 Found 73 hits with Last Name = 'velvadapu' and Initial = 'v'
Found 73 hits with Last Name = 'velvadapu' and Initial = 'v' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM370419
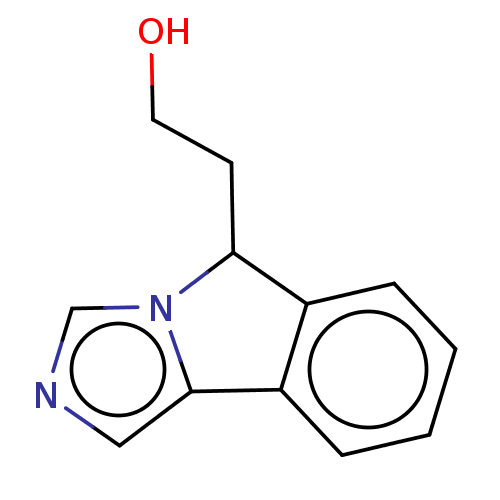
(2-(5H-imidazo[5,1-a]isoindol-5-yl)ethanol | US1023...)Show InChI InChI=1S/C12H12N2O/c15-6-5-11-9-3-1-2-4-10(9)12-7-13-8-14(11)12/h1-4,7-8,11,15H,5-6H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human TDO using L-tryptophan as substrate preincubated for 5 mins followed by substrate addition and measured after 15 mins... |
ACS Med Chem Lett 11: 541-549 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00004
BindingDB Entry DOI: 10.7270/Q2RR22JS |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50511732
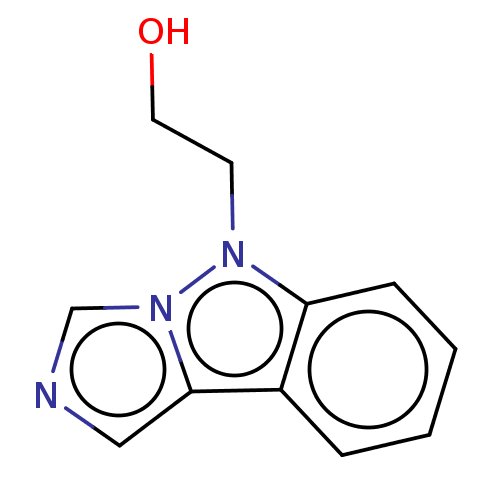
(CHEMBL4434743) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human TDO using L-tryptophan as substrate preincubated for 5 mins followed by substrate addition and measured after 15 mins... |
ACS Med Chem Lett 11: 541-549 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00004
BindingDB Entry DOI: 10.7270/Q2RR22JS |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50511733
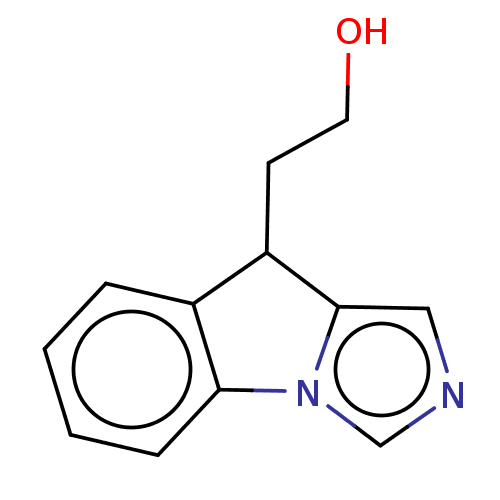
(CHEMBL4443553)Show InChI InChI=1S/C12H12N2O/c15-6-5-10-9-3-1-2-4-11(9)14-8-13-7-12(10)14/h1-4,7-8,10,15H,5-6H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human TDO using L-tryptophan as substrate preincubated for 5 mins followed by substrate addition and measured after 15 mins... |
ACS Med Chem Lett 11: 541-549 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00004
BindingDB Entry DOI: 10.7270/Q2RR22JS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50511717
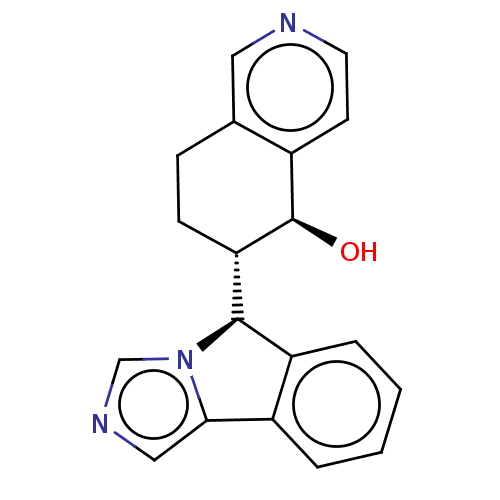
