)30(20(7)8)38-27(42)14-19(5)6)25(40)15-28(43)35-22(11)32(46)36-24(13-18(3)4)26(41)16-29(44)45/h17-26,30-31,40-41H,12-16H2,1-11H3,(H,35,43)(H,36,46)(H,37,47)(H,38,42)(H,39,48)(H,44,45)/t22-,23-,24-,25-,26-,30-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
Article
PubMed
| n/a | n/a | 0.173 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin D |
J Med Chem 52: 5732-47 (2009)
Article DOI: 10.1021/jm9009394
BindingDB Entry DOI: 10.7270/Q2BG2PXP |
More data for this
Ligand-Target Pair | |
Cathepsin E
(Homo sapiens (Human)) | BDBM912

((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CC(C)C)C(C)C)C(C)C)[C@@H](O)CC(O)=O |r| Show InChI InChI=1S/C34H63N5O9/c1-17(2)12-23(37-33(47)31(21(9)10)39-34(48)30(20(7)8)38-27(42)14-19(5)6)25(40)15-28(43)35-22(11)32(46)36-24(13-18(3)4)26(41)16-29(44)45/h17-26,30-31,40-41H,12-16H2,1-11H3,(H,35,43)(H,36,46)(H,37,47)(H,38,42)(H,39,48)(H,44,45)/t22-,23-,24-,25-,26-,30-,31-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.181 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin E |
J Med Chem 52: 5732-47 (2009)
Article DOI: 10.1021/jm9009394
BindingDB Entry DOI: 10.7270/Q2BG2PXP |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591016
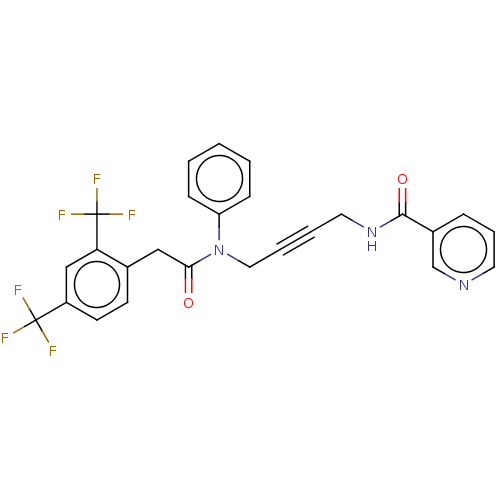
(CHEMBL5175156)Show SMILES FC(F)(F)c1ccc(CC(=O)N(CC#CCNC(=O)c2cccnc2)c2ccccc2)c(c1)C(F)(F)F | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00998
BindingDB Entry DOI: 10.7270/Q2C53QVS |
More data for this
Ligand-Target Pair | |
Cathepsin E
(Homo sapiens (Human)) | BDBM912

((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CC(C)C)C(C)C)C(C)C)[C@@H](O)CC(O)=O |r| Show InChI InChI=1S/C34H63N5O9/c1-17(2)12-23(37-33(47)31(21(9)10)39-34(48)30(20(7)8)38-27(42)14-19(5)6)25(40)15-28(43)35-22(11)32(46)36-24(13-18(3)4)26(41)16-29(44)45/h17-26,30-31,40-41H,12-16H2,1-11H3,(H,35,43)(H,36,46)(H,37,47)(H,38,42)(H,39,48)(H,44,45)/t22-,23-,24-,25-,26-,30-,31-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human C-terminal His10-tagged cathepsin E (Gln18 to Pro396 residues) using Mca-Gly-Lys-Pro-Ile-Leu-PhePhe-Arg-Leu-Lys-(Dnp)... |
J Nat Prod 80: 2969-2986 (2017)
Article DOI: 10.1021/acs.jnatprod.7b00551
BindingDB Entry DOI: 10.7270/Q2JS9SXJ |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM912

((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CC(C)C)C(C)C)C(C)C)[C@@H](O)CC(O)=O |r| Show InChI InChI=1S/C34H63N5O9/c1-17(2)12-23(37-33(47)31(21(9)10)39-34(48)30(20(7)8)38-27(42)14-19(5)6)25(40)15-28(43)35-22(11)32(46)36-24(13-18(3)4)26(41)16-29(44)45/h17-26,30-31,40-41H,12-16H2,1-11H3,(H,35,43)(H,36,46)(H,37,47)(H,38,42)(H,39,48)(H,44,45)/t22-,23-,24-,25-,26-,30-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
Article
PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of human liver cathepsin D using Mca-Gly-Lys-Pro-Ile-Leu-PhePhe-Arg-Leu-Lys-(Dnp)-D-Arg-NH2 as substrate pretreated for 15 mins followed b... |
J Nat Prod 80: 2969-2986 (2017)
