 Found 34 hits with Last Name = 'pottiez' and Initial = 'v'
Found 34 hits with Last Name = 'pottiez' and Initial = 'v' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Insulin-degrading enzyme
(Homo sapiens (Human)) | BDBM50427703

(CHEMBL2324220)Show SMILES NC(N)=NCCC[C@H](NC(=O)[C@@H](CC(=O)NO)Cc1ccc2ccccc2c1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCC(O)=O)C(N)=O |r,wU:7.7,45.48,11.11,wD:31.32,(15.26,-7.72,;16.62,-8.43,;17.92,-7.6,;16.69,-9.97,;15.39,-10.79,;15.46,-12.33,;14.16,-13.16,;14.24,-14.7,;12.94,-15.53,;11.61,-14.76,;11.61,-13.22,;10.27,-15.53,;10.27,-17.07,;8.94,-17.84,;7.6,-17.07,;8.94,-19.38,;7.6,-20.16,;8.97,-14.76,;8.94,-13.22,;7.58,-12.47,;7.55,-10.93,;8.87,-10.13,;8.84,-8.59,;10.15,-7.8,;11.51,-8.53,;11.55,-10.08,;10.22,-10.88,;10.26,-12.42,;15.6,-15.4,;15.66,-16.95,;16.9,-14.58,;18.26,-15.29,;18.34,-16.83,;19.7,-17.53,;21.15,-17.03,;22.08,-18.26,;21.2,-19.52,;21.54,-21.02,;20.42,-22.07,;18.95,-21.62,;18.6,-20.12,;19.73,-19.07,;19.56,-14.46,;19.49,-12.92,;20.92,-15.17,;22.22,-14.34,;22.16,-12.8,;23.45,-11.98,;23.38,-10.44,;22.02,-9.73,;24.68,-9.61,;23.58,-15.05,;24.88,-14.22,;23.66,-16.59,)| Show InChI InChI=1S/C37H45N9O8/c38-33(50)28(13-14-32(48)49)43-36(53)30(18-25-20-42-27-9-4-3-8-26(25)27)45-35(52)29(10-5-15-41-37(39)40)44-34(51)24(19-31(47)46-54)17-21-11-12-22-6-1-2-7-23(22)16-21/h1-4,6-9,11-12,16,20,24,28-30,42,54H,5,10,13-15,17-19H2,(H2,38,50)(H,43,53)(H,44,51)(H,45,52)(H,46,47)(H,48,49)(H4,39,40,41)/t24-,28+,29+,30+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Lille
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDE expressed in CHO cells in presence of [125I]-insulin by HTRF assay |
Eur J Med Chem 179: 557-566 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.057
BindingDB Entry DOI: 10.7270/Q2XS5ZT5 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM21641

(2-(2-benzyl-3-sulfanylpropanamido)acetic acid | CH...)Show InChI InChI=1S/C12H15NO3S/c14-11(15)7-13-12(16)10(8-17)6-9-4-2-1-3-5-9/h1-5,10,17H,6-8H2,(H,13,16)(H,14,15) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of human NEP-mediated amyloid beta hydrolysis |
Eur J Med Chem 79: 184-93 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.009
BindingDB Entry DOI: 10.7270/Q27H1M34 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Insulin-degrading enzyme
(Homo sapiens (Human)) | BDBM50525490
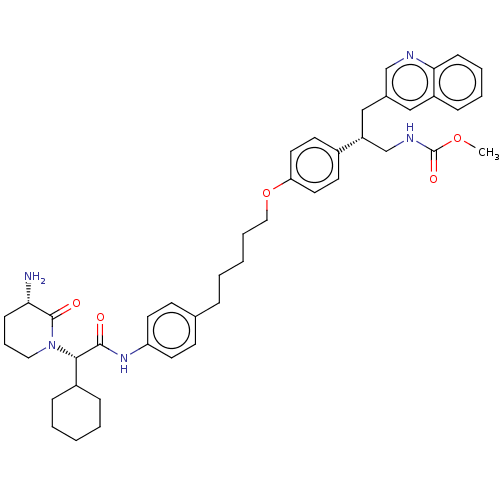
(CHEMBL4437643)Show SMILES COC(=O)NC[C@@H](Cc1cnc2ccccc2c1)c1ccc(OCCCCCc2ccc(NC(=O)[C@H](C3CCCCC3)N3CCC[C@H](N)C3=O)cc2)cc1 |r| Show InChI InChI=1S/C44H55N5O5/c1-53-44(52)47-30-36(28-32-27-35-14-7-8-16-40(35)46-29-32)33-19-23-38(24-20-33)54-26-9-3-4-11-31-17-21-37(22-18-31)48-42(50)41(34-12-5-2-6-13-34)49-25-10-15-39(45)43(49)51/h7-8,14,16-24,27,29,34,36,39,41H,2-6,9-13,15,25-26,28,30,45H2,1H3,(H,47,52)(H,48,50)/t36-,39+,41+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Lille
Curated by ChEMBL
| Assay Description
Inhibition of recombinant IDE exosite (unknown origin) expressed in Escherichia coli using insulin as substrate incubated for 4 hrs by AlphaLisa assa... |
Eur J Med Chem 179: 557-566 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.057
BindingDB Entry DOI: 10.7270/Q2XS5ZT5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM21642