(CHEMBL4437257)Show SMILES [H][C@@]1(c2ccccc2-c2cncn12)[C@]1([H])CCc2cnccc2[C@H]1O |r| Show InChI InChI=1S/C19H17N3O/c23-19-13-7-8-20-9-12(13)5-6-16(19)18-15-4-2-1-3-14(15)17-10-21-11-22(17)18/h1-4,7-11,16,18-19,23H,5-6H2/t16-,18+,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
ACS Med Chem Lett 11: 541-549 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00004
BindingDB Entry DOI: 10.7270/Q2RR22JS |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM370555
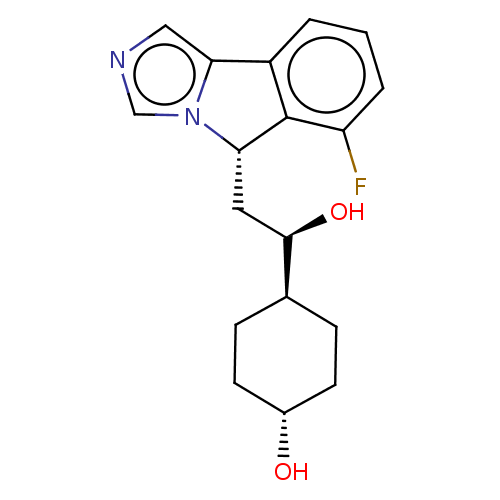
((1R,4r)-4-((R)-2-((S)-6-fluoro-5H-imidazo[5,1- a]i...)Show SMILES O[C@H](C[C@H]1c2c(cccc2F)-c2cncn12)[C@H]1CC[C@H](O)CC1 |r,wU:19.22,wD:3.2,1.0,16.19,(-.99,-3.03,;-.22,-1.7,;-.99,-.37,;-2.53,-.37,;-3.37,.92,;-4.85,.53,;-5.94,1.61,;-5.54,3.1,;-4.06,3.5,;-2.97,2.41,;-1.48,2.81,;-4.93,-1.01,;-5.9,-2.21,;-5.06,-3.5,;-3.58,-3.1,;-3.5,-1.56,;1.32,-1.7,;2.09,-3.03,;3.63,-3.03,;4.4,-1.7,;5.94,-1.7,;3.63,-.37,;2.09,-.37,)| Show InChI InChI=1S/C18H21FN2O2/c19-14-3-1-2-13-16-9-20-10-21(16)15(18(13)14)8-17(23)11-4-6-12(22)7-5-11/h1-3,9-12,15,17,22-23H,4-8H2/t11-,12-,15-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 (unknown origin) |
ACS Med Chem Lett 11: 541-549 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00004
BindingDB Entry DOI: 10.7270/Q2RR22JS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50511719
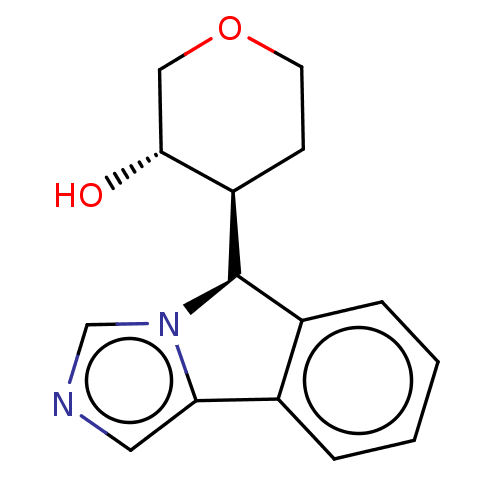
(CHEMBL4548068)Show SMILES [H][C@@]1(c2ccccc2-c2cncn12)[C@]1([H])CCOC[C@H]1O |r| Show InChI InChI=1S/C15H16N2O2/c18-14-8-19-6-5-12(14)15-11-4-2-1-3-10(11)13-7-16-9-17(13)15/h1-4,7,9,12,14-15,18H,5-6,8H2/t12-,14-,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
ACS Med Chem Lett 11: 541-549 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00004
BindingDB Entry DOI: 10.7270/Q2RR22JS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50511715