Article DOI: 10.1021/acs.jnatprod.7b00551
BindingDB Entry DOI: 10.7270/Q2JS9SXJ |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM912

((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CC(C)C)C(C)C)C(C)C)[C@@H](O)CC(O)=O |r| Show InChI InChI=1S/C34H63N5O9/c1-17(2)12-23(37-33(47)31(21(9)10)39-34(48)30(20(7)8)38-27(42)14-19(5)6)25(40)15-28(43)35-22(11)32(46)36-24(13-18(3)4)26(41)16-29(44)45/h17-26,30-31,40-41H,12-16H2,1-11H3,(H,35,43)(H,36,46)(H,37,47)(H,38,42)(H,39,48)(H,44,45)/t22-,23-,24-,25-,26-,30-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
Article
PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of human liver cathepsin D using Mca-Gly-Lys-Pro-Ile-Leu-Phe-Phe-Arg-Leu-Lys-(Dnp)-D-Arg-NH2 as substrate preincubated for 15 mins followe... |
Bioorg Med Chem 24: 3276-82 (2016)
Article DOI: 10.1016/j.bmc.2016.04.062
BindingDB Entry DOI: 10.7270/Q2QZ2CWR |
More data for this
Ligand-Target Pair | |
Cathepsin E
(Homo sapiens (Human)) | BDBM50302109

(CHEMBL568553 | Grassystatin B)Show SMILES CC[C@H](NC(=O)[C@@H](NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](OC(=O)[C@@H](OC(=O)[C@H](C(C)C)N(C)C)C(C)C)C(C)C)[C@@H](C)O)C(=O)N(C)[C@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)OC |r| Show InChI InChI=1S/C59H97N9O16/c1-17-38(55(77)67(15)43(28-37-22-19-18-20-23-37)56(78)68-25-21-24-42(68)57(79)82-16)61-53(75)47(36(12)69)65-46(72)30-44(70)39(26-31(2)3)62-52(74)41(29-45(60)71)63-51(73)40(27-32(4)5)64-54(76)49(34(8)9)83-59(81)50(35(10)11)84-58(80)48(33(6)7)66(13)14/h18-20,22-23,31-36,38-44,47-50,69-70H,17,21,24-30H2,1-16H3,(H2,60,71)(H,61,75)(H,62,74)(H,63,73)(H,64,76)(H,65,72)/t36-,38+,39+,40+,41+,42+,43-,44+,47+,48+,49-,50+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.354 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin E |
J Med Chem 52: 5732-47 (2009)
Article DOI: 10.1021/jm9009394
BindingDB Entry DOI: 10.7270/Q2BG2PXP |
More data for this
Ligand-Target Pair | |
Cathepsin E
(Homo sapiens (Human)) | BDBM50302109

(CHEMBL568553 | Grassystatin B)Show SMILES CC[C@H](NC(=O)[C@@H](NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](OC(=O)[C@@H](OC(=O)[C@H](C(C)C)N(C)C)C(C)C)C(C)C)[C@@H](C)O)C(=O)N(C)[C@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)OC |r| Show InChI InChI=1S/C59H97N9O16/c1-17-38(55(77)67(15)43(28-37-22-19-18-20-23-37)56(78)68-25-21-24-42(68)57(79)82-16)61-53(75)47(36(12)69)65-46(72)30-44(70)39(26-31(2)3)62-52(74)41(29-45(60)71)63-51(73)40(27-32(4)5)64-54(76)49(34(8)9)83-59(81)50(35(10)11)84-58(80)48(33(6)7)66(13)14/h18-20,22-23,31-36,38-44,47-50,69-70H,17,21,24-30H2,1-16H3,(H2,60,71)(H,61,75)(H,62,74)(H,63,73)(H,64,76)(H,65,72)/t36-,38+,39+,40+,41+,42+,43-,44+,47+,48+,49-,50+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human C-terminal His10-tagged cathepsin E (Gln18 to Pro396 residues) using Mca-Gly-Lys-Pro-Ile-Leu-PhePhe-Arg-Leu-Lys-(Dnp)... |
J Nat Prod 80: 2969-2986 (2017)
Article DOI: 10.1021/acs.jnatprod.7b00551