((2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolid...)Show InChI InChI=1S/C9H15NO3S/c1-6(5-14)8(11)10-4-2-3-7(10)9(12)13/h6-7,14H,2-5H2,1H3,(H,12,13)/t6-,7+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of human ACE-mediated amyloid beta hydrolysis |
Eur J Med Chem 79: 184-93 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.009
BindingDB Entry DOI: 10.7270/Q27H1M34 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Insulin-degrading enzyme
(Homo sapiens (Human)) | BDBM34233

(2-Phenyl-benzo[d]isoselenazol-3-one | 2-Phenyl-ben...)Show InChI InChI=1S/C13H9NOSe/c15-13-11-8-4-5-9-12(11)16-14(13)10-6-2-1-3-7-10/h1-9H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Lille
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDE expressed in Escherichia coli BL21 (DE3) cells using insulin as substrate preincubated for 10 mins followed by su... |
Eur J Med Chem 179: 557-566 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.057
BindingDB Entry DOI: 10.7270/Q2XS5ZT5 |
More data for this
Ligand-Target Pair | |
Insulin-degrading enzyme
(Homo sapiens (Human)) | BDBM34233

(2-Phenyl-benzo[d]isoselenazol-3-one | 2-Phenyl-ben...)Show InChI InChI=1S/C13H9NOSe/c15-13-11-8-4-5-9-12(11)16-14(13)10-6-2-1-3-7-10/h1-9H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Lille
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDE expressed in Escherichia coli BL21 (DE3) cells using ATTO 655- Cys-Lys-Leu-Val-Phe-Phe-Ala-Glu-Asp-Trp as substra... |
Eur J Med Chem 179: 557-566 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.057
BindingDB Entry DOI: 10.7270/Q2XS5ZT5 |
More data for this
Ligand-Target Pair | |
Insulin-degrading enzyme
(Mus musculus (Mouse)) | BDBM50525489

(CHEMBL4556893)Show SMILES NCCCC[C@@H]1NC(=O)\C=C\C(=O)N[C@@H](CCCCNC(=O)[C@@H](Cc2ccc(cc2)C(=O)c2ccccc2)NC(=O)[C@H](CC2CCCCC2)NC1=O)C(N)=O |r,t:9| Show InChI InChI=1S/C41H55N7O7/c42-23-9-7-16-32-40(54)48-34(25-27-11-3-1-4-12-27)41(55)47-33(26-28-17-19-30(20-18-28)37(51)29-13-5-2-6-14-29)39(53)44-24-10-8-15-31(38(43)52)45-35(49)21-22-36(50)46-32/h2,5-6,13-14,17-22,27,31-34H,1,3-4,7-12,15-16,23-26,42H2,(H2,43,52)(H,44,53)(H,45,49)(H,46,50)(H,47,55)(H,48,54)/b22-21+/t31-,32-,33+,34-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Lille
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged mouse recombinant IDE Escherichia coli BL21 (DE3) cells using fluorogenic peptide Mca-RPPGFSAFK(Dnp)-OH as subst... |
Eur J Med Chem 179: 557-566 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.057
BindingDB Entry DOI: 10.7270/Q2XS5ZT5 |
More data for this
Ligand-Target Pair | |
Insulin-degrading enzyme
(Homo sapiens (Human)) | BDBM50462574
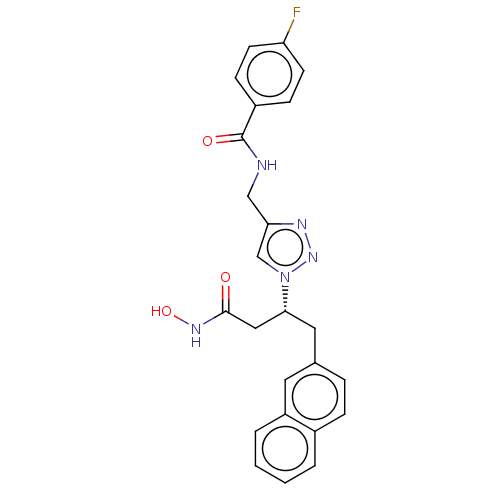
(CHEMBL4241044)Show SMILES ONC(=O)C[C@@H](Cc1ccc2ccccc2c1)n1cc(CNC(=O)c2ccc(F)cc2)nn1 |r| Show InChI InChI=1S/C24H22FN5O3/c25-20-9-7-18(8-10-20)24(32)26-14-21-15-30(29-27-21)22(13-23(31)28-33)12-16-5-6-17-3-1-2-4-19(17)11-16/h1-11,15,22,33H,12-14H2,(H,26,32)(H,28,31)/t22-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Lille
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDE expressed in Escherichia coli BL21 (DE3) cells using insulin as substrate preincubated for 10 mins followed by su... |
Eur J Med Chem 179: 557-566 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.057
BindingDB Entry DOI: 10.7270/Q2XS5ZT5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Insulin-degrading enzyme
(Homo sapiens (Human)) | BDBM50462574

