 Found 1156 hits with Last Name = 'salmaso' and Initial = 'v'
Found 1156 hits with Last Name = 'salmaso' and Initial = 'v' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
P2Y purinoceptor 14
(Homo sapiens (Human)) | BDBM50512416

(CHEMBL4447162)Show SMILES Nc1ccc2c(-c3ccc(cc3C(O)=O)C(=O)NCCCCCCn3cc(CCCCN4CCC(CC4)c4ccc(cc4)-c4cc(cc5cc(ccc45)-c4ccc(cc4)C(F)(F)F)C(O)=O)nn3)c3ccc(=N)c(c3oc2c1S(O)(=O)=O)S(O)(=O)=O |(41.72,-13.14,;41.86,-14.67,;40.6,-15.57,;40.74,-17.1,;42.13,-17.74,;42.28,-19.27,;41.03,-20.17,;41.18,-21.7,;39.93,-22.59,;38.53,-21.95,;38.38,-20.42,;39.63,-19.53,;39.47,-17.99,;37.94,-17.87,;39.87,-16.5,;37.28,-22.84,;37.42,-24.37,;35.88,-22.2,;34.62,-23.09,;33.22,-22.45,;31.97,-23.35,;30.57,-22.71,;29.31,-23.6,;27.91,-22.96,;26.66,-23.85,;25.2,-23.36,;24.28,-24.6,;22.74,-24.59,;21.98,-23.25,;20.44,-23.23,;19.69,-21.89,;18.15,-21.87,;17.36,-23.2,;15.83,-23.18,;15.07,-21.84,;15.84,-20.51,;17.39,-20.53,;13.54,-21.84,;12.76,-23.17,;11.22,-23.15,;10.46,-21.81,;11.23,-20.49,;12.77,-20.49,;8.92,-21.8,;8.16,-20.45,;6.61,-20.45,;5.83,-21.78,;6.59,-23.12,;5.82,-24.44,;6.58,-25.78,;8.12,-25.8,;8.9,-24.47,;8.14,-23.13,;5.79,-27.1,;4.25,-27.09,;3.47,-28.41,;4.23,-29.76,;5.78,-29.76,;6.55,-28.44,;3.45,-31.08,;1.91,-31.07,;4.21,-32.42,;2.67,-32.41,;5.84,-19.11,;4.3,-19.1,;6.62,-17.78,;25.17,-25.85,;26.64,-25.39,;43.68,-19.91,;43.82,-21.43,;45.21,-22.07,;46.46,-21.19,;47.86,-21.84,;46.33,-19.66,;44.93,-19.02,;44.8,-17.49,;43.39,-16.85,;43.26,-15.32,;44.51,-14.44,;45.91,-15.09,;43.74,-13.1,;45.28,-13.09,;47.58,-18.77,;48.98,-19.41,;46.8,-17.43,;48.35,-17.43,)| Show InChI InChI=1S/C62H58F3N7O12S2/c63-62(64,65)44-16-12-37(13-17-44)40-14-18-46-42(31-40)32-43(60(74)75)34-50(46)39-10-8-36(9-11-39)38-24-29-71(30-25-38)27-6-3-7-45-35-72(70-69-45)28-5-2-1-4-26-68-59(73)41-15-19-47(51(33-41)61(76)77)54-48-20-22-52(66)57(85(78,79)80)55(48)84-56-49(54)21-23-53(67)58(56)86(81,82)83/h8-23,31-35,38,66H,1-7,24-30,67H2,(H,68,73)(H,74,75)(H,76,77)(H,78,79,80)(H,81,82,83) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Inhibition of human P2Y14R |
J Med Chem 63: 9563-9589 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00745
BindingDB Entry DOI: 10.7270/Q20R9SZP |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Mus musculus) | BDBM50517301
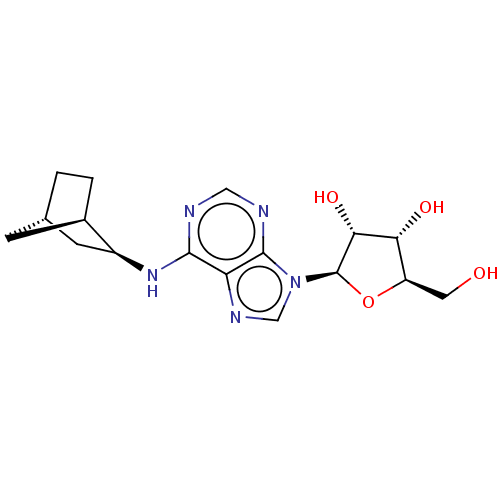
(CHEMBL1877326)Show SMILES [H][C@]12CC[C@]([H])(C1)[C@H](C2)Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |TLB:9:7:6:3.2| Show InChI InChI=1S/C17H23N5O4/c23-5-11-13(24)14(25)17(26-11)22-7-20-12-15(18-6-19-16(12)22)21-10-4-8-1-2-9(10)3-8/h6-11,13-14,17,23-25H,1-5H2,(H,18,19,21)/t8-,9+,10-,11+,13+,14+,17+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical College of Wisconsin
Curated by ChEMBL
| Assay Description
Displacement of [3H]N6-R-phenylisopropyladenosine from mouse A1A adenosine receptor expressed in CHO cell membranes after 60 mins by scintillation pr... |
J Med Chem 62: 1502-1522 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01662
BindingDB Entry DOI: 10.7270/Q2TM7FGK |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Mus musculus) | BDBM50517302
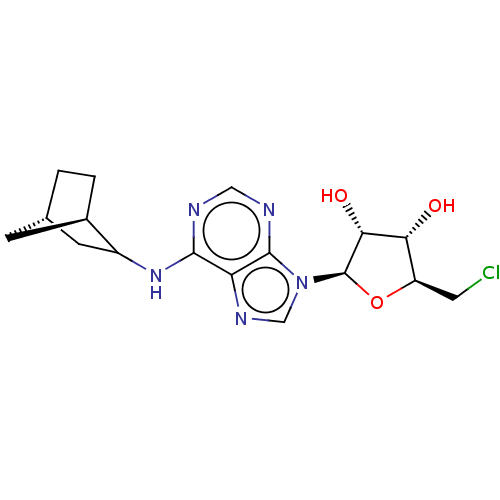
(CHEMBL4562258)Show SMILES [H][C@]12CC[C@]([H])(C1)C(C2)Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CCl)[C@@H](O)[C@H]1O |r,TLB:9:7:3.2:6| Show InChI InChI=1S/C17H22ClN5O3/c18-5-11-13(24)14(25)17(26-11)23-7-21-12-15(19-6-20-16(12)23)22-10-4-8-1-2-9(10)3-8/h6-11,13-14,17,24-25H,1-5H2,(H,19,20,22)/t8-,9+,10?,11+,13+,14+,17+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical College of Wisconsin
Curated by ChEMBL
| Assay Description
Displacement of [3H]N6-R-phenylisopropyladenosine from mouse A1A adenosine receptor expressed in CHO cell membranes after 60 mins by scintillation pr... |
J Med Chem 62: 1502-1522 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01662
BindingDB Entry DOI: 10.7270/Q2TM7FGK |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Mus musculus) | BDBM50085658