BindingDB Entry DOI: 10.7270/Q2JS9SXJ |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591017
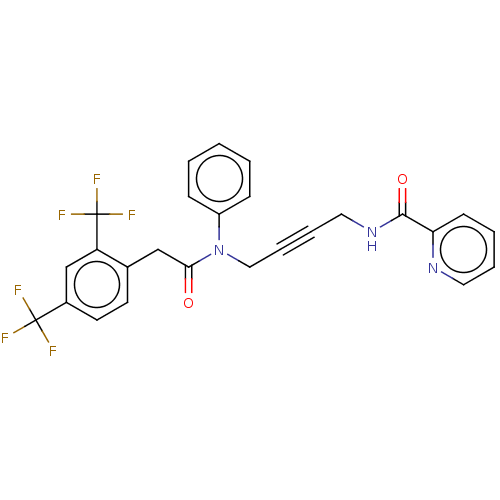
(CHEMBL5206022)Show SMILES FC(F)(F)c1ccc(CC(=O)N(CC#CCNC(=O)c2ccccn2)c2ccccc2)c(c1)C(F)(F)F | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00998
BindingDB Entry DOI: 10.7270/Q2C53QVS |
More data for this
Ligand-Target Pair | |
Cathepsin E
(Homo sapiens (Human)) | BDBM50283681
(CHEMBL4163078)Show SMILES CC[C@H](C)[C@H](NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](CCC(N)=O)N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@H](C)OC(=O)[C@H](Cc1ccccc1)N(C)C)C(=O)N[C@@H](C)C(=O)N(C)[C@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)OC |r| Show InChI InChI=1S/C59H91N9O13/c1-14-37(6)51(54(74)61-38(7)55(75)67(12)46(32-40-22-17-15-18-23-40)57(77)68-29-21-26-45(68)58(78)80-13)64-50(71)34-48(69)42(30-35(2)3)62-53(73)44(27-28-49(60)70)66(11)56(76)43(31-36(4)5)63-52(72)39(8)81-59(79)47(65(9)10)33-41-24-19-16-20-25-41/h15-20,22-25,35-39,42-48,51,69H,14,21,26-34H2,1-13H3,(H2,60,70)(H,61,74)(H,62,73)(H,63,72)(H,64,71)/t37-,38-,39-,42-,43-,44-,45-,46+,47-,48-,51-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human C-terminal His10-tagged cathepsin E (Gln18 to Pro396 residues) using Mca-Gly-Lys-Pro-Ile-Leu-PhePhe-Arg-Leu-Lys-(Dnp)... |
J Nat Prod 80: 2969-2986 (2017)
Article DOI: 10.1021/acs.jnatprod.7b00551
BindingDB Entry DOI: 10.7270/Q2JS9SXJ |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591022
(CHEMBL5197367)Show SMILES FC(F)(F)c1ccc(CC(=O)N(CC#Cc2cccc(=O)[nH]2)c2ccccc2)c(c1)C(F)(F)F | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00998
BindingDB Entry DOI: 10.7270/Q2C53QVS |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591035
(CHEMBL5187422)Show SMILES Nc1ccc(nn1)C#CCN(C(=O)Cc1ncc(cc1C(F)(F)F)C(F)(F)F)c1ccc(F)cc1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00998
BindingDB Entry DOI: 10.7270/Q2C53QVS |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591030
(CHEMBL5174509)Show SMILES Fc1ccc(cc1)N(CC#Cc1ccc(cn1)C1CCNC1)C(=O)Cc1ncc(cc1C(F)(F)F)C(F)(F)F | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00998
BindingDB Entry DOI: 10.7270/Q2C53QVS |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591026
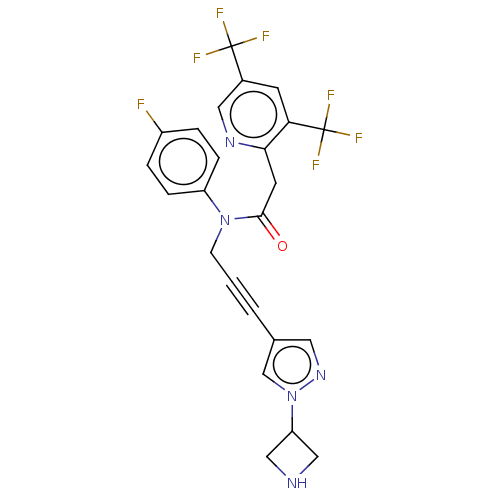
(CHEMBL5197173)Show SMILES Fc1ccc(cc1)N(CC#Cc1cnn(c1)C1CNC1)C(=O)Cc1ncc(cc1C(F)(F)F)C(F)(F)F | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00998
BindingDB Entry DOI: 10.7270/Q2C53QVS |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591015
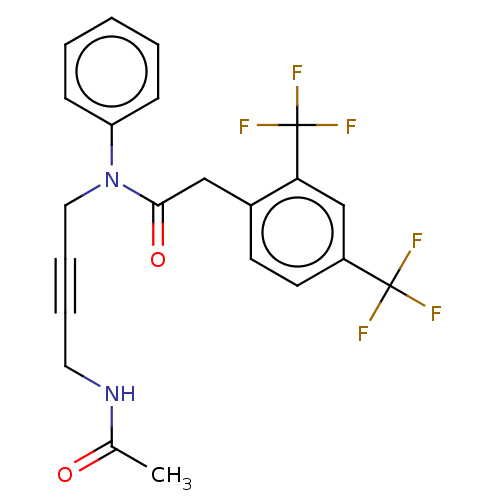
(CHEMBL5170373)Show SMILES CC(=O)NCC#CCN(C(=O)Cc1ccc(cc1C(F)(F)F)C(F)(F)F)c1ccccc1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00998
BindingDB Entry DOI: 10.7270/Q2C53QVS |
More data for this
Ligand-Target Pair | |
Cathepsin E
(Homo sapiens (Human)) | BDBM50302107
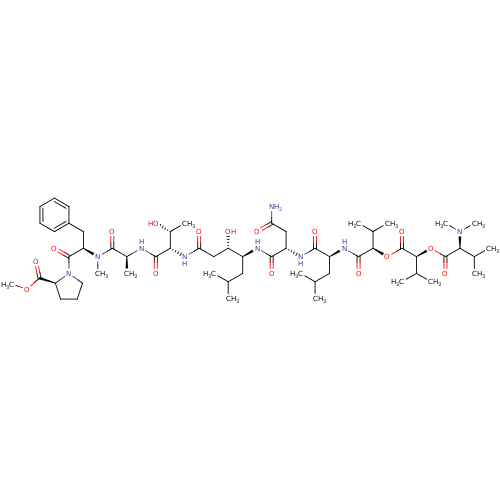