(CHEMBL4241044)Show SMILES ONC(=O)C[C@@H](Cc1ccc2ccccc2c1)n1cc(CNC(=O)c2ccc(F)cc2)nn1 |r| Show InChI InChI=1S/C24H22FN5O3/c25-20-9-7-18(8-10-20)24(32)26-14-21-15-30(29-27-21)22(13-23(31)28-33)12-16-5-6-17-3-1-2-4-19(17)11-16/h1-11,15,22,33H,12-14H2,(H,26,32)(H,28,31)/t22-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Lille
Curated by ChEMBL
| Assay Description
Inhibition of wild type human IDE catalytic site using insulin as substrate preincubated for 10 mins followed by substrate addition and measured afte... |
Eur J Med Chem 179: 557-566 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.057
BindingDB Entry DOI: 10.7270/Q2XS5ZT5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Insulin-degrading enzyme
(Homo sapiens (Human)) | BDBM50005641

(CHEMBL3235416)Show SMILES CNC(=O)[C@H](Cc1c[nH]cn1)NC(=O)CN(CCCc1ccccc1)CC(O)=O |r| Show InChI InChI=1S/C20H27N5O4/c1-21-20(29)17(10-16-11-22-14-23-16)24-18(26)12-25(13-19(27)28)9-5-8-15-6-3-2-4-7-15/h2-4,6-7,11,14,17H,5,8-10,12-13H2,1H3,(H,21,29)(H,22,23)(H,24,26)(H,27,28)/t17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDE-mediated amyloid beta (16 to 23) hydrolysis using ATTO 655-Cys-Lys-Leu-Val-Phe-Phe-Ala-Glu-Asp-Trp as substrate p... |
Eur J Med Chem 79: 184-93 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.009
BindingDB Entry DOI: 10.7270/Q27H1M34 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Insulin-degrading enzyme
(Homo sapiens (Human)) | BDBM50005641

(CHEMBL3235416)Show SMILES CNC(=O)[C@H](Cc1c[nH]cn1)NC(=O)CN(CCCc1ccccc1)CC(O)=O |r| Show InChI InChI=1S/C20H27N5O4/c1-21-20(29)17(10-16-11-22-14-23-16)24-18(26)12-25(13-19(27)28)9-5-8-15-6-3-2-4-7-15/h2-4,6-7,11,14,17H,5,8-10,12-13H2,1H3,(H,21,29)(H,22,23)(H,24,26)(H,27,28)/t17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Lille
Curated by ChEMBL
| Assay Description
Inhibition of IDE exosite and catalytic site (unknown origin) |
Eur J Med Chem 179: 557-566 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.057
BindingDB Entry DOI: 10.7270/Q2XS5ZT5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Insulin-degrading enzyme
(Homo sapiens (Human)) | BDBM50462574