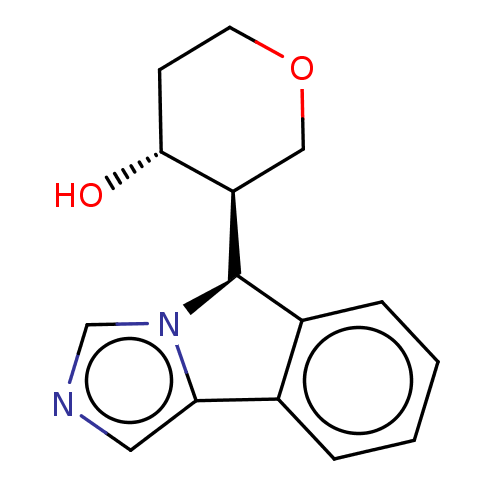
(CHEMBL4540245)Show SMILES [H][C@@]1(c2ccccc2-c2cncn12)[C@]1([H])COCC[C@H]1O |r| Show InChI InChI=1S/C15H16N2O2/c18-14-5-6-19-8-12(14)15-11-4-2-1-3-10(11)13-7-16-9-17(13)15/h1-4,7,9,12,14-15,18H,5-6,8H2/t12-,14-,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
ACS Med Chem Lett 11: 541-549 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00004
BindingDB Entry DOI: 10.7270/Q2RR22JS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50511728
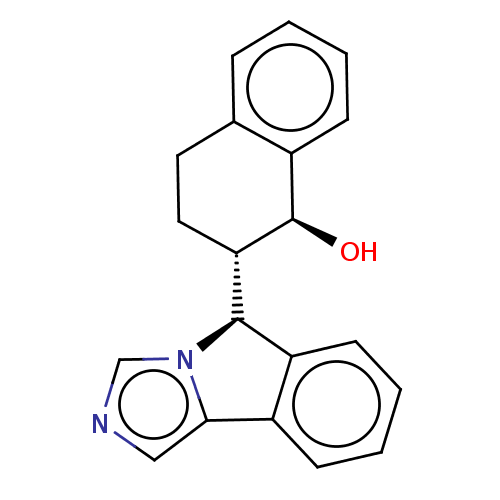
(CHEMBL4577396)Show SMILES [H][C@@]1(c2ccccc2-c2cncn12)[C@]1([H])CCc2ccccc2[C@H]1O |r| Show InChI InChI=1S/C20H18N2O/c23-20-14-6-2-1-5-13(14)9-10-17(20)19-16-8-4-3-7-15(16)18-11-21-12-22(18)19/h1-8,11-12,17,19-20,23H,9-10H2/t17-,19+,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 840 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
ACS Med Chem Lett 11: 541-549 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00004
BindingDB Entry DOI: 10.7270/Q2RR22JS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50511729
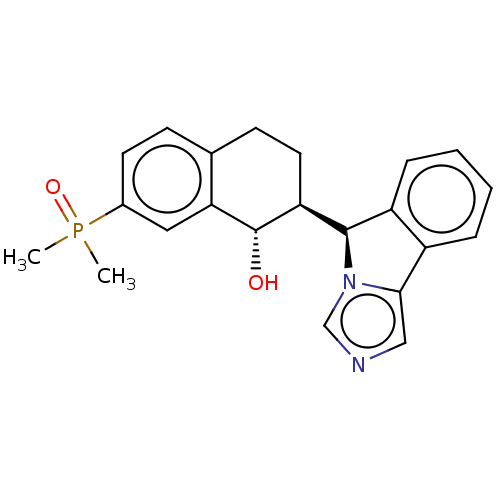
(CHEMBL4589215)Show SMILES [H][C@@]1(c2ccccc2-c2cncn12)[C@]1([H])CCc2ccc(cc2[C@H]1O)P(C)(C)=O |r| Show InChI InChI=1S/C22H23N2O2P/c1-27(2,26)15-9-7-14-8-10-18(22(25)19(14)11-15)21-17-6-4-3-5-16(17)20-12-23-13-24(20)21/h3-7,9,11-13,18,21-22,25H,8,10H2,1-2H3/t18-,21+,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
ACS Med Chem Lett 11: 541-549 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00004
BindingDB Entry DOI: 10.7270/Q2RR22JS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50511727
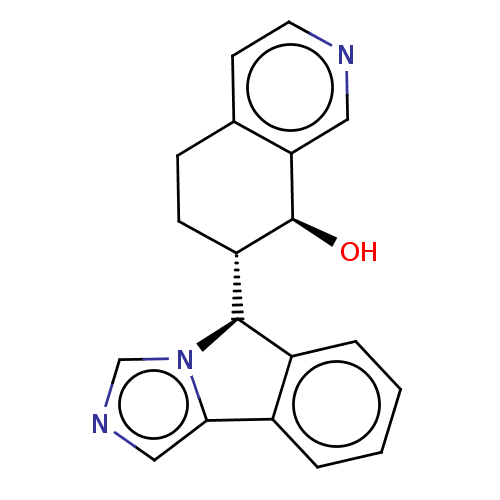
(CHEMBL4447185)Show SMILES [H][C@@]1(c2ccccc2-c2cncn12)[C@]1([H])CCc2ccncc2[C@H]1O |r| Show InChI InChI=1S/C19H17N3O/c23-19-15(6-5-12-7-8-20-9-16(12)19)18-14-4-2-1-3-13(14)17-10-21-11-22(17)18/h1-4,7-11,15,18-19,23H,5-6H2/t15-,18+,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