((2R,3R,4S,5R)-2-(2-Chloro-6-cyclopentylamino-purin...)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NC3CCCC3)nc(Cl)nc12 |r| Show InChI InChI=1S/C15H20ClN5O4/c16-15-19-12(18-7-3-1-2-4-7)9-13(20-15)21(6-17-9)14-11(24)10(23)8(5-22)25-14/h6-8,10-11,14,22-24H,1-5H2,(H,18,19,20)/t8-,10-,11-,14-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical College of Wisconsin
Curated by ChEMBL
| Assay Description
Displacement of [3H]N6-R-phenylisopropyladenosine from mouse A1A adenosine receptor expressed in CHO cell membranes after 60 mins by scintillation pr... |
J Med Chem 62: 1502-1522 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01662
BindingDB Entry DOI: 10.7270/Q2TM7FGK |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Mus musculus) | BDBM25400

((2R,3R,4S,5R)-2-[6-(cyclopentylamino)-9H-purin-9-y...)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NC3CCCC3)ncnc12 Show InChI InChI=1S/C15H21N5O4/c21-5-9-11(22)12(23)15(24-9)20-7-18-10-13(16-6-17-14(10)20)19-8-3-1-2-4-8/h6-9,11-12,15,21-23H,1-5H2,(H,16,17,19)/t9-,11-,12-,15-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical College of Wisconsin
Curated by ChEMBL
| Assay Description
Displacement of [3H]N6-R-phenylisopropyladenosine from mouse A1A adenosine receptor expressed in CHO cell membranes after 60 mins by scintillation pr... |
J Med Chem 62: 1502-1522 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01662
BindingDB Entry DOI: 10.7270/Q2TM7FGK |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50561600
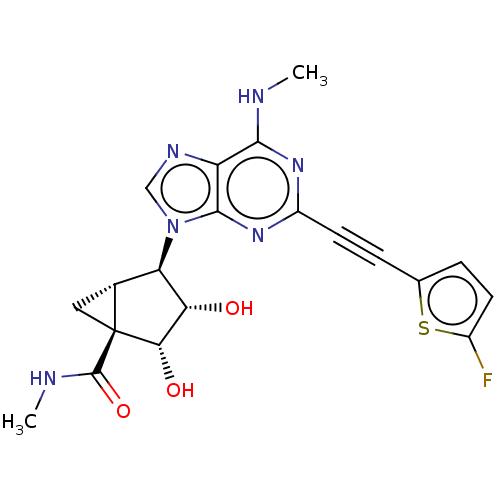
(CHEMBL4788488)Show SMILES [H][C@]12C[C@]1([C@@H](O)[C@@H](O)[C@@H]2n1cnc2c(NC)nc(nc12)C#Cc1ccc(F)s1)C(=O)NC |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125I]N6-(4-amino-3-iodobenzyl)adenosine-5'-N-methyluronamide from human adrenergic A3 receptor expressed in CHO cell membranes asses... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00637
BindingDB Entry DOI: 10.7270/Q20C50H1 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM50517301

(CHEMBL1877326)Show SMILES [H][C@]12CC[C@]([H])(C1)[C@H](C2)Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |TLB:9:7:6:3.2| Show InChI InChI=1S/C17H23N5O4/c23-5-11-13(24)14(25)17(26-11)22-7-20-12-15(18-6-19-16(12)22)21-10-4-8-1-2-9(10)3-8/h6-11,13-14,17,23-25H,1-5H2,(H,18,19,21)/t8-,9+,10-,11+,13+,14+,17+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical College of Wisconsin
Curated by ChEMBL
| Assay Description
Displacement of [3H]N6-R-phenylisopropyladenosine from rat A1A adenosine receptor expressed in CHO cell membranes after 60 mins by scintillation prox... |
J Med Chem 62: 1502-1522 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01662
BindingDB Entry DOI: 10.7270/Q2TM7FGK |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50118812

((2S,3S,4R,5R)-3,4-Dihydroxy-5-[6-(3-iodo-benzylami...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCc3cccc(I)c3)ncnc12 |r| Show InChI InChI=1S/C18H19IN6O4/c1-20-17(28)14-12(26)13(27)18(29-14)25-8-24-11-15(22-7-23-16(11)25)21-6-9-3-2-4-10(19)5-9/h2-5,7-8,12-14,18,26-27H,6H2,1H3,(H,20,28)(H,21,22,23)/t12-,13+,14-,18+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125I]I-AB-MECA from human adenosine A3 receptor expressed in HEK293 cells by radioligand binding assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00685
BindingDB Entry DOI: 10.7270/Q2930Z2X |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Mus musculus) | BDBM50517299

(CHEMBL4533718)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NC(C3CCC3)C3CCC3)ncnc12 |r| Show InChI InChI=1S/C19H27N5O4/c25-7-12-15(26)16(27)19(28-12)24-9-22-14-17(20-8-21-18(14)24)23-13(10-3-1-4-10)11-5-2-6-11/h8-13,15-16,19,25-27H,1-7H2,(H,20,21,23)/t12-,15-,16-,19-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical College of Wisconsin
Curated by ChEMBL
| Assay Description
Displacement of [3H]N6-R-phenylisopropyladenosine from mouse A1A adenosine receptor expressed in CHO cell membranes after 60 mins by scintillation pr... |
J Med Chem 62: 1502-1522 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01662
BindingDB Entry DOI: 10.7270/Q2TM7FGK |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50517301

(CHEMBL1877326)Show SMILES [H][C@]12CC[C@]([H])(C1)[C@H](C2)Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |TLB:9:7:6:3.2| Show InChI InChI=1S/C17H23N5O4/c23-5-11-13(24)14(25)17(26-11)22-7-20-12-15(18-6-19-16(12)22)21-10-4-8-1-2-9(10)3-8/h6-11,13-14,17,23-25H,1-5H2,(H,18,19,21)/t8-,9+,10-,11+,13+,14+,17+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical College of Wisconsin
Curated by ChEMBL
| Assay Description
Displacement of [3H]N6-R-phenylisopropyladenosine from human A1A adenosine receptor expressed in CHO cell membranes after 60 mins by scintillation pr... |
J Med Chem 62: 1502-1522 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01662
BindingDB Entry DOI: 10.7270/Q2TM7FGK |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Mus musculus) | BDBM50602667

(CHEMBL5196916)Show SMILES CNC(=O)[C@@]12C[C@@H]1[C@H]([C@H](O)[C@@H]2O)n1cnc2c(NCc3ccc(O)c(OC)c3)nc(nc12)C#Cc1ccc(Cl)s1 |r| | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113983
BindingDB Entry DOI: 10.7270/Q29027V8 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 14
(Homo sapiens (Human)) | BDBM50456168