(CHEMBL4241044)Show SMILES ONC(=O)C[C@@H](Cc1ccc2ccccc2c1)n1cc(CNC(=O)c2ccc(F)cc2)nn1 |r| Show InChI InChI=1S/C24H22FN5O3/c25-20-9-7-18(8-10-20)24(32)26-14-21-15-30(29-27-21)22(13-23(31)28-33)12-16-5-6-17-3-1-2-4-19(17)11-16/h1-11,15,22,33H,12-14H2,(H,26,32)(H,28,31)/t22-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Lille
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDE harboring C110L/C171S/C178A/C257V/C414L/C573N/C590S/C789S/C812A/C819A/C904S/C966N/C974A mutant expressed in Esche... |
Eur J Med Chem 179: 557-566 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.057
BindingDB Entry DOI: 10.7270/Q2XS5ZT5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Insulin-degrading enzyme
(Homo sapiens (Human)) | BDBM50005640
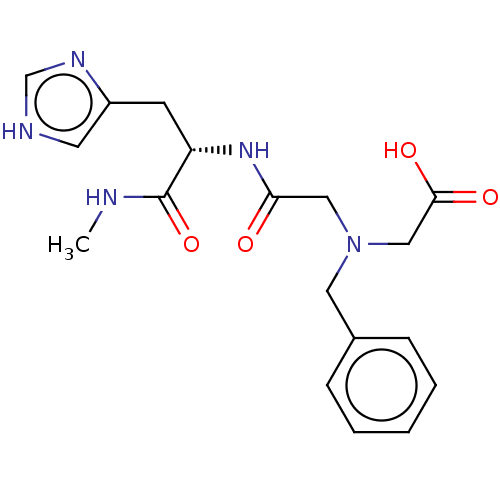
(CHEMBL3235415)Show SMILES CNC(=O)[C@H](Cc1c[nH]cn1)NC(=O)CN(CC(O)=O)Cc1ccccc1 |r| Show InChI InChI=1S/C18H23N5O4/c1-19-18(27)15(7-14-8-20-12-21-14)22-16(24)10-23(11-17(25)26)9-13-5-3-2-4-6-13/h2-6,8,12,15H,7,9-11H2,1H3,(H,19,27)(H,20,21)(H,22,24)(H,25,26)/t15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDE-mediated amyloid beta (16 to 23) hydrolysis using ATTO 655-Cys-Lys-Leu-Val-Phe-Phe-Ala-Glu-Asp-Trp as substrate p... |
Eur J Med Chem 79: 184-93 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.009
BindingDB Entry DOI: 10.7270/Q27H1M34 |
More data for this
Ligand-Target Pair | |
Insulin-degrading enzyme
(Homo sapiens (Human)) | BDBM50005638
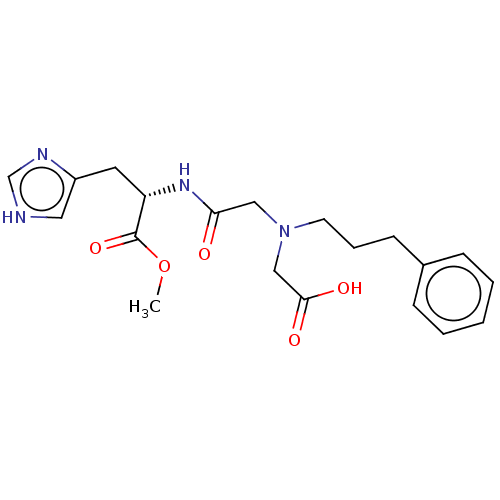
(CHEMBL3235414)Show SMILES COC(=O)[C@H](Cc1c[nH]cn1)NC(=O)CN(CCCc1ccccc1)CC(O)=O |r| Show InChI InChI=1S/C20H26N4O5/c1-29-20(28)17(10-16-11-21-14-22-16)23-18(25)12-24(13-19(26)27)9-5-8-15-6-3-2-4-7-15/h2-4,6-7,11,14,17H,5,8-10,12-13H2,1H3,(H,21,22)(H,23,25)(H,26,27)/t17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDE-mediated amyloid beta (16 to 23) hydrolysis using ATTO 655-Cys-Lys-Leu-Val-Phe-Phe-Ala-Glu-Asp-Trp as substrate p... |
Eur J Med Chem 79: 184-93 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.009
BindingDB Entry DOI: 10.7270/Q27H1M34 |
More data for this
Ligand-Target Pair | |
Insulin-degrading enzyme
(Homo sapiens (Human)) | BDBM50005637
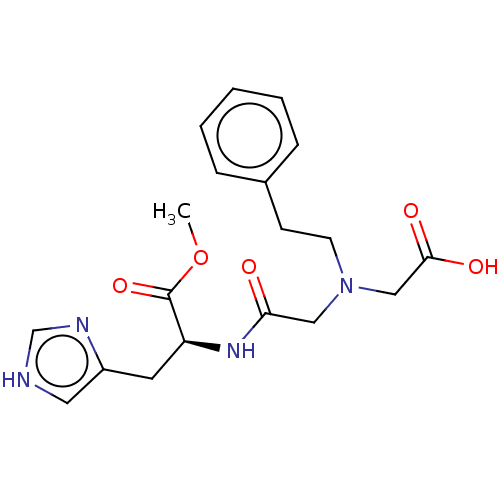
(CHEMBL3235413)Show SMILES COC(=O)[C@H](Cc1c[nH]cn1)NC(=O)CN(CCc1ccccc1)CC(O)=O |r| Show InChI InChI=1S/C19H24N4O5/c1-28-19(27)16(9-15-10-20-13-21-15)22-17(24)11-23(12-18(25)26)8-7-14-5-3-2-4-6-14/h2-6,10,13,16H,7-9,11-12H2,1H3,(H,20,21)(H,22,24)(H,25,26)/t16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDE-mediated amyloid beta (16 to 23) hydrolysis using ATTO 655-Cys-Lys-Leu-Val-Phe-Phe-Ala-Glu-Asp-Trp as substrate p... |
Eur J Med Chem 79: 184-93 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.009
BindingDB Entry DOI: 10.7270/Q27H1M34 |
More data for this
Ligand-Target Pair | |
Insulin-degrading enzyme
(Homo sapiens (Human)) | BDBM50005636
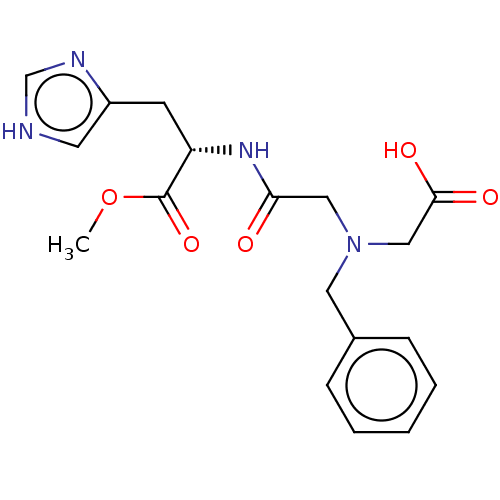
(CHEMBL3235412)Show SMILES COC(=O)[C@H](Cc1c[nH]cn1)NC(=O)CN(CC(O)=O)Cc1ccccc1 |r| Show InChI InChI=1S/C18H22N4O5/c1-27-18(26)15(7-14-8-19-12-20-14)21-16(23)10-22(11-17(24)25)9-13-5-3-2-4-6-13/h2-6,8,12,15H,7,9-11H2,1H3,(H,19,20)(H,21,23)(H,24,25)/t15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDE-mediated amyloid beta (16 to 23) hydrolysis using ATTO 655-Cys-Lys-Leu-Val-Phe-Phe-Ala-Glu-Asp-Trp as substrate p... |
Eur J Med Chem 79: 184-93 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.009
BindingDB Entry DOI: 10.7270/Q27H1M34 |
More data for this
Ligand-Target Pair | |
Insulin-degrading enzyme
(Homo sapiens (Human)) | BDBM50005641