(CHEMBL567893 | Grassystatin A)Show SMILES COC(=O)[C@@H]1CCCN1C(=O)[C@@H](Cc1ccccc1)N(C)C(=O)[C@H](C)NC(=O)[C@@H](NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](OC(=O)[C@@H](OC(=O)[C@H](C(C)C)N(C)C)C(C)C)C(C)C)[C@@H](C)O |r| Show InChI InChI=1S/C58H95N9O16/c1-30(2)25-38(43(69)29-45(71)64-46(36(12)68)52(74)60-35(11)54(76)66(15)42(27-37-21-18-17-19-22-37)55(77)67-24-20-23-41(67)56(78)81-16)61-51(73)40(28-44(59)70)62-50(72)39(26-31(3)4)63-53(75)48(33(7)8)82-58(80)49(34(9)10)83-57(79)47(32(5)6)65(13)14/h17-19,21-22,30-36,38-43,46-49,68-69H,20,23-29H2,1-16H3,(H2,59,70)(H,60,74)(H,61,73)(H,62,72)(H,63,75)(H,64,71)/t35-,36+,38-,39-,40-,41-,42+,43-,46-,47-,48+,49-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.886 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin E |
J Med Chem 52: 5732-47 (2009)
Article DOI: 10.1021/jm9009394
BindingDB Entry DOI: 10.7270/Q2BG2PXP |
More data for this
Ligand-Target Pair | |
Cathepsin E
(Homo sapiens (Human)) | BDBM50302107

(CHEMBL567893 | Grassystatin A)Show SMILES COC(=O)[C@@H]1CCCN1C(=O)[C@@H](Cc1ccccc1)N(C)C(=O)[C@H](C)NC(=O)[C@@H](NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](OC(=O)[C@@H](OC(=O)[C@H](C(C)C)N(C)C)C(C)C)C(C)C)[C@@H](C)O |r| Show InChI InChI=1S/C58H95N9O16/c1-30(2)25-38(43(69)29-45(71)64-46(36(12)68)52(74)60-35(11)54(76)66(15)42(27-37-21-18-17-19-22-37)55(77)67-24-20-23-41(67)56(78)81-16)61-51(73)40(28-44(59)70)62-50(72)39(26-31(3)4)63-53(75)48(33(7)8)82-58(80)49(34(9)10)83-57(79)47(32(5)6)65(13)14/h17-19,21-22,30-36,38-43,46-49,68-69H,20,23-29H2,1-16H3,(H2,59,70)(H,60,74)(H,61,73)(H,62,72)(H,63,75)(H,64,71)/t35-,36+,38-,39-,40-,41-,42+,43-,46-,47-,48+,49-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human C-terminal His10-tagged cathepsin E (Gln18 to Pro396 residues) using Mca-Gly-Lys-Pro-Ile-Leu-PhePhe-Arg-Leu-Lys-(Dnp)... |
J Nat Prod 80: 2969-2986 (2017)
Article DOI: 10.1021/acs.jnatprod.7b00551
BindingDB Entry DOI: 10.7270/Q2JS9SXJ |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591028
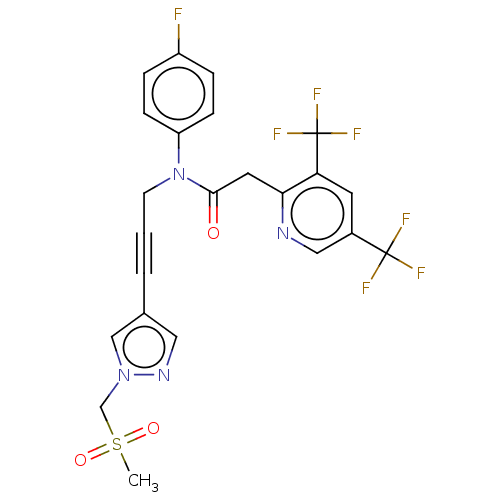
(CHEMBL5194637)Show SMILES CS(=O)(=O)Cn1cc(cn1)C#CCN(C(=O)Cc1ncc(cc1C(F)(F)F)C(F)(F)F)c1ccc(F)cc1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00998
BindingDB Entry DOI: 10.7270/Q2C53QVS |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591027
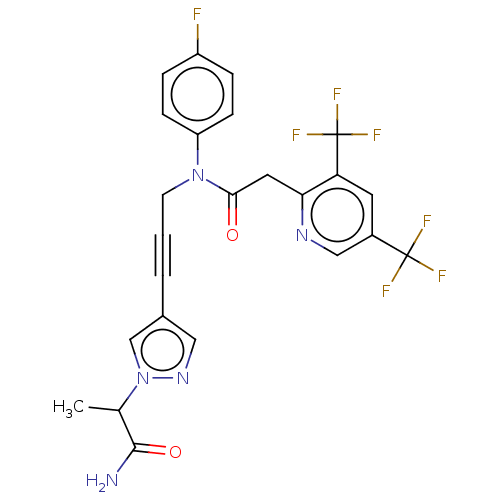
(CHEMBL5197021)Show SMILES CC(C(N)=O)n1cc(cn1)C#CCN(C(=O)Cc1ncc(cc1C(F)(F)F)C(F)(F)F)c1ccc(F)cc1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.990 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00998
BindingDB Entry DOI: 10.7270/Q2C53QVS |
More data for this
Ligand-Target Pair | |
Membrane-associated tyrosine- and threonine-specific cdc2-inhibitory kinase
(Homo sapiens (Human)) | BDBM50598850
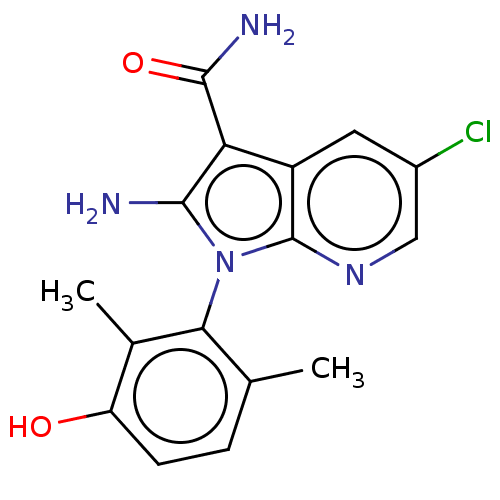
(CHEMBL5196713)Show SMILES Cc1ccc(O)c(C)c1-n1c(N)c(C(N)=O)c2cc(Cl)cnc12 |(.92,2.78,;1.81,1.93,;3.29,2.36,;4.41,1.3,;4.04,-.2,;4.93,-1.05,;2.56,-.63,;2.27,-1.83,;1.45,.43,;-.05,.11,;-.68,-1.3,;-.06,-2.36,;-2.21,-1.13,;-3.24,-2.28,;-4.44,-2.03,;-2.86,-3.45,;-2.53,.37,;-3.87,1.14,;-3.87,2.68,;-4.93,3.3,;-2.53,3.45,;-1.2,2.68,;-1.2,1.14,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00552