(CHEMBL1800685)Show SMILES OC(=O)c1cc(-c2ccc(cc2)C2CCNCC2)c2ccc(cc2c1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C29H24F3NO2/c30-29(31,32)25-8-5-19(6-9-25)22-7-10-26-23(15-22)16-24(28(34)35)17-27(26)21-3-1-18(2-4-21)20-11-13-33-14-12-20/h1-10,15-17,20,33H,11-14H2,(H,34,35) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human P2Y14 expressed in CHO cells assessed as inhibition of forskolin-induced increase of cAMP accumulation incubated for 15 ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01964
BindingDB Entry DOI: 10.7270/Q2611470 |
More data for this
Ligand-Target Pair | |
5'-nucleotidase
(Homo sapiens (Human)) | BDBM50583394

(CHEMBL5081267)Show SMILES CCN(CC)CC.CCN(CC)CC.Cn1c(=O)n(cc\c1=N\OCc1ccc(I)cc1)[C@@H]1O[C@H](COP(O)(=O)CP(O)(O)=O)[C@@H](O)[C@H]1O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.436 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human soluble CD73 assessed as inhibition constant |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01852
BindingDB Entry DOI: 10.7270/Q2ZS31D5 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50299701

(CHEMBL574602 | N6-Methyl-2-phenylethynyl-5'-N-meth...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NC)nc(nc12)C#Cc1ccccc1 |r| Show InChI InChI=1S/C20H20N6O4/c1-21-17-13-18(25-12(24-17)9-8-11-6-4-3-5-7-11)26(10-23-13)20-15(28)14(27)16(30-20)19(29)22-2/h3-7,10,14-16,20,27-28H,1-2H3,(H,22,29)(H,21,24,25)/t14-,15+,16-,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human A3AR assessed as inhibitor constant |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00637
BindingDB Entry DOI: 10.7270/Q20C50H1 |
More data for this
Ligand-Target Pair | |
5'-nucleotidase
(Homo sapiens (Human)) | BDBM50583393
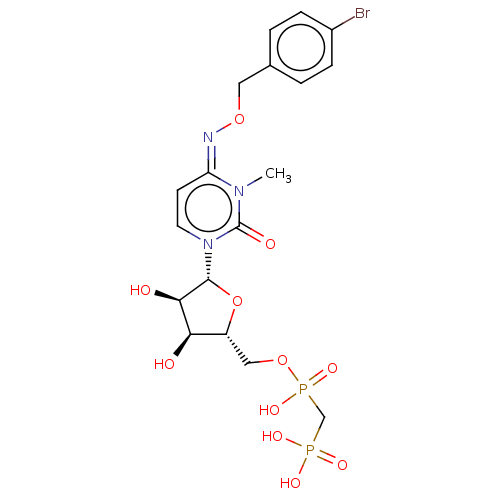
(CHEMBL5083600)Show SMILES CCN(CC)CC.CCN(CC)CC.Cn1c(=O)n(cc\c1=N\OCc1ccc(Br)cc1)[C@@H]1O[C@H](COP(O)(=O)CP(O)(O)=O)[C@@H](O)[C@H]1O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.441 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human soluble CD73 assessed as inhibition constant |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01852
BindingDB Entry DOI: 10.7270/Q2ZS31D5 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Mus musculus) | BDBM50517307
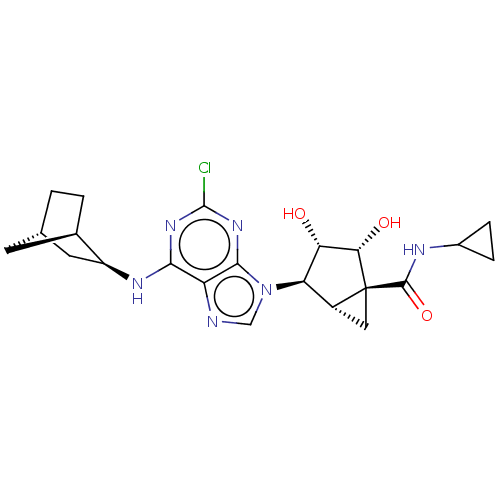
(CHEMBL4463802)Show SMILES [H][C@]12C[C@]1([C@@H](O)[C@@H](O)[C@@H]2n1cnc2c(N[C@H]3C[C@@]4([H])CC[C@]3([H])C4)nc(Cl)nc12)C(=O)NC1CC1 |r,TLB:14:15:20.19:23| Show InChI InChI=1S/C22H27ClN6O3/c23-21-27-18(26-13-6-9-1-2-10(13)5-9)14-19(28-21)29(8-24-14)15-12-7-22(12,17(31)16(15)30)20(32)25-11-3-4-11/h8-13,15-17,30-31H,1-7H2,(H,25,32)(H,26,27,28)/t9-,10+,12+,13-,15+,16-,17-,22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical College of Wisconsin
Curated by ChEMBL
| Assay Description
Displacement of [3H]N6-R-phenylisopropyladenosine from mouse A1A adenosine receptor expressed in CHO cell membranes after 60 mins by scintillation pr... |
J Med Chem 62: 1502-1522 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01662
BindingDB Entry DOI: 10.7270/Q2TM7FGK |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Mus musculus) | BDBM21220

((2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-N-ethyl-3,...)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 Show InChI InChI=1S/C12H16N6O4/c1-2-14-11(21)8-6(19)7(20)12(22-8)18-4-17-5-9(13)15-3-16-10(5)18/h3-4,6-8,12,19-20H,2H2,1H3,(H,14,21)(H2,13,15,16)/t6-,7+,8-,12+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical College of Wisconsin
Curated by ChEMBL
| Assay Description
Displacement of [3H]N6-R-phenylisopropyladenosine from mouse A1A adenosine receptor expressed in CHO cell membranes after 60 mins by scintillation pr... |
J Med Chem 62: 1502-1522 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01662
BindingDB Entry DOI: 10.7270/Q2TM7FGK |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50517302