(CHEMBL3235416)Show SMILES CNC(=O)[C@H](Cc1c[nH]cn1)NC(=O)CN(CCCc1ccccc1)CC(O)=O |r| Show InChI InChI=1S/C20H27N5O4/c1-21-20(29)17(10-16-11-22-14-23-16)24-18(26)12-25(13-19(27)28)9-5-8-15-6-3-2-4-7-15/h2-4,6-7,11,14,17H,5,8-10,12-13H2,1H3,(H,21,29)(H,22,23)(H,24,26)(H,27,28)/t17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDE-mediated amyloid beta (1 to 40) hydrolysis preincubated for 10 mins measured after 30 mins by spectrophotometer a... |
Eur J Med Chem 79: 184-93 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.009
BindingDB Entry DOI: 10.7270/Q27H1M34 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Insulin-degrading enzyme
(Homo sapiens (Human)) | BDBM50350471
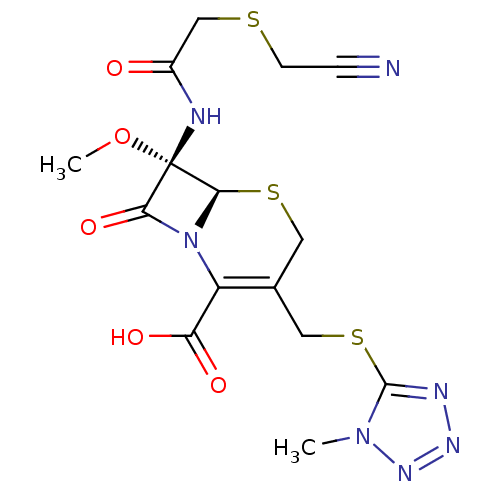
(CEFMETAZOLE)Show SMILES CO[C@]1(NC(=O)CSCC#N)[C@H]2SCC(CSc3nnnn3C)=C(N2C1=O)C(O)=O |r,c:23| Show InChI InChI=1S/C15H17N7O5S3/c1-21-14(18-19-20-21)30-6-8-5-29-13-15(27-2,17-9(23)7-28-4-3-16)12(26)22(13)10(8)11(24)25/h13H,4-7H2,1-2H3,(H,17,23)(H,24,25)/t13-,15+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Lille
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDE expressed in Escherichia coli BL21 (DE3) cells using ATTO 655- Cys-Lys-Leu-Val-Phe-Phe-Ala-Glu-Asp-Trp as substra... |
Eur J Med Chem 179: 557-566 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.057
BindingDB Entry DOI: 10.7270/Q2XS5ZT5 |
More data for this
Ligand-Target Pair | |
Insulin-degrading enzyme
(Homo sapiens (Human)) | BDBM50070209
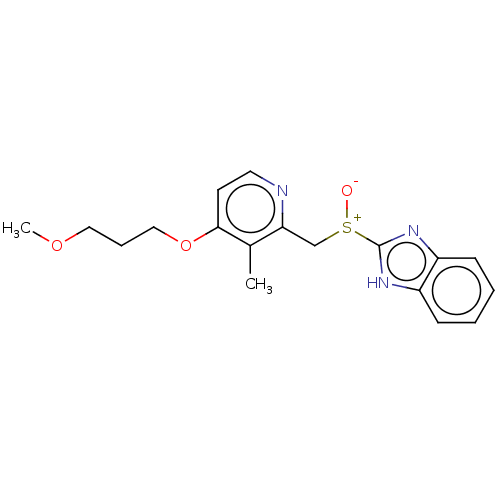
(Aciphex | CHEBI:8768 | LY-307640 | Rabeprazole)Show SMILES COCCCOc1ccnc(C[S+]([O-])c2nc3ccccc3[nH]2)c1C | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Lille
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDE expressed in Escherichia coli BL21 (DE3) cells using ATTO 655- Cys-Lys-Leu-Val-Phe-Phe-Ala-Glu-Asp-Trp as substra... |
Eur J Med Chem 179: 557-566 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.057
BindingDB Entry DOI: 10.7270/Q2XS5ZT5 |
More data for this
Ligand-Target Pair | |
N(G),N(G)-dimethylarginine dimethylaminohydrolase 1
(Homo sapiens (Human)) | BDBM50070209