BindingDB Entry DOI: 10.7270/Q2028WKQ |
More data for this
Ligand-Target Pair | |
Membrane-associated tyrosine- and threonine-specific cdc2-inhibitory kinase
(Homo sapiens (Human)) | BDBM50598854
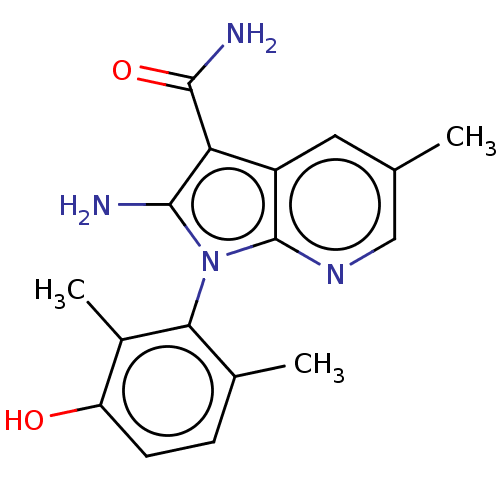
(CHEMBL5174684)Show SMILES Cc1cnc2n(c(N)c(C(N)=O)c2c1)-c1c(C)ccc(O)c1C |(-4.93,3.3,;-3.87,2.68,;-2.53,3.45,;-1.2,2.68,;-1.2,1.14,;-.05,.11,;-.68,-1.3,;-.06,-2.36,;-2.21,-1.13,;-3.24,-2.28,;-4.44,-2.03,;-2.86,-3.45,;-2.53,.37,;-3.87,1.14,;1.45,.43,;1.81,1.93,;.92,2.78,;3.29,2.36,;4.41,1.3,;4.04,-.2,;4.93,-1.05,;2.56,-.63,;2.27,-1.83,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00552
BindingDB Entry DOI: 10.7270/Q2028WKQ |
More data for this
Ligand-Target Pair | |
Membrane-associated tyrosine- and threonine-specific cdc2-inhibitory kinase
(Homo sapiens (Human)) | BDBM50598845

(CHEMBL5201803)Show SMILES Cc1ccc(O)c(C)c1-n1c(N)c(C(N)=O)c2nc(cnc12)C1CC1 |(3.4,-2.47,;3.69,-1.27,;5.17,-.84,;5.53,.66,;4.42,1.72,;4.71,2.92,;2.94,1.29,;2.05,2.14,;2.58,-.21,;1.07,-.53,;.45,-1.94,;1.06,-3.01,;-1.09,-1.78,;-2.11,-2.92,;-3.32,-2.67,;-1.73,-4.1,;-1.4,-.27,;-2.74,.5,;-2.74,2.04,;-1.4,2.81,;-.07,2.04,;-.07,.5,;-4.07,2.81,;-5.53,2.81,;-4.76,4.1,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00552
BindingDB Entry DOI: 10.7270/Q2028WKQ |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591019
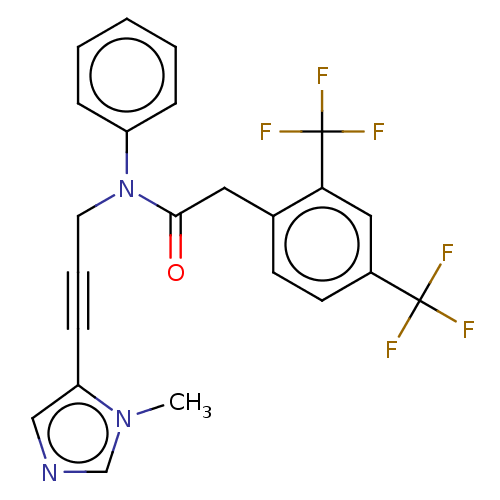
(CHEMBL5187337)Show SMILES Cn1cncc1C#CCN(C(=O)Cc1ccc(cc1C(F)(F)F)C(F)(F)F)c1ccccc1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00998
BindingDB Entry DOI: 10.7270/Q2C53QVS |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591024

(CHEMBL5188048)Show SMILES FC(F)(F)c1ccc(CC(=O)N(CC#Cc2cccnn2)c2ccccc2)c(c1)C(F)(F)F | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00998
BindingDB Entry DOI: 10.7270/Q2C53QVS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
DNA polymerase theta
(Homo sapiens) | BDBM50591033
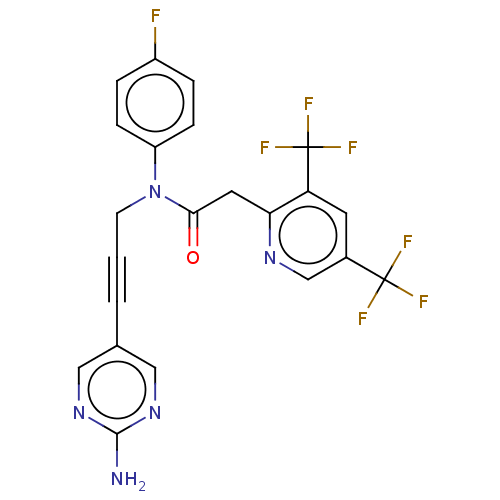
(CHEMBL5181369)Show SMILES Nc1ncc(cn1)C#CCN(C(=O)Cc1ncc(cc1C(F)(F)F)C(F)(F)F)c1ccc(F)cc1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00998
BindingDB Entry DOI: 10.7270/Q2C53QVS |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591029
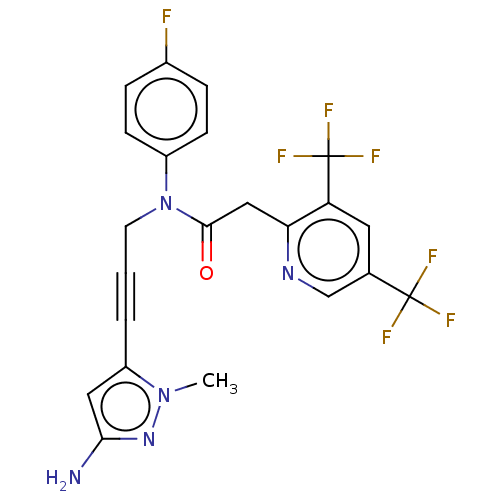
(CHEMBL5174376)Show SMILES Cn1nc(N)cc1C#CCN(C(=O)Cc1ncc(cc1C(F)(F)F)C(F)(F)F)c1ccc(F)cc1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00998
BindingDB Entry DOI: 10.7270/Q2C53QVS |
More data for this
Ligand-Target Pair | |
Membrane-associated tyrosine- and threonine-specific cdc2-inhibitory kinase
(Homo sapiens (Human)) | BDBM50598851