(CHEMBL4562258)Show SMILES [H][C@]12CC[C@]([H])(C1)C(C2)Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CCl)[C@@H](O)[C@H]1O |r,TLB:9:7:3.2:6| Show InChI InChI=1S/C17H22ClN5O3/c18-5-11-13(24)14(25)17(26-11)23-7-21-12-15(19-6-20-16(12)23)22-10-4-8-1-2-9(10)3-8/h6-11,13-14,17,24-25H,1-5H2,(H,19,20,22)/t8-,9+,10?,11+,13+,14+,17+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical College of Wisconsin
Curated by ChEMBL
| Assay Description
Displacement of [3H]N6-R-phenylisopropyladenosine from human A1A adenosine receptor expressed in CHO cell membranes after 60 mins by scintillation pr... |
J Med Chem 62: 1502-1522 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01662
BindingDB Entry DOI: 10.7270/Q2TM7FGK |
More data for this
Ligand-Target Pair | |
5'-nucleotidase
(Homo sapiens (Human)) | BDBM50583396
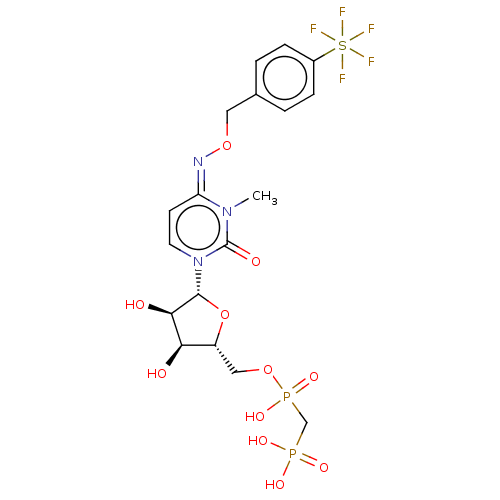
(CHEMBL5074970)Show SMILES CCN(CC)CC.CCN(CC)CC.Cn1c(=O)n(cc\c1=N\OCc1ccc(cc1)S(F)(F)(F)(F)F)[C@@H]1O[C@H](COP(O)(=O)CP(O)(O)=O)[C@@H](O)[C@H]1O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.511 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human soluble CD73 assessed as inhibition constant |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01852
BindingDB Entry DOI: 10.7270/Q2ZS31D5 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Mus musculus) | BDBM50517289
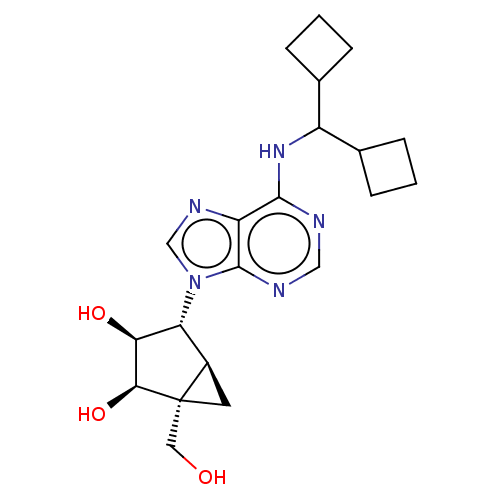
(CHEMBL4436786)Show SMILES [H][C@]12C[C@@]1(CO)[C@@H](O)[C@@H](O)[C@@H]2n1cnc2c(NC(C3CCC3)C3CCC3)ncnc12 |r| Show InChI InChI=1S/C21H29N5O3/c27-8-21-7-13(21)16(17(28)18(21)29)26-10-24-15-19(22-9-23-20(15)26)25-14(11-3-1-4-11)12-5-2-6-12/h9-14,16-18,27-29H,1-8H2,(H,22,23,25)/t13-,16-,17+,18+,21+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical College of Wisconsin
Curated by ChEMBL
| Assay Description
Displacement of [3H]N6-R-phenylisopropyladenosine from mouse A1A adenosine receptor expressed in CHO cell membranes after 60 mins by scintillation pr... |
J Med Chem 62: 1502-1522 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01662
BindingDB Entry DOI: 10.7270/Q2TM7FGK |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50602675
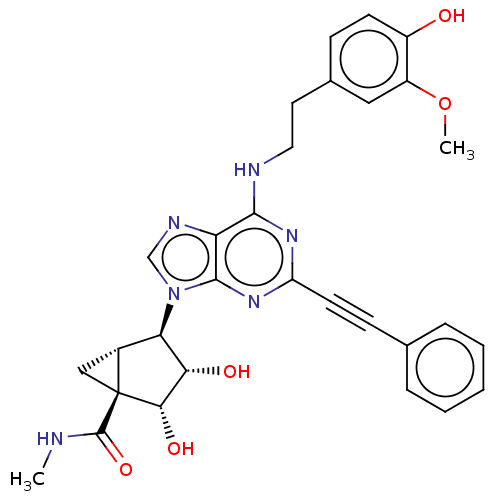
(CHEMBL5200403)Show SMILES CNC(=O)[C@@]12C[C@@H]1[C@H]([C@H](O)[C@@H]2O)n1cnc2c(NCCc3ccc(O)c(OC)c3)nc(nc12)C#Cc1ccccc1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.563 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113983
BindingDB Entry DOI: 10.7270/Q29027V8 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50602673
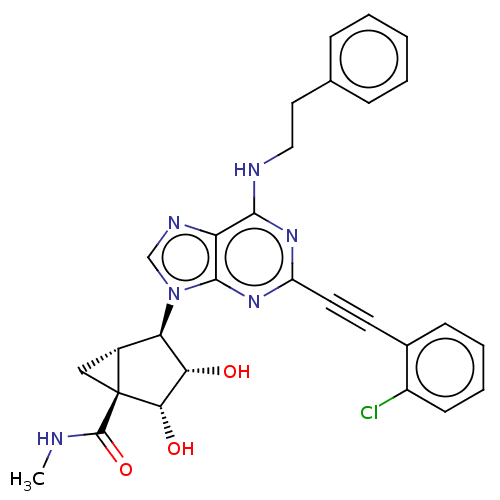
(CHEMBL5203749)Show SMILES CNC(=O)[C@@]12C[C@@H]1[C@H]([C@H](O)[C@@H]2O)n1cnc2c(NCCc3ccccc3)nc(nc12)C#Cc1ccccc1Cl |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113983
BindingDB Entry DOI: 10.7270/Q29027V8 |
More data for this
Ligand-Target Pair | |
5'-nucleotidase
(Homo sapiens (Human)) | BDBM50583400
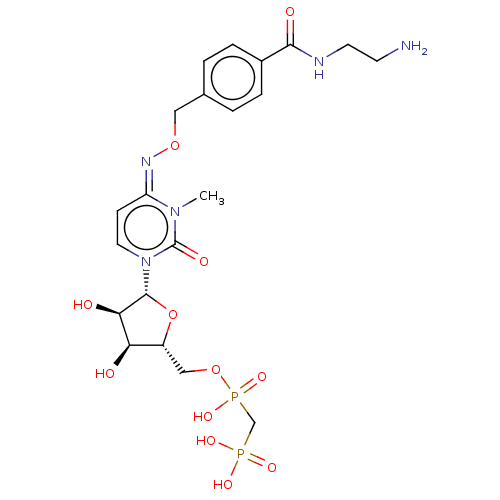
(CHEMBL5078828)Show SMILES CCN(CC)CC.CCN(CC)CC.Cn1c(=O)n(cc\c1=N\OCc1ccc(cc1)C(=O)NCCN)[C@@H]1O[C@H](COP(O)(=O)CP(O)(O)=O)[C@@H](O)[C@H]1O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.626 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human soluble CD73 assessed as inhibition constant |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01852
BindingDB Entry DOI: 10.7270/Q2ZS31D5 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Mus musculus) | BDBM50517300