(Aciphex | CHEBI:8768 | LY-307640 | Rabeprazole)Show SMILES COCCCOc1ccnc(C[S+]([O-])c2nc3ccccc3[nH]2)c1C | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Lille
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DDAH1 reduction in L-citrulline formation using ADMA as substrate |
Eur J Med Chem 179: 557-566 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.057
BindingDB Entry DOI: 10.7270/Q2XS5ZT5 |
More data for this
Ligand-Target Pair | |
Insulin-degrading enzyme
(Homo sapiens (Human)) | BDBM15236

(3,5,7-trihydroxy-2-(3,4,5-trihydroxyphenyl)-4H-chr...)Show SMILES Oc1cc(O)c2c(c1)oc(-c1cc(O)c(O)c(O)c1)c(O)c2=O Show InChI InChI=1S/C15H10O8/c16-6-3-7(17)11-10(4-6)23-15(14(22)13(11)21)5-1-8(18)12(20)9(19)2-5/h1-4,16-20,22H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Lille
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDE expressed in Escherichia coli BL21 (DE3) cells using ATTO 655- Cys-Lys-Leu-Val-Phe-Phe-Ala-Glu-Asp-Trp as substra... |
Eur J Med Chem 179: 557-566 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.057
BindingDB Entry DOI: 10.7270/Q2XS5ZT5 |
More data for this
Ligand-Target Pair | |
Insulin-degrading enzyme
(Homo sapiens (Human)) | BDBM50477463
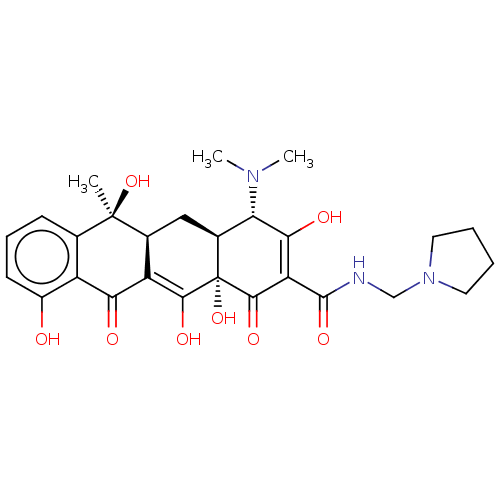
(CHEBI:63334 | Rolitetracycline | SO-15659 | Syntet...)Show SMILES [H][C@@]12C[C@@]3([H])C(C(=O)c4c(O)cccc4[C@@]3(C)O)=C(O)[C@]1(O)C(=O)C(C(=O)NCN1CCCC1)=C(O)[C@H]2N(C)C |t:19,37| Show InChI InChI=1S/C27H33N3O8/c1-26(37)13-7-6-8-16(31)17(13)21(32)18-14(26)11-15-20(29(2)3)22(33)19(24(35)27(15,38)23(18)34)25(36)28-12-30-9-4-5-10-30/h6-8,14-15,20,31,33-34,37-38H,4-5,9-12H2,1-3H3,(H,28,36)/t14-,15-,20-,26+,27-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Lille
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDE expressed in Escherichia coli BL21 (DE3) cells using ATTO 655- Cys-Lys-Leu-Val-Phe-Phe-Ala-Glu-Asp-Trp as substra... |
Eur J Med Chem 179: 557-566 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.057
BindingDB Entry DOI: 10.7270/Q2XS5ZT5 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50005641

(CHEMBL3235416)Show SMILES CNC(=O)[C@H](Cc1c[nH]cn1)NC(=O)CN(CCCc1ccccc1)CC(O)=O |r| Show InChI InChI=1S/C20H27N5O4/c1-21-20(29)17(10-16-11-22-14-23-16)24-18(26)12-25(13-19(27)28)9-5-8-15-6-3-2-4-7-15/h2-4,6-7,11,14,17H,5,8-10,12-13H2,1H3,(H,21,29)(H,22,23)(H,24,26)(H,27,28)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MMP1-mediated amyloid beta hydrolysis using DNP-Pro-Cha-Gly- Cys(Me)-His-Ala- Lys(n-Me-Abz)-NH2 as substrate after 40... |
Eur J Med Chem 79: 184-93 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.009
BindingDB Entry DOI: 10.7270/Q27H1M34 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50005641