(CHEMBL5199076)Show SMILES Cc1cc2c(C(N)=O)c(N)n(-c3c(C)ccc(O)c3C)c2nc1C |(-4.93,2.68,;-3.87,2.07,;-3.87,.52,;-2.53,-.25,;-2.21,-1.75,;-3.24,-2.9,;-4.44,-2.64,;-2.86,-4.07,;-.68,-1.91,;-.06,-2.98,;-.05,-.5,;1.45,-.18,;1.81,1.31,;.92,2.16,;3.29,1.75,;4.41,.68,;4.04,-.81,;4.93,-1.66,;2.56,-1.25,;2.27,-2.44,;-1.2,.52,;-1.2,2.07,;-2.53,2.84,;-2.53,4.07,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00552
BindingDB Entry DOI: 10.7270/Q2028WKQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50399459

(CHEMBL2179805)Show SMILES CC(C)c1ccc(NC(=O)c2cccc(c2)-c2nn([C@@H]3CCCN(C3)C(=O)C=C)c3ncnc(N)c23)cc1C |r| Show InChI InChI=1S/C30H33N7O2/c1-5-25(38)36-13-7-10-23(16-36)37-29-26(28(31)32-17-33-29)27(35-37)20-8-6-9-21(15-20)30(39)34-22-11-12-24(18(2)3)19(4)14-22/h5-6,8-9,11-12,14-15,17-18,23H,1,7,10,13,16H2,2-4H3,(H,34,39)(H2,31,32,33)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Covalent inhibition of ITK in presence of 5 uM ATP |
J Med Chem 55: 10047-63 (2012)
Article DOI: 10.1021/jm301190s
BindingDB Entry DOI: 10.7270/Q2M32WWD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50399459

(CHEMBL2179805)Show SMILES CC(C)c1ccc(NC(=O)c2cccc(c2)-c2nn([C@@H]3CCCN(C3)C(=O)C=C)c3ncnc(N)c23)cc1C |r| Show InChI InChI=1S/C30H33N7O2/c1-5-25(38)36-13-7-10-23(16-36)37-29-26(28(31)32-17-33-29)27(35-37)20-8-6-9-21(15-20)30(39)34-22-11-12-24(18(2)3)19(4)14-22/h5-6,8-9,11-12,14-15,17-18,23H,1,7,10,13,16H2,2-4H3,(H,34,39)(H2,31,32,33)/t23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BTK in presence of 50 uM ATP |
J Med Chem 55: 10047-63 (2012)
Article DOI: 10.1021/jm301190s
BindingDB Entry DOI: 10.7270/Q2M32WWD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50399459

(CHEMBL2179805)Show SMILES CC(C)c1ccc(NC(=O)c2cccc(c2)-c2nn([C@@H]3CCCN(C3)C(=O)C=C)c3ncnc(N)c23)cc1C |r| Show InChI InChI=1S/C30H33N7O2/c1-5-25(38)36-13-7-10-23(16-36)37-29-26(28(31)32-17-33-29)27(35-37)20-8-6-9-21(15-20)30(39)34-22-11-12-24(18(2)3)19(4)14-22/h5-6,8-9,11-12,14-15,17-18,23H,1,7,10,13,16H2,2-4H3,(H,34,39)(H2,31,32,33)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Covalent inhibition of ITK in presence of 1 mM ATP |
J Med Chem 55: 10047-63 (2012)
Article DOI: 10.1021/jm301190s
BindingDB Entry DOI: 10.7270/Q2M32WWD |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591032
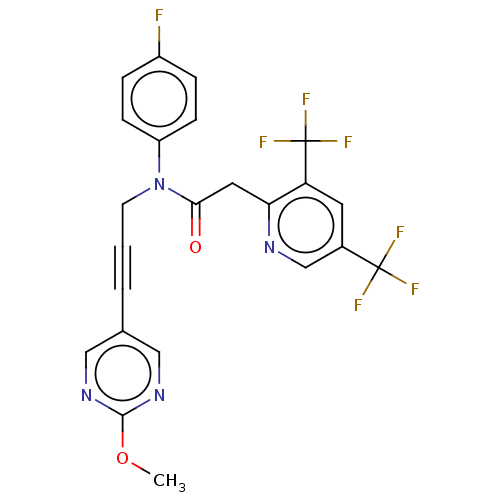
(CHEMBL5200250)Show SMILES COc1ncc(cn1)C#CCN(C(=O)Cc1ncc(cc1C(F)(F)F)C(F)(F)F)c1ccc(F)cc1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00998
BindingDB Entry DOI: 10.7270/Q2C53QVS |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591021
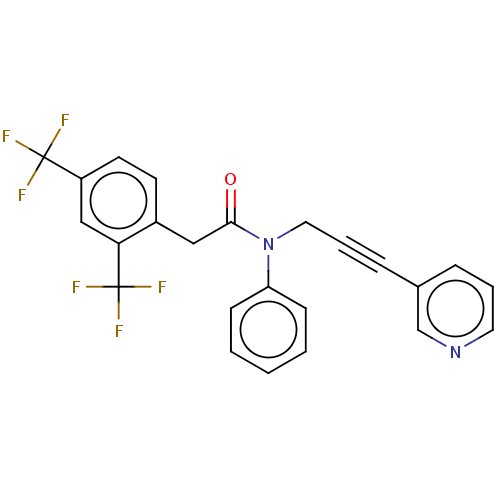
(CHEMBL5174283)Show SMILES FC(F)(F)c1ccc(CC(=O)N(CC#Cc2cccnc2)c2ccccc2)c(c1)C(F)(F)F | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00998
BindingDB Entry DOI: 10.7270/Q2C53QVS |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591020
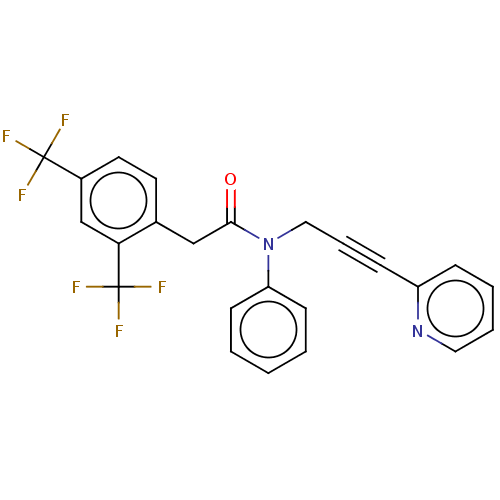
(CHEMBL5195161)Show SMILES FC(F)(F)c1ccc(CC(=O)N(CC#Cc2ccccn2)c2ccccc2)c(c1)C(F)(F)F | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00998
BindingDB Entry DOI: 10.7270/Q2C53QVS |
More data for this