(CHEMBL4468006)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NC(C3CCC3)C3CCC3)nc(Cl)nc12 |r| Show InChI InChI=1S/C19H26ClN5O4/c20-19-23-16(22-12(9-3-1-4-9)10-5-2-6-10)13-17(24-19)25(8-21-13)18-15(28)14(27)11(7-26)29-18/h8-12,14-15,18,26-28H,1-7H2,(H,22,23,24)/t11-,14-,15-,18-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical College of Wisconsin
Curated by ChEMBL
| Assay Description
Displacement of [3H]N6-R-phenylisopropyladenosine from mouse A1A adenosine receptor expressed in CHO cell membranes after 60 mins by scintillation pr... |
J Med Chem 62: 1502-1522 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01662
BindingDB Entry DOI: 10.7270/Q2TM7FGK |
More data for this
Ligand-Target Pair | |
5'-nucleotidase
(Homo sapiens (Human)) | BDBM50583392
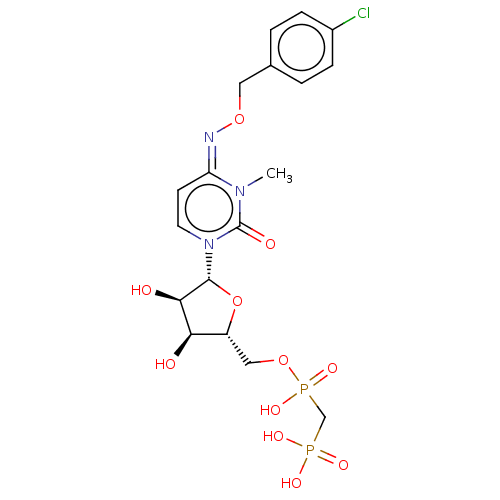
(CHEMBL5080841)Show SMILES CCN(CC)CC.CCN(CC)CC.Cn1c(=O)n(cc\c1=N\OCc1ccc(Cl)cc1)[C@@H]1O[C@H](COP(O)(=O)CP(O)(O)=O)[C@@H](O)[C@H]1O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.673 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human soluble CD73 assessed as inhibition constant |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01852
BindingDB Entry DOI: 10.7270/Q2ZS31D5 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Mus musculus) | BDBM50179180
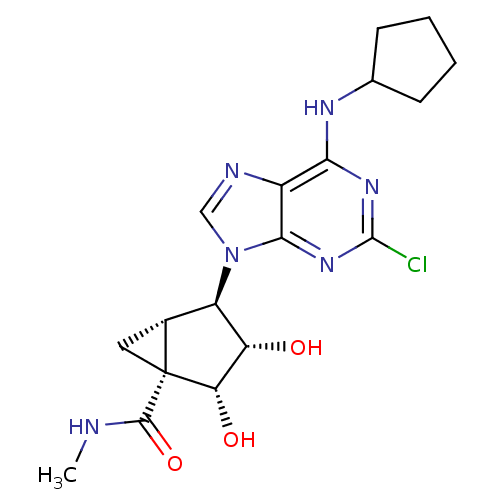
((1S,2R,3S,4R,5S)-4-(2-chloro-6-(cyclopentylamino)-...)Show SMILES CNC(=O)[C@]12C[C@@H]1[C@H]([C@H](O)[C@@H]2O)n1cnc2c(NC3CCCC3)nc(Cl)nc12 Show InChI InChI=1S/C18H23ClN6O3/c1-20-16(28)18-6-9(18)11(12(26)13(18)27)25-7-21-10-14(22-8-4-2-3-5-8)23-17(19)24-15(10)25/h7-9,11-13,26-27H,2-6H2,1H3,(H,20,28)(H,22,23,24)/t9-,11-,12+,13+,18-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical College of Wisconsin
Curated by ChEMBL
| Assay Description
Displacement of [3H]N6-R-phenylisopropyladenosine from mouse A1A adenosine receptor expressed in CHO cell membranes after 60 mins by scintillation pr... |
J Med Chem 62: 1502-1522 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01662
BindingDB Entry DOI: 10.7270/Q2TM7FGK |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50069812

(CHEMBL3407784)Show SMILES [H][C@]12C[C@]1([C@@H](O)[C@@H](O)[C@@H]2n1cnc2c(NC)nc(nc12)C#Cc1ccc(Cl)s1)C(=O)NC |r| Show InChI InChI=1S/C20H19ClN6O3S/c1-22-17-13-18(26-12(25-17)6-4-9-3-5-11(21)31-9)27(8-24-13)14-10-7-20(10,19(30)23-2)16(29)15(14)28/h3,5,8,10,14-16,28-29H,7H2,1-2H3,(H,23,30)(H,22,25,26)/t10-,14-,15+,16+,20+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human A3AR assessed as inhibitor constant |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00637
BindingDB Entry DOI: 10.7270/Q20C50H1 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Mus musculus) | BDBM50517298

(CHEMBL4439013)Show SMILES O[C@@H]1[C@@H](CCl)O[C@H]([C@@H]1O)n1cnc2c(NC(C3CCC3)C3CCC3)ncnc12 |r| Show InChI InChI=1S/C19H26ClN5O3/c20-7-12-15(26)16(27)19(28-12)25-9-23-14-17(21-8-22-18(14)25)24-13(10-3-1-4-10)11-5-2-6-11/h8-13,15-16,19,26-27H,1-7H2,(H,21,22,24)/t12-,15-,16-,19-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical College of Wisconsin
Curated by ChEMBL
| Assay Description
Displacement of [3H]N6-R-phenylisopropyladenosine from mouse A1A adenosine receptor expressed in CHO cell membranes after 60 mins by scintillation pr... |
J Med Chem 62: 1502-1522 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01662
BindingDB Entry DOI: 10.7270/Q2TM7FGK |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50561595
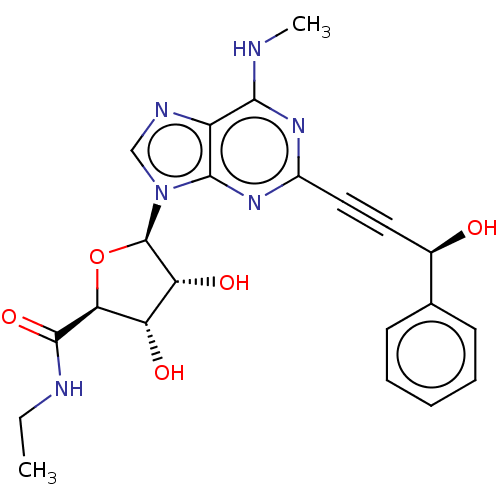
(CHEMBL4755127)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NC)nc(nc12)C#C[C@@H](O)c1ccccc1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human A3AR assessed as inhibitor constant |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00637
BindingDB Entry DOI: 10.7270/Q20C50H1 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50517291
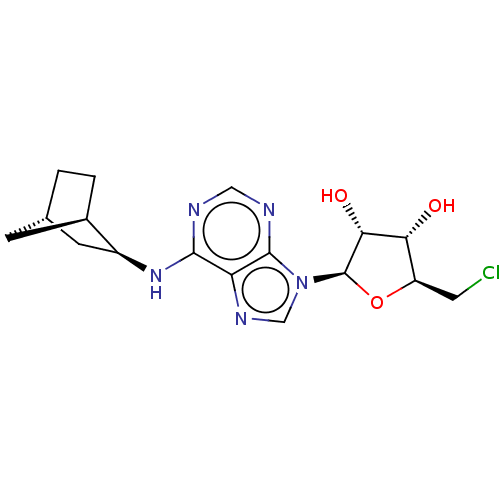
(CHEMBL4541086)Show SMILES [H][C@]12CC[C@]([H])(C1)[C@H](C2)Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CCl)[C@@H](O)[C@H]1O |r,TLB:9:7:3.2:6| Show InChI InChI=1S/C17H22ClN5O3/c18-5-11-13(24)14(25)17(26-11)23-7-21-12-15(19-6-20-16(12)23)22-10-4-8-1-2-9(10)3-8/h6-11,13-14,17,24-25H,1-5H2,(H,19,20,22)/t8-,9+,10-,11+,13+,14+,17+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 0.760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical College of Wisconsin
Curated by ChEMBL
| Assay Description
Displacement of [3H]N6-R-phenylisopropyladenosine from human A1A adenosine receptor expressed in CHO cell membranes after 60 mins by scintillation pr... |
J Med Chem 62: 1502-1522 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01662
BindingDB Entry DOI: 10.7270/Q2TM7FGK |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50085658