(CHEMBL3235416)Show SMILES CNC(=O)[C@H](Cc1c[nH]cn1)NC(=O)CN(CCCc1ccccc1)CC(O)=O |r| Show InChI InChI=1S/C20H27N5O4/c1-21-20(29)17(10-16-11-22-14-23-16)24-18(26)12-25(13-19(27)28)9-5-8-15-6-3-2-4-7-15/h2-4,6-7,11,14,17H,5,8-10,12-13H2,1H3,(H,21,29)(H,22,23)(H,24,26)(H,27,28)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant TACE-mediated amyloid beta hydrolysis after 5 mins by fluorimetry assay |
Eur J Med Chem 79: 184-93 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.009
BindingDB Entry DOI: 10.7270/Q27H1M34 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50005641

(CHEMBL3235416)Show SMILES CNC(=O)[C@H](Cc1c[nH]cn1)NC(=O)CN(CCCc1ccccc1)CC(O)=O |r| Show InChI InChI=1S/C20H27N5O4/c1-21-20(29)17(10-16-11-22-14-23-16)24-18(26)12-25(13-19(27)28)9-5-8-15-6-3-2-4-7-15/h2-4,6-7,11,14,17H,5,8-10,12-13H2,1H3,(H,21,29)(H,22,23)(H,24,26)(H,27,28)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ACE2-mediated amyloid beta hydrolysis using Mca-Tyr-Val-Ala-Asp-Pro-Ala-Lys-(DNP)-OH as substrate after 20 mins by fl... |
Eur J Med Chem 79: 184-93 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.009
BindingDB Entry DOI: 10.7270/Q27H1M34 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50005641

(CHEMBL3235416)Show SMILES CNC(=O)[C@H](Cc1c[nH]cn1)NC(=O)CN(CCCc1ccccc1)CC(O)=O |r| Show InChI InChI=1S/C20H27N5O4/c1-21-20(29)17(10-16-11-22-14-23-16)24-18(26)12-25(13-19(27)28)9-5-8-15-6-3-2-4-7-15/h2-4,6-7,11,14,17H,5,8-10,12-13H2,1H3,(H,21,29)(H,22,23)(H,24,26)(H,27,28)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of human ACE-mediated amyloid beta hydrolysis |
Eur J Med Chem 79: 184-93 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.009
BindingDB Entry DOI: 10.7270/Q27H1M34 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50005641

(CHEMBL3235416)Show SMILES CNC(=O)[C@H](Cc1c[nH]cn1)NC(=O)CN(CCCc1ccccc1)CC(O)=O |r| Show InChI InChI=1S/C20H27N5O4/c1-21-20(29)17(10-16-11-22-14-23-16)24-18(26)12-25(13-19(27)28)9-5-8-15-6-3-2-4-7-15/h2-4,6-7,11,14,17H,5,8-10,12-13H2,1H3,(H,21,29)(H,22,23)(H,24,26)(H,27,28)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MMP13-mediated amyloid beta hydrolysis after 10 mins by fluorimetry assay |
Eur J Med Chem 79: 184-93 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.009
BindingDB Entry DOI: 10.7270/Q27H1M34 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50005641

(CHEMBL3235416)Show SMILES CNC(=O)[C@H](Cc1c[nH]cn1)NC(=O)CN(CCCc1ccccc1)CC(O)=O |r| Show InChI InChI=1S/C20H27N5O4/c1-21-20(29)17(10-16-11-22-14-23-16)24-18(26)12-25(13-19(27)28)9-5-8-15-6-3-2-4-7-15/h2-4,6-7,11,14,17H,5,8-10,12-13H2,1H3,(H,21,29)(H,22,23)(H,24,26)(H,27,28)/t17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of human NEP-mediated amyloid beta hydrolysis |
Eur J Med Chem 79: 184-93 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.009
BindingDB Entry DOI: 10.7270/Q27H1M34 |
More data for this
Ligand-Target Pair | |
Insulin-degrading enzyme
(Homo sapiens (Human)) | BDBM34233

(2-Phenyl-benzo[d]isoselenazol-3-one | 2-Phenyl-ben...)Show InChI InChI=1S/C13H9NOSe/c15-13-11-8-4-5-9-12(11)16-14(13)10-6-2-1-3-7-10/h1-9H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Lille
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDE harboring C110L/C171S/C178A/C257V/C414L/C573N/C590S/C789S/C812A/C819A/C904S/C966N/C974A mutant expressed in Esche... |
Eur J Med Chem 179: 557-566 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.057
BindingDB Entry DOI: 10.7270/Q2XS5ZT5 |
More data for this
Ligand-Target Pair | |
Endothelin-converting enzyme 1
(Homo sapiens (Human)) | BDBM50005641