ACS Med Chem Lett 11: 541-549 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00004
BindingDB Entry DOI: 10.7270/Q2RR22JS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50511716
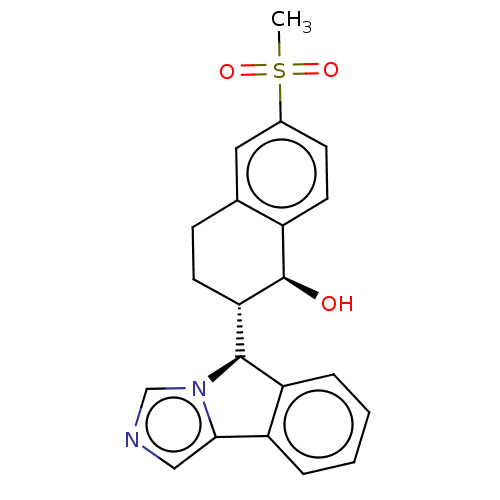
(CHEMBL4539108)Show SMILES [H][C@@]1(c2ccccc2-c2cncn12)[C@]1([H])CCc2cc(ccc2[C@H]1O)S(C)(=O)=O |r| Show InChI InChI=1S/C21H20N2O3S/c1-27(25,26)14-7-9-15-13(10-14)6-8-18(21(15)24)20-17-5-3-2-4-16(17)19-11-22-12-23(19)20/h2-5,7,9-12,18,20-21,24H,6,8H2,1H3/t18-,20+,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
ACS Med Chem Lett 11: 541-549 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00004
BindingDB Entry DOI: 10.7270/Q2RR22JS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50511726
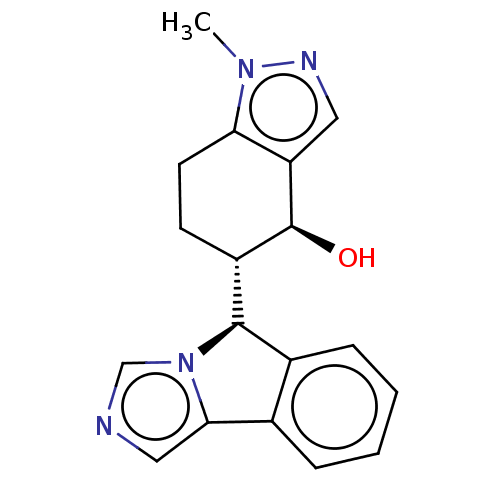
(CHEMBL4440730)Show SMILES [H][C@@]1(c2ccccc2-c2cncn12)[C@]1([H])CCc2c(cnn2C)[C@H]1O |r| Show InChI InChI=1S/C18H18N4O/c1-21-15-7-6-13(18(23)14(15)8-20-21)17-12-5-3-2-4-11(12)16-9-19-10-22(16)17/h2-5,8-10,13,17-18,23H,6-7H2,1H3/t13-,17+,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
ACS Med Chem Lett 11: 541-549 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00004
BindingDB Entry DOI: 10.7270/Q2RR22JS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50511710
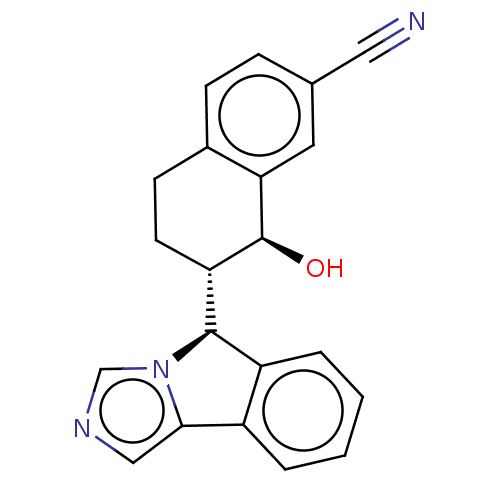
(CHEMBL4541824)Show SMILES [H][C@@]1(c2ccccc2-c2cncn12)[C@]1([H])CCc2ccc(cc2[C@H]1O)C#N |r| Show InChI InChI=1S/C21H17N3O/c22-10-13-5-6-14-7-8-17(21(25)18(14)9-13)20-16-4-2-1-3-15(16)19-11-23-12-24(19)20/h1-6,9,11-12,17,20-21,25H,7-8H2/t17-,20+,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
ACS Med Chem Lett 11: 541-549 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00004