((2R,3R,4S,5R)-2-(2-Chloro-6-cyclopentylamino-purin...)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NC3CCCC3)nc(Cl)nc12 |r| Show InChI InChI=1S/C15H20ClN5O4/c16-15-19-12(18-7-3-1-2-4-7)9-13(20-15)21(6-17-9)14-11(24)10(23)8(5-22)25-14/h6-8,10-11,14,22-24H,1-5H2,(H,18,19,20)/t8-,10-,11-,14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical College of Wisconsin
Curated by ChEMBL
| Assay Description
Displacement of [3H]N6-R-phenylisopropyladenosine from human A1A adenosine receptor expressed in CHO cell membranes after 60 mins by scintillation pr... |
J Med Chem 62: 1502-1522 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01662
BindingDB Entry DOI: 10.7270/Q2TM7FGK |
More data for this
Ligand-Target Pair | |
5'-nucleotidase
(Homo sapiens (Human)) | BDBM50583397
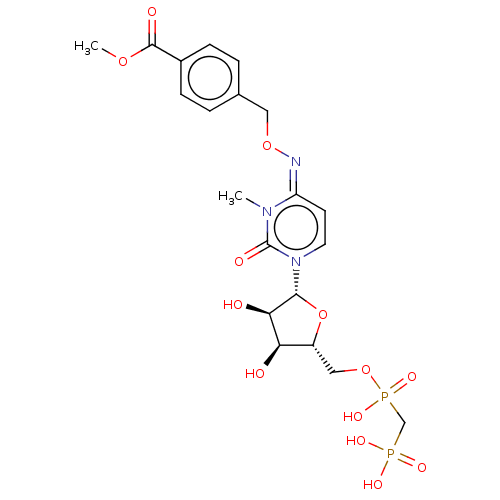
(CHEMBL5086866)Show SMILES CCN(CC)CC.CCN(CC)CC.COC(=O)c1ccc(CO\N=c2\ccn([C@@H]3O[C@H](COP(O)(=O)CP(O)(O)=O)[C@@H](O)[C@H]3O)c(=O)n2C)cc1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.848 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human soluble CD73 assessed as inhibition constant |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01852
BindingDB Entry DOI: 10.7270/Q2ZS31D5 |
More data for this
Ligand-Target Pair | |
5'-nucleotidase
(Homo sapiens (Human)) | BDBM50583389
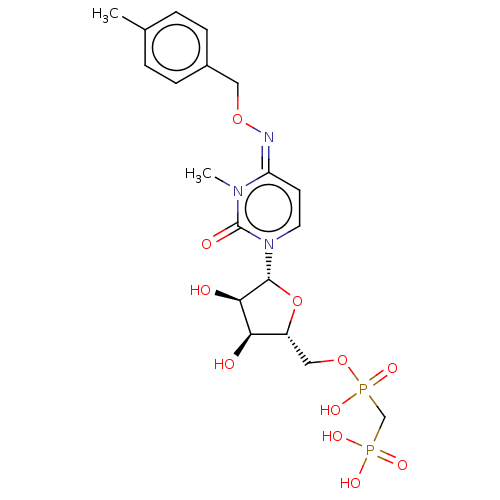
(CHEMBL5076916)Show SMILES CCN(CC)CC.CCN(CC)CC.Cc1ccc(CO\N=c2\ccn([C@@H]3O[C@H](COP(O)(=O)CP(O)(O)=O)[C@@H](O)[C@H]3O)c(=O)n2C)cc1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.894 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human soluble CD73 assessed as inhibition constant |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01852
BindingDB Entry DOI: 10.7270/Q2ZS31D5 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50561602

(CHEMBL4752619)Show SMILES [H][C@]12C[C@]1([C@@H](O)[C@@H](O)[C@@H]2n1cnc2c(NC)cc(nc12)C#Cc1ccc(F)s1)C(=O)NC |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.896 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125I]N6-(4-amino-3-iodobenzyl)adenosine-5'-N-methyluronamide from human adrenergic A3 receptor expressed in CHO cell membranes asses... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00637
BindingDB Entry DOI: 10.7270/Q20C50H1 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Mus musculus) | BDBM50602689

(CHEMBL5178712)Show SMILES CNC(=O)[C@@]12C[C@@H]1[C@H]([C@H](O)[C@@H]2O)n1cnc2c(NCCc3ccc(O)c(O)c3)nc(nc12)C#Cc1ccc(Cl)s1 |r| | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113983
BindingDB Entry DOI: 10.7270/Q29027V8 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50118812

((2S,3S,4R,5R)-3,4-Dihydroxy-5-[6-(3-iodo-benzylami...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCc3cccc(I)c3)ncnc12 |r| Show InChI InChI=1S/C18H19IN6O4/c1-20-17(28)14-12(26)13(27)18(29-14)25-8-24-11-15(22-7-23-16(11)25)21-6-9-3-2-4-10(19)5-9/h2-5,7-8,12-14,18,26-27H,6H2,1H3,(H,20,28)(H,21,22,23)/t12-,13+,14-,18+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]HMRS7799 from human adenosine A3 receptor expressed in HEK293 cells by radioligand binding assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00685
BindingDB Entry DOI: 10.7270/Q2930Z2X |
More data for this
Ligand-Target Pair | |
5'-nucleotidase
(Homo sapiens (Human)) | BDBM50583391
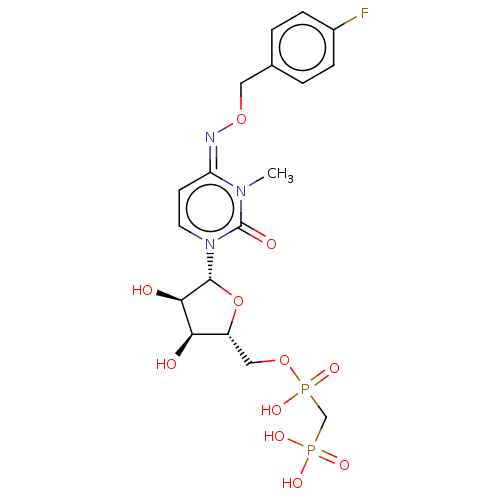
(CHEMBL5081442)Show SMILES CCN(CC)CC.CCN(CC)CC.Cn1c(=O)n(cc\c1=N\OCc1ccc(F)cc1)[C@@H]1O[C@H](COP(O)(=O)CP(O)(O)=O)[C@@H](O)[C@H]1O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human soluble CD73 assessed as inhibition constant |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01852
BindingDB Entry DOI: 10.7270/Q2ZS31D5 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50561598