Ligand-Target Pair | |
Membrane-associated tyrosine- and threonine-specific cdc2-inhibitory kinase
(Homo sapiens (Human)) | BDBM50598855
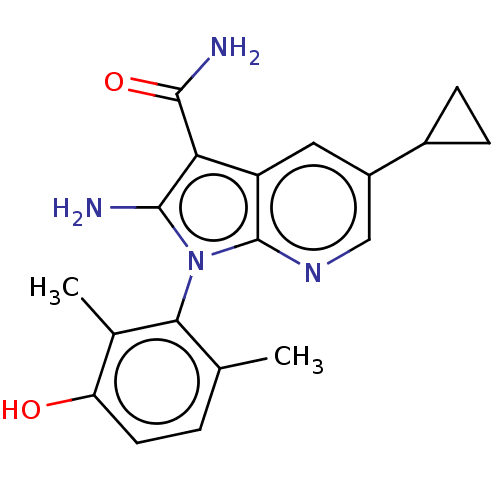
(CHEMBL5190904)Show SMILES Cc1ccc(O)c(C)c1-n1c(N)c(C(N)=O)c2cc(cnc12)C1CC1 |(1.78,2.14,;2.68,1.29,;4.15,1.72,;5.27,.66,;4.9,-.84,;5.8,-1.69,;3.43,-1.27,;3.13,-2.47,;2.32,-.21,;.81,-.53,;.18,-1.94,;.8,-3.01,;-1.35,-1.78,;-2.38,-2.92,;-3.58,-2.67,;-1.99,-4.09,;-1.67,-.27,;-3,.5,;-3,2.04,;-1.67,2.81,;-.33,2.04,;-.33,.5,;-4.34,2.81,;-5.8,2.81,;-5.03,4.09,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00552
BindingDB Entry DOI: 10.7270/Q2028WKQ |
More data for this
Ligand-Target Pair | |
Membrane-associated tyrosine- and threonine-specific cdc2-inhibitory kinase
(Homo sapiens (Human)) | BDBM50598832
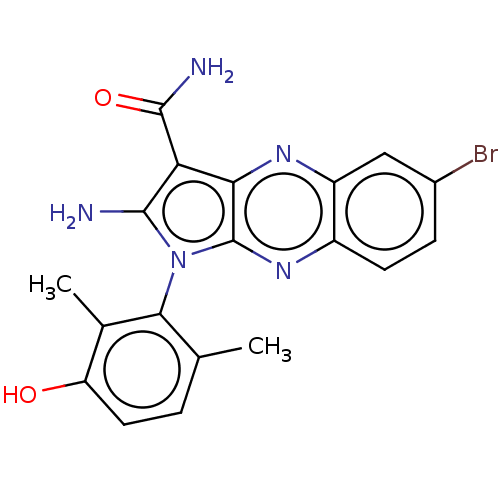
(CHEMBL5185146)Show SMILES Cc1ccc(O)c(C)c1-n1c(N)c(C(N)=O)c2nc3cc(Br)ccc3nc12 |(3.2,-2.98,;3.49,-1.78,;4.97,-1.35,;5.34,.14,;4.22,1.21,;4.51,2.41,;2.74,.78,;1.85,1.63,;2.38,-.72,;.88,-1.04,;.25,-2.45,;.87,-3.52,;-1.28,-2.29,;-2.31,-3.43,;-3.51,-3.18,;-1.93,-4.61,;-1.6,-.78,;-2.93,-.01,;-2.93,1.53,;-4.27,2.3,;-4.27,3.84,;-5.34,4.45,;-2.93,4.61,;-1.6,3.84,;-1.6,2.3,;-.27,1.53,;-.27,-.01,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00552
BindingDB Entry DOI: 10.7270/Q2028WKQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Membrane-associated tyrosine- and threonine-specific cdc2-inhibitory kinase
(Homo sapiens (Human)) | BDBM50598836

(CHEMBL5190822)Show SMILES Cc1ccc(O)c(C)c1-n1c(N)c(C(N)=O)c2nc3cccc(C4=CCCC4)c3nc12 |t:24,(2.67,-3.9,;2.96,-2.7,;4.44,-2.27,;4.8,-.77,;3.69,.29,;3.98,1.49,;2.21,-.14,;1.32,.71,;1.85,-1.64,;.34,-1.96,;-.28,-3.37,;.33,-4.44,;-1.81,-3.21,;-2.84,-4.35,;-4.05,-4.1,;-2.46,-5.53,;-2.13,-1.7,;-3.47,-.93,;-3.47,.61,;-4.8,1.38,;-4.8,2.92,;-3.47,3.69,;-2.13,2.92,;-.8,3.69,;-.66,5.21,;.85,5.53,;1.62,4.19,;.59,3.05,;-2.13,1.38,;-.8,.61,;-.8,-.93,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00552
BindingDB Entry DOI: 10.7270/Q2028WKQ |
More data for this
Ligand-Target Pair | |
Membrane-associated tyrosine- and threonine-specific cdc2-inhibitory kinase
(Homo sapiens (Human)) | BDBM50598835
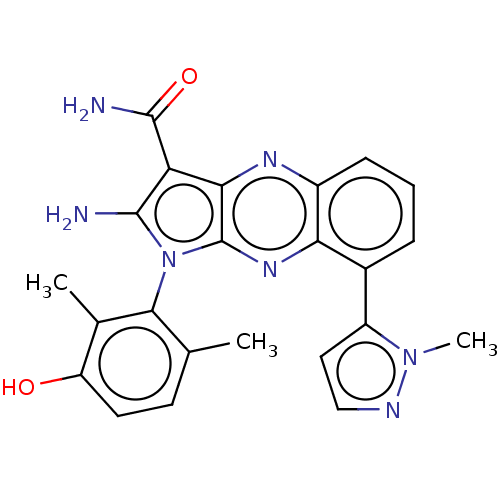
(CHEMBL5169345)Show SMILES Cc1ccc(O)c(C)c1-n1c(N)c(C(N)=O)c2nc3cccc(-c4ccnn4C)c3nc12 |(2.67,-4.15,;2.96,-2.95,;4.44,-2.52,;4.8,-1.03,;3.69,.04,;3.98,1.24,;2.21,-.39,;1.32,.45,;1.85,-1.89,;.34,-2.21,;-.28,-3.62,;.33,-4.69,;-1.81,-3.46,;-2.84,-4.61,;-4.05,-4.35,;-2.46,-5.78,;-2.13,-1.95,;-3.47,-1.18,;-3.47,.36,;-4.8,1.13,;-4.8,2.67,;-3.47,3.44,;-2.13,2.67,;-.8,3.44,;.59,2.8,;1.62,3.94,;.85,5.27,;-.66,4.96,;-1.58,5.78,;-2.13,1.13,;-.8,.36,;-.8,-1.18,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00552
BindingDB Entry DOI: 10.7270/Q2028WKQ |
More data for this
Ligand-Target Pair | |
Membrane-associated tyrosine- and threonine-specific cdc2-inhibitory kinase
(Homo sapiens (Human)) | BDBM50598833