BindingDB Entry DOI: 10.7270/Q2RR22JS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50511731
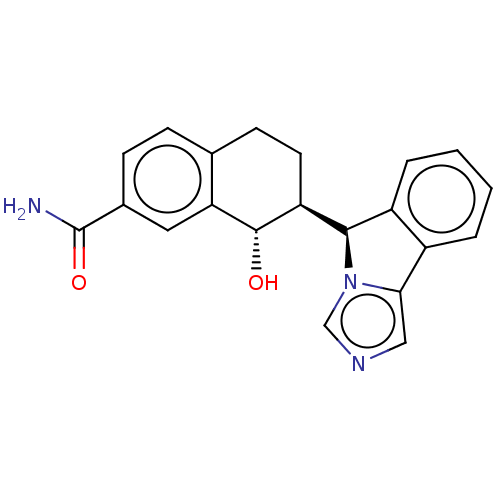
(CHEMBL4544695)Show SMILES [H][C@@]1(c2ccccc2-c2cncn12)[C@]1([H])CCc2ccc(cc2[C@H]1O)C(N)=O |r| Show InChI InChI=1S/C21H19N3O2/c22-21(26)13-6-5-12-7-8-16(20(25)17(12)9-13)19-15-4-2-1-3-14(15)18-10-23-11-24(18)19/h1-6,9-11,16,19-20,25H,7-8H2,(H2,22,26)/t16-,19+,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
ACS Med Chem Lett 11: 541-549 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00004
BindingDB Entry DOI: 10.7270/Q2RR22JS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50511714
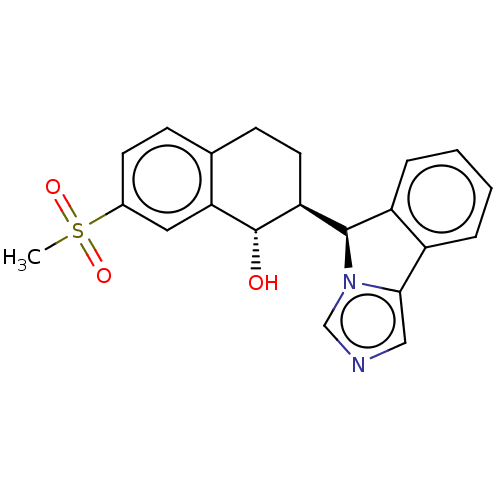
(CHEMBL4520696)Show SMILES [H][C@@]1(c2ccccc2-c2cncn12)[C@]1([H])CCc2ccc(cc2[C@H]1O)S(C)(=O)=O |r| Show InChI InChI=1S/C21H20N2O3S/c1-27(25,26)14-8-6-13-7-9-17(21(24)18(13)10-14)20-16-5-3-2-4-15(16)19-11-22-12-23(19)20/h2-6,8,10-12,17,20-21,24H,7,9H2,1H3/t17-,20+,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
ACS Med Chem Lett 11: 541-549 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00004
BindingDB Entry DOI: 10.7270/Q2RR22JS |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50511736
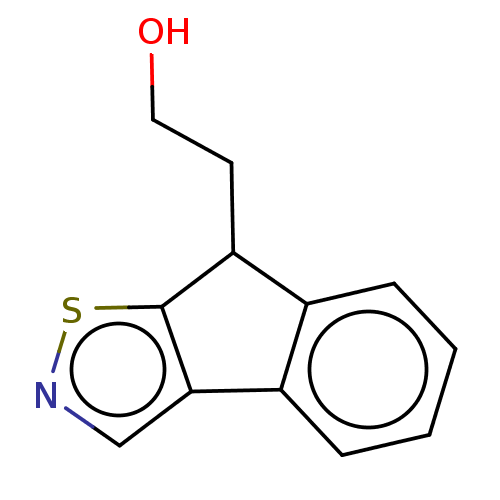
(CHEMBL4572482)Show InChI InChI=1S/C12H11NOS/c14-6-5-10-8-3-1-2-4-9(8)11-7-13-15-12(10)11/h1-4,7,10,14H,5-6H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human TDO using L-tryptophan as substrate preincubated for 5 mins followed by substrate addition and measured after 15 mins... |
ACS Med Chem Lett 11: 541-549 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00004
BindingDB Entry DOI: 10.7270/Q2RR22JS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50511730
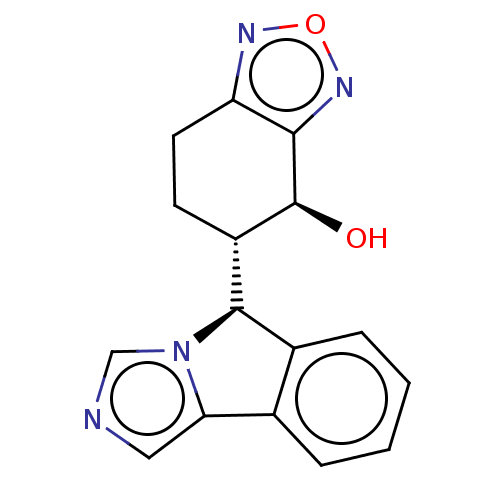