(CHEMBL4791368)Show SMILES [H][C@]12C[C@]1([C@@H](O)[C@@H](O)[C@@H]2n1cnc2c(NCCC)nc(nc12)C#Cc1ccc(F)s1)C(=O)NC |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125I]N6-(4-amino-3-iodobenzyl)adenosine-5'-N-methyluronamide from human adrenergic A3 receptor expressed in CHO cell membranes asses... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00637
BindingDB Entry DOI: 10.7270/Q20C50H1 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Mus musculus) | BDBM50517304
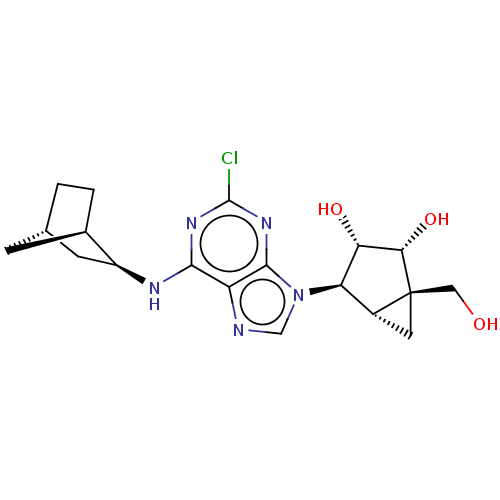
(CHEMBL4587144)Show SMILES [H][C@]12C[C@@]1(CO)[C@@H](O)[C@@H](O)[C@@H]2n1cnc2c(N[C@H]3C[C@@]4([H])CC[C@]3([H])C4)nc(Cl)nc12 |r,TLB:16:17:22.21:25| Show InChI InChI=1S/C19H24ClN5O3/c20-18-23-16(22-11-4-8-1-2-9(11)3-8)12-17(24-18)25(7-21-12)13-10-5-19(10,6-26)15(28)14(13)27/h7-11,13-15,26-28H,1-6H2,(H,22,23,24)/t8-,9+,10+,11-,13+,14-,15-,19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical College of Wisconsin
Curated by ChEMBL
| Assay Description
Displacement of [3H]N6-R-phenylisopropyladenosine from mouse A1A adenosine receptor expressed in CHO cell membranes after 60 mins by scintillation pr... |
J Med Chem 62: 1502-1522 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01662
BindingDB Entry DOI: 10.7270/Q2TM7FGK |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Mus musculus) | BDBM50517290
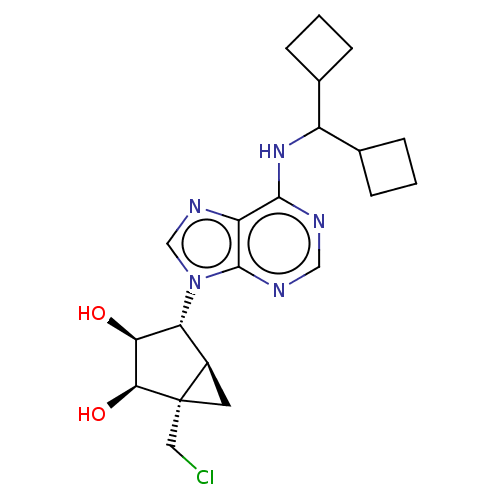
(CHEMBL4554295)Show SMILES [H][C@]12C[C@@]1(CCl)[C@@H](O)[C@@H](O)[C@@H]2n1cnc2c(NC(C3CCC3)C3CCC3)ncnc12 |r| Show InChI InChI=1S/C21H28ClN5O2/c22-8-21-7-13(21)16(17(28)18(21)29)27-10-25-15-19(23-9-24-20(15)27)26-14(11-3-1-4-11)12-5-2-6-12/h9-14,16-18,28-29H,1-8H2,(H,23,24,26)/t13-,16-,17+,18+,21+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical College of Wisconsin
Curated by ChEMBL
| Assay Description
Displacement of [3H]N6-R-phenylisopropyladenosine from mouse A1A adenosine receptor expressed in CHO cell membranes after 60 mins by scintillation pr... |
J Med Chem 62: 1502-1522 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01662
BindingDB Entry DOI: 10.7270/Q2TM7FGK |
More data for this
Ligand-Target Pair | |
5'-nucleotidase
(Homo sapiens (Human)) | BDBM50583395
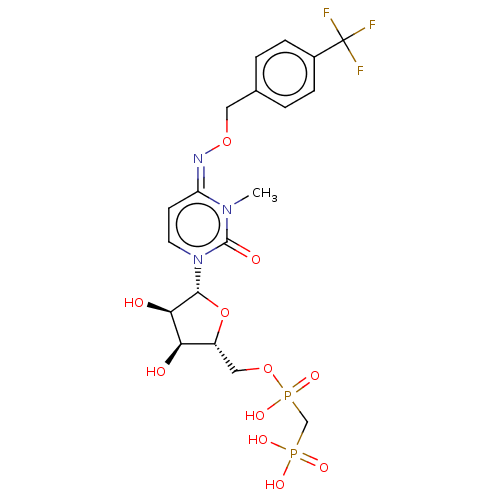
(CHEMBL5084248)Show SMILES CCN(CC)CC.CCN(CC)CC.Cn1c(=O)n(cc\c1=N\OCc1ccc(cc1)C(F)(F)F)[C@@H]1O[C@H](COP(O)(=O)CP(O)(O)=O)[C@@H](O)[C@H]1O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human soluble CD73 assessed as inhibition constant |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01852
BindingDB Entry DOI: 10.7270/Q2ZS31D5 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50517307