(CHEMBL3235416)Show SMILES CNC(=O)[C@H](Cc1c[nH]cn1)NC(=O)CN(CCCc1ccccc1)CC(O)=O |r| Show InChI InChI=1S/C20H27N5O4/c1-21-20(29)17(10-16-11-22-14-23-16)24-18(26)12-25(13-19(27)28)9-5-8-15-6-3-2-4-7-15/h2-4,6-7,11,14,17H,5,8-10,12-13H2,1H3,(H,21,29)(H,22,23)(H,24,26)(H,27,28)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ECE1-mediated amyloid beta hydrolysis after 45 mins by fluorimetry assay |
Eur J Med Chem 79: 184-93 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.009
BindingDB Entry DOI: 10.7270/Q27H1M34 |
More data for this
Ligand-Target Pair | |
Insulin-degrading enzyme
(Homo sapiens (Human)) | BDBM50005646
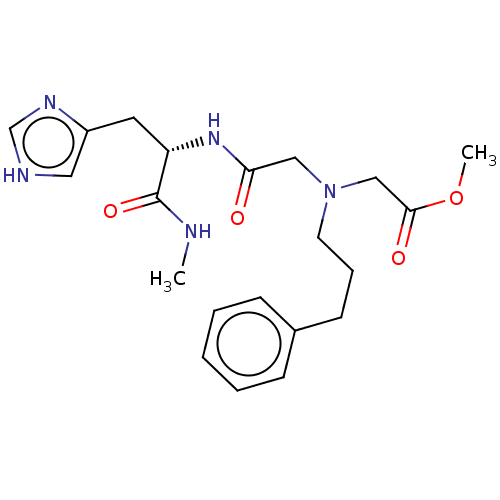
(CHEMBL3235419)Show SMILES CNC(=O)[C@H](Cc1c[nH]cn1)NC(=O)CN(CCCc1ccccc1)CC(=O)OC |r| Show InChI InChI=1S/C21H29N5O4/c1-22-21(29)18(11-17-12-23-15-24-17)25-19(27)13-26(14-20(28)30-2)10-6-9-16-7-4-3-5-8-16/h3-5,7-8,12,15,18H,6,9-11,13-14H2,1-2H3,(H,22,29)(H,23,24)(H,25,27)/t18-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDE-mediated amyloid beta (16 to 23) hydrolysis using ATTO 655-Cys-Lys-Leu-Val-Phe-Phe-Ala-Glu-Asp-Trp as substrate p... |
Eur J Med Chem 79: 184-93 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.009
BindingDB Entry DOI: 10.7270/Q27H1M34 |
More data for this
Ligand-Target Pair | |
Insulin-degrading enzyme
(Homo sapiens (Human)) | BDBM50005645

(CHEMBL3235418)Show SMILES COC(=O)[C@H](Cc1c[nH]cn1)NC(=O)CN(CC(O)=O)C(=O)c1ccccc1 |r| Show InChI InChI=1S/C18H20N4O6/c1-28-18(27)14(7-13-8-19-11-20-13)21-15(23)9-22(10-16(24)25)17(26)12-5-3-2-4-6-12/h2-6,8,11,14H,7,9-10H2,1H3,(H,19,20)(H,21,23)(H,24,25)/t14-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDE-mediated amyloid beta (16 to 23) hydrolysis using ATTO 655-Cys-Lys-Leu-Val-Phe-Phe-Ala-Glu-Asp-Trp as substrate p... |
Eur J Med Chem 79: 184-93 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.009
BindingDB Entry DOI: 10.7270/Q27H1M34 |
More data for this
Ligand-Target Pair | |
Insulin-degrading enzyme
(Homo sapiens (Human)) | BDBM50005643

(CHEMBL3235417)Show SMILES COC(=O)[C@H](Cc1ccccc1)NC(=O)CN(CC(O)=O)Cc1ccccc1 |r| Show InChI InChI=1S/C21H24N2O5/c1-28-21(27)18(12-16-8-4-2-5-9-16)22-19(24)14-23(15-20(25)26)13-17-10-6-3-7-11-17/h2-11,18H,12-15H2,1H3,(H,22,24)(H,25,26)/t18-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDE-mediated amyloid beta (16 to 23) hydrolysis using ATTO 655-Cys-Lys-Leu-Val-Phe-Phe-Ala-Glu-Asp-Trp as substrate p... |
Eur J Med Chem 79: 184-93 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.009
BindingDB Entry DOI: 10.7270/Q27H1M34 |
More data for this
Ligand-Target Pair | |
Insulin-degrading enzyme
(Homo sapiens (Human)) | BDBM50005641

(CHEMBL3235416)Show SMILES CNC(=O)[C@H](Cc1c[nH]cn1)NC(=O)CN(CCCc1ccccc1)CC(O)=O |r| Show InChI InChI=1S/C20H27N5O4/c1-21-20(29)17(10-16-11-22-14-23-16)24-18(26)12-25(13-19(27)28)9-5-8-15-6-3-2-4-7-15/h2-4,6-7,11,14,17H,5,8-10,12-13H2,1H3,(H,21,29)(H,22,23)(H,24,26)(H,27,28)/t17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 530 | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Induction of human recombinant IDE-mediated insulin hydrolysis preincubated for 10 mins measured after 30 mins by spectrophotometer analysis |
Eur J Med Chem 79: 184-93 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.009
BindingDB Entry DOI: 10.7270/Q27H1M34 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data