(CHEMBL4590789)Show SMILES [H][C@@]1(c2ccccc2-c2cncn12)[C@]1([H])CCc2nonc2[C@H]1O |r| Show InChI InChI=1S/C16H14N4O2/c21-16-11(5-6-12-14(16)19-22-18-12)15-10-4-2-1-3-9(10)13-7-17-8-20(13)15/h1-4,7-8,11,15-16,21H,5-6H2/t11-,15+,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
ACS Med Chem Lett 11: 541-549 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00004
BindingDB Entry DOI: 10.7270/Q2RR22JS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50511718
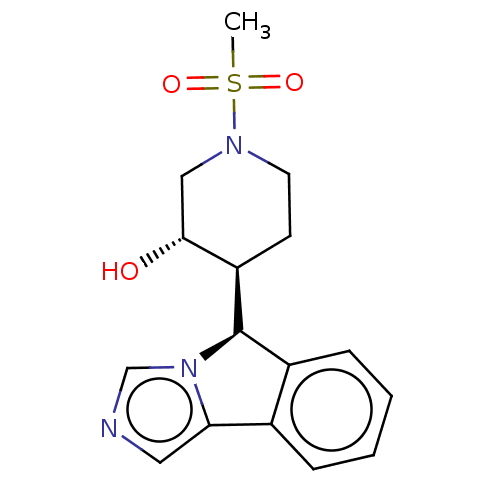
(CHEMBL4542307)Show SMILES [H][C@@]1(c2ccccc2-c2cncn12)[C@]1([H])CCN(C[C@H]1O)S(C)(=O)=O |r| Show InChI InChI=1S/C16H19N3O3S/c1-23(21,22)18-7-6-13(15(20)9-18)16-12-5-3-2-4-11(12)14-8-17-10-19(14)16/h2-5,8,10,13,15-16,20H,6-7,9H2,1H3/t13-,15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
ACS Med Chem Lett 11: 541-549 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00004
BindingDB Entry DOI: 10.7270/Q2RR22JS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50511712
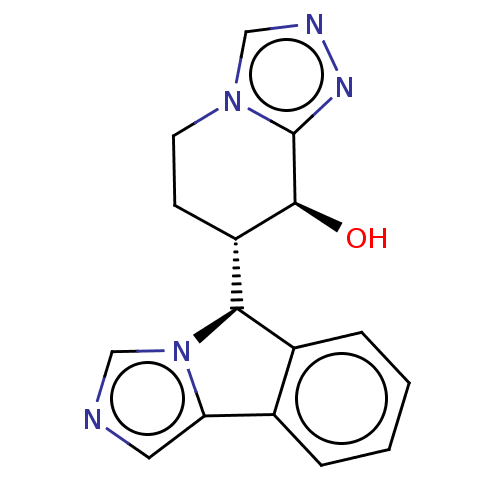
(CHEMBL4529817)Show SMILES [H][C@@]1(c2ccccc2-c2cncn12)[C@]1([H])CCn2cnnc2[C@H]1O |r| Show InChI InChI=1S/C16H15N5O/c22-15-12(5-6-20-9-18-19-16(15)20)14-11-4-2-1-3-10(11)13-7-17-8-21(13)14/h1-4,7-9,12,14-15,22H,5-6H2/t12-,14+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
ACS Med Chem Lett 11: 541-549 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00004
BindingDB Entry DOI: 10.7270/Q2RR22JS |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50126143

(Epacadostat | INCB-024360)Show SMILES NS(=O)(=O)NCCNc1nonc1\C(Nc1ccc(F)c(Br)c1)=N\O Show InChI InChI=1S/C11H13BrFN7O4S/c12-7-5-6(1-2-8(7)13)17-11(18-21)9-10(20-24-19-9)15-3-4-16-25(14,22)23/h1-2,5,16,21H,3-4H2,(H,15,20)(H,17,18)(H2,14,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of TDO (unknown origin) |