(CHEMBL4463802)Show SMILES [H][C@]12C[C@]1([C@@H](O)[C@@H](O)[C@@H]2n1cnc2c(N[C@H]3C[C@@]4([H])CC[C@]3([H])C4)nc(Cl)nc12)C(=O)NC1CC1 |r,TLB:14:15:20.19:23| Show InChI InChI=1S/C22H27ClN6O3/c23-21-27-18(26-13-6-9-1-2-10(13)5-9)14-19(28-21)29(8-24-14)15-12-7-22(12,17(31)16(15)30)20(32)25-11-3-4-11/h8-13,15-17,30-31H,1-7H2,(H,25,32)(H,26,27,28)/t9-,10+,12+,13-,15+,16-,17-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical College of Wisconsin
Curated by ChEMBL
| Assay Description
Displacement of [3H]N6-R-phenylisopropyladenosine from human A1A adenosine receptor expressed in CHO cell membranes after 60 mins by scintillation pr... |
J Med Chem 62: 1502-1522 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01662
BindingDB Entry DOI: 10.7270/Q2TM7FGK |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50437189
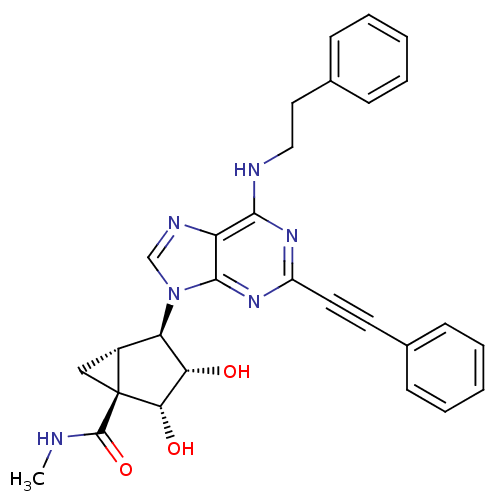
(CHEMBL2402024)Show SMILES CNC(=O)[C@@]12C[C@@H]1[C@H]([C@H](O)[C@@H]2O)n1cnc2c(NCCc3ccccc3)nc(nc12)C#Cc1ccccc1 |r| Show InChI InChI=1S/C29H28N6O3/c1-30-28(38)29-16-20(29)23(24(36)25(29)37)35-17-32-22-26(31-15-14-19-10-6-3-7-11-19)33-21(34-27(22)35)13-12-18-8-4-2-5-9-18/h2-11,17,20,23-25,36-37H,14-16H2,1H3,(H,30,38)(H,31,33,34)/t20-,23-,24+,25+,29+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113983
BindingDB Entry DOI: 10.7270/Q29027V8 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50470471
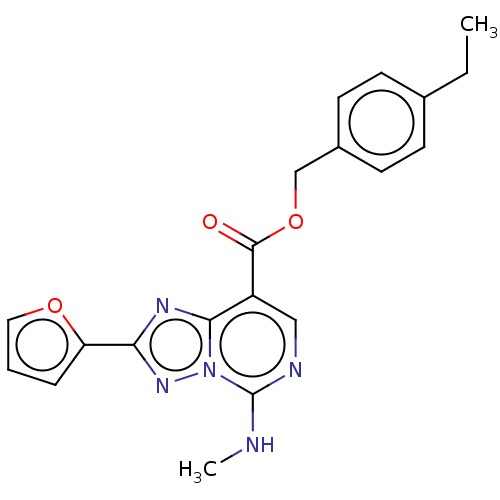
(CHEMBL4294479)Show SMILES CCc1ccc(COC(=O)c2cnc(NC)n3nc(nc23)-c2ccco2)cc1 Show InChI InChI=1S/C20H19N5O3/c1-3-13-6-8-14(9-7-13)12-28-19(26)15-11-22-20(21-2)25-18(15)23-17(24-25)16-5-4-10-27-16/h4-11H,3,12H2,1-2H3,(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Trieste
Curated by ChEMBL
| Assay Description
Displacement of [3H]HEMADO from human adenosine A3 receptor expressed in CHO cell membranes after 3 hrs by micro beta scintillation counting method |
Eur J Med Chem 157: 837-851 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.042
BindingDB Entry DOI: 10.7270/Q2H41V45 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Mus musculus) | BDBM50602679

(CHEMBL5180037)Show SMILES CNC(=O)[C@@]12C[C@@H]1[C@H]([C@H](O)[C@@H]2O)n1cnc2c(NCCc3ccc(O)c(OC)c3)nc(nc12)C#Cc1cccs1 |r| | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113983
BindingDB Entry DOI: 10.7270/Q29027V8 |
More data for this
Ligand-Target Pair | |
5'-nucleotidase
(Homo sapiens (Human)) | BDBM50583388
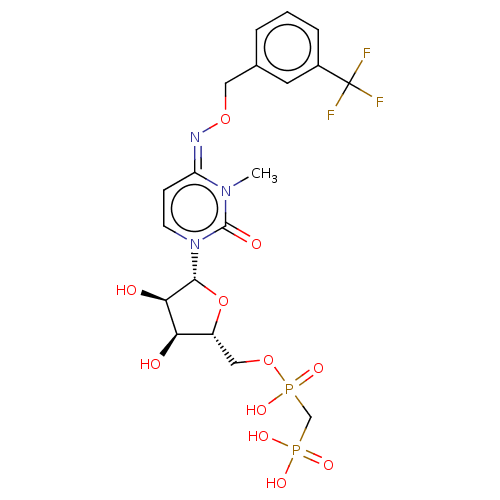
(CHEMBL5081604)Show SMILES CCN(CC)CC.CCN(CC)CC.Cn1c(=O)n(cc\c1=N\OCc1cccc(c1)C(F)(F)F)[C@@H]1O[C@H](COP(O)(=O)CP(O)(O)=O)[C@@H](O)[C@H]1O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human soluble CD73 assessed as inhibition constant |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01852
BindingDB Entry DOI: 10.7270/Q2ZS31D5 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Mus musculus) | BDBM50602690

(CHEMBL5187659)Show SMILES CNC(=O)[C@@]12C[C@@H]1[C@H]([C@H](O)[C@@H]2O)n1cnc2c(NCCc3ccc(O)c(OC)c3)nc(nc12)C#Cc1ccc(Br)s1 |r| | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113983
BindingDB Entry DOI: 10.7270/Q29027V8 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM21221

((2S,3S,4R,5R)-5-(2-chloro-6-{[(3-iodophenyl)methyl...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCc3cccc(I)c3)nc(Cl)nc12 Show InChI InChI=1S/C18H18ClIN6O4/c1-21-16(29)13-11(27)12(28)17(30-13)26-7-23-10-14(24-18(19)25-15(10)26)22-6-8-3-2-4-9(20)5-8/h2-5,7,11-13,17,27-28H,6H2,1H3,(H,21,29)(H,22,24,25)/t11-,12+,13-,17+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human A3AR assessed as inhibitor constant |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00637
BindingDB Entry DOI: 10.7270/Q20C50H1 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM21221

((2S,3S,4R,5R)-5-(2-chloro-6-{[(3-iodophenyl)methyl...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCc3cccc(I)c3)nc(Cl)nc12 Show InChI InChI=1S/C18H18ClIN6O4/c1-21-16(29)13-11(27)12(28)17(30-13)26-7-23-10-14(24-18(19)25-15(10)26)22-6-8-3-2-4-9(20)5-8/h2-5,7,11-13,17,27-28H,6H2,1H3,(H,21,29)(H,22,24,25)/t11-,12+,13-,17+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Trieste
Curated by ChEMBL
| Assay Description
Displacement of [3H]HEMADO from human adenosine A3 receptor expressed in CHO cell membranes after 3 hrs by micro beta scintillation counting method |
Eur J Med Chem 157: 837-851 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.042
BindingDB Entry DOI: 10.7270/Q2H41V45 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Mus musculus) | BDBM50602675

(CHEMBL5200403)Show SMILES CNC(=O)[C@@]12C[C@@H]1[C@H]([C@H](O)[C@@H]2O)n1cnc2c(NCCc3ccc(O)c(OC)c3)nc(nc12)C#Cc1ccccc1 |r| | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113983
BindingDB Entry DOI: 10.7270/Q29027V8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data