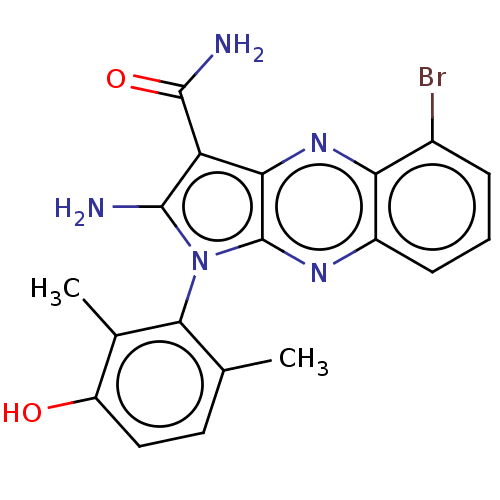
(CHEMBL5181222)Show SMILES Cc1ccc(O)c(C)c1-n1c(N)c(C(N)=O)c2nc3c(Br)cccc3nc12 |(3.2,-2.98,;3.49,-1.78,;4.97,-1.35,;5.34,.14,;4.22,1.21,;4.51,2.41,;2.74,.78,;1.85,1.63,;2.38,-.72,;.88,-1.04,;.25,-2.45,;.87,-3.52,;-1.28,-2.29,;-2.31,-3.43,;-3.51,-3.18,;-1.93,-4.61,;-1.6,-.78,;-2.93,-.01,;-2.93,1.53,;-4.27,2.3,;-5.34,1.68,;-4.27,3.84,;-2.93,4.61,;-1.6,3.84,;-1.6,2.3,;-.27,1.53,;-.27,-.01,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00552
BindingDB Entry DOI: 10.7270/Q2028WKQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Membrane-associated tyrosine- and threonine-specific cdc2-inhibitory kinase
(Homo sapiens (Human)) | BDBM50598830
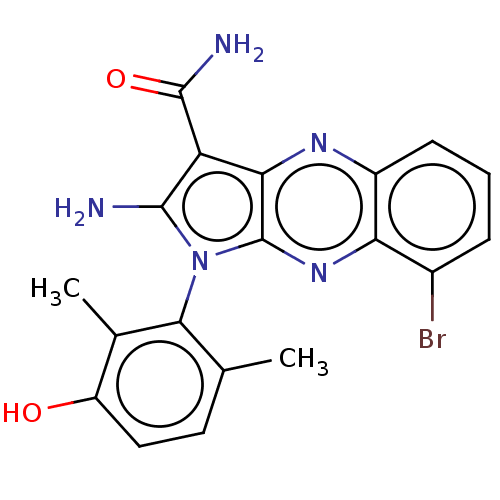
(CHEMBL5186220)Show SMILES Cc1ccc(O)c(C)c1-n1c(N)c(C(N)=O)c2nc3cccc(Br)c3nc12 |(2.67,-2.98,;2.96,-1.78,;4.44,-1.35,;4.8,.14,;3.69,1.21,;3.98,2.41,;2.21,.78,;1.32,1.63,;1.85,-.72,;.34,-1.04,;-.28,-2.45,;.33,-3.52,;-1.81,-2.29,;-2.84,-3.43,;-4.05,-3.18,;-2.46,-4.61,;-2.13,-.78,;-3.47,-.01,;-3.47,1.53,;-4.8,2.3,;-4.8,3.84,;-3.47,4.61,;-2.13,3.84,;-1.07,4.45,;-2.13,2.3,;-.8,1.53,;-.8,-.01,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00552
BindingDB Entry DOI: 10.7270/Q2028WKQ |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591023
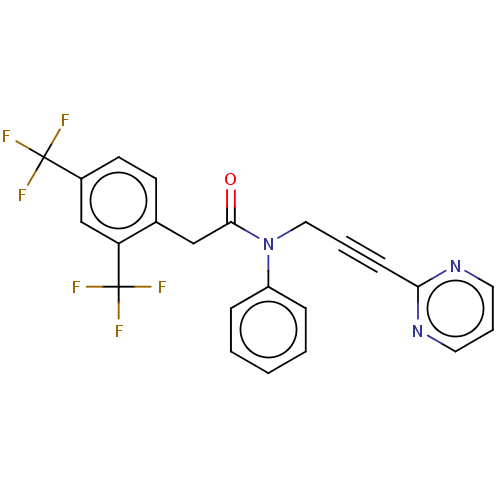
(CHEMBL5194194)Show SMILES FC(F)(F)c1ccc(CC(=O)N(CC#Cc2ncccn2)c2ccccc2)c(c1)C(F)(F)F | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00998
BindingDB Entry DOI: 10.7270/Q2C53QVS |
More data for this
Ligand-Target Pair | |
Membrane-associated tyrosine- and threonine-specific cdc2-inhibitory kinase
(Homo sapiens (Human)) | BDBM50598834
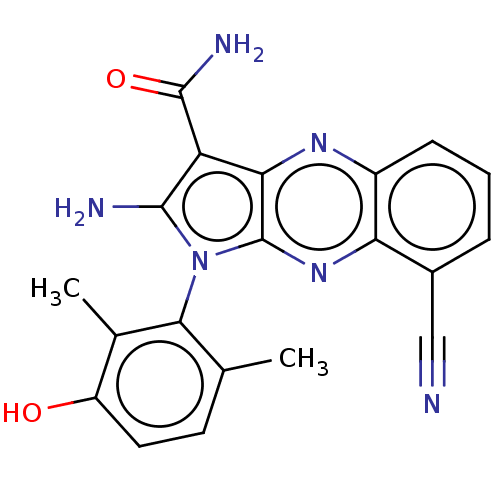
(CHEMBL5198360)Show SMILES Cc1ccc(O)c(C)c1-n1c(N)c(C(N)=O)c2nc3cccc(C#N)c3nc12 |(2.67,-3.29,;2.96,-2.09,;4.44,-1.66,;4.8,-.16,;3.69,.9,;3.98,2.1,;2.21,.47,;1.32,1.32,;1.85,-1.03,;.34,-1.35,;-.28,-2.76,;.33,-3.83,;-1.81,-2.6,;-2.84,-3.74,;-4.05,-3.49,;-2.46,-4.91,;-2.13,-1.09,;-3.47,-.32,;-3.47,1.22,;-4.8,1.99,;-4.8,3.53,;-3.47,4.3,;-2.13,3.53,;-.8,4.3,;.27,4.91,;-2.13,1.99,;-.8,1.22,;-.8,-.32,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00552
BindingDB Entry DOI: 10.7270/Q2028WKQ |
More data for this
Ligand-Target Pair | |
Membrane-associated tyrosine- and threonine-specific cdc2-inhibitory kinase
(Homo sapiens (Human)) | BDBM50598838
(CHEMBL5172086)Show SMILES Cc1ccc(O)c(C)c1-n1c(N)c(C(N)=O)c2nc3cc(ccc3nc12)-c1ccnn1C |(4.54,-3.9,;4.84,-2.71,;6.31,-2.27,;6.68,-.78,;5.56,.29,;5.86,1.48,;4.09,-.15,;3.19,.7,;3.73,-1.65,;2.22,-1.97,;1.59,-3.37,;2.21,-4.44,;.06,-3.21,;-.97,-4.36,;-2.17,-4.1,;-.58,-5.53,;-.26,-1.71,;-1.59,-.94,;-1.59,.6,;-2.93,1.37,;-2.93,2.91,;-1.59,3.68,;-.26,2.91,;-.26,1.37,;1.07,.6,;1.07,-.94,;-4.26,3.68,;-4.4,5.21,;-5.91,5.53,;-6.68,4.2,;-5.65,3.05,;-5.9,1.84,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00552
BindingDB Entry DOI: 10.7270/Q2028WKQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data