ACS Med Chem Lett 11: 541-549 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00004
BindingDB Entry DOI: 10.7270/Q2RR22JS |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50285416
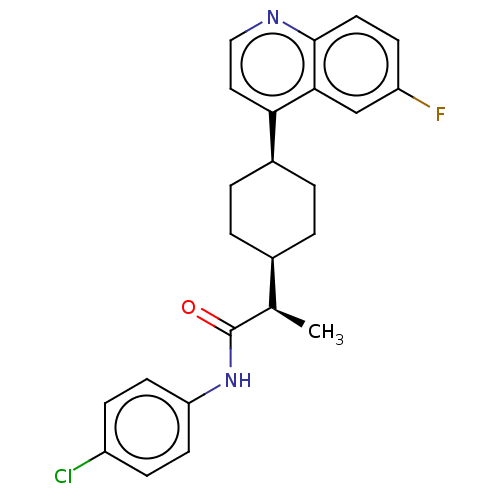
(CHEMBL4161733)Show SMILES [H][C@]1(CC[C@@H](CC1)c1ccnc2ccc(F)cc12)[C@@H](C)C(=O)Nc1ccc(Cl)cc1 |r,wU:18.21,1.0,wD:4.7,(66.16,-9.22,;66.17,-7.68,;66.16,-6.15,;67.5,-5.38,;68.84,-6.15,;68.83,-7.69,;67.5,-8.46,;70.18,-5.39,;71.5,-6.16,;72.84,-5.4,;72.84,-3.85,;71.5,-3.08,;71.5,-1.55,;70.17,-.79,;68.84,-1.56,;67.5,-.8,;68.85,-3.09,;70.18,-3.85,;64.83,-8.46,;64.84,-10,;63.5,-7.69,;63.49,-6.14,;62.16,-8.46,;60.83,-7.69,;59.49,-8.47,;58.15,-7.69,;58.16,-6.14,;56.82,-5.38,;59.49,-5.38,;60.83,-6.14,)| Show InChI InChI=1S/C24H24ClFN2O/c1-15(24(29)28-20-9-6-18(25)7-10-20)16-2-4-17(5-3-16)21-12-13-27-23-11-8-19(26)14-22(21)23/h6-17H,2-5H2,1H3,(H,28,29)/t15-,16-,17+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of TDO (unknown origin) |
ACS Med Chem Lett 11: 541-549 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00004
BindingDB Entry DOI: 10.7270/Q2RR22JS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50511711
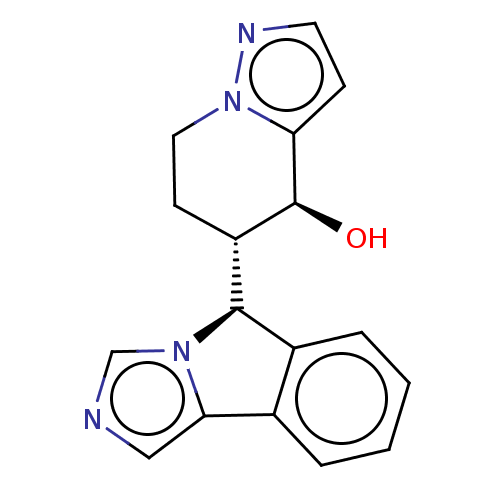
(CHEMBL4439294)Show SMILES [H][C@@]1(c2ccccc2-c2cncn12)[C@]1([H])CCn2nccc2[C@H]1O |r| Show InChI InChI=1S/C17H16N4O/c22-17-13(6-8-21-14(17)5-7-19-21)16-12-4-2-1-3-11(12)15-9-18-10-20(15)16/h1-5,7,9-10,13,16-17,22H,6,8H2/t13-,16+,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
ACS Med Chem Lett 11: 541-549 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00004
BindingDB Entry DOI: 10.7270/Q2RR22JS |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM309529

(3-(5-fluoro-1H-indol-3- yl)pyrrolidine-2,5-dione |...) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of TDO (unknown origin) |
ACS Med Chem Lett 11: 541-549 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00004
BindingDB Entry DOI: 10.7270/Q2RR22JS |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50511734
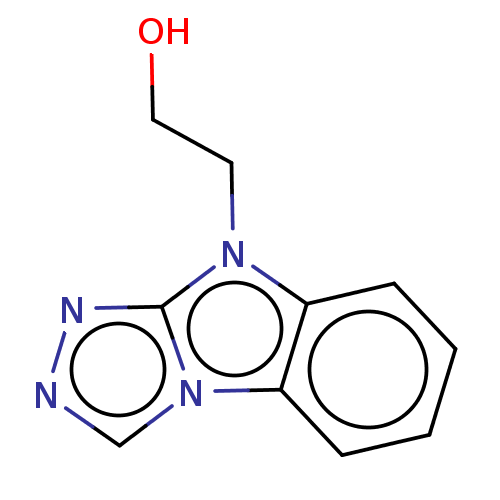
(CHEMBL4441431)Show InChI InChI=1S/C10H10N4O/c15-6-5-13-8-3-1-2-4-9(8)14-7-11-12-10(13)14/h1-4,7,15H,5-6H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human TDO using L-tryptophan as substrate preincubated for 5 mins followed by substrate addition and measured after 15 mins... |
ACS Med Chem Lett 11: 541-549 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00004
BindingDB Entry DOI: 10.7270/Q2RR22JS |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50511735
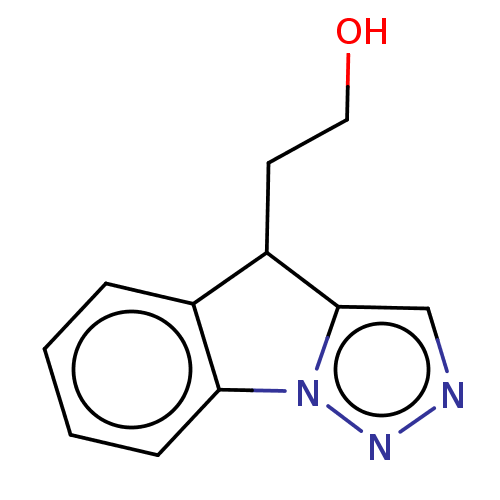
(CHEMBL4593520)Show InChI InChI=1S/C11H11N3O/c15-6-5-9-8-3-1-2-4-10(8)14-11(9)7-12-13-14/h1-4,7,9,15H,5-6H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human TDO using L-tryptophan as substrate preincubated for 5 mins followed by substrate addition and measured after 15 mins... |
ACS Med Chem Lett 11: 541-549 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00004
BindingDB Entry DOI: 10.7270/Q2RR22JS |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50511712

(CHEMBL4529817)Show SMILES [H][C@@]1(c2ccccc2-c2cncn12)[C@]1([H])CCn2cnnc2[C@H]1O |r| Show InChI InChI=1S/C16H15N5O/c22-15-12(5-6-20-9-18-19-16(15)20)14-11-4-2-1-3-10(11)13-7-17-8-21(13)14/h1-4,7-9,12,14-15,22H,5-6H2/t12-,14+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in human A172 cells after 24 hrs in presence of IFNgamma by NFK green reagent based assay |
ACS Med Chem Lett 11: 541-549 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00004
BindingDB Entry DOI: 10.7270/Q2RR22JS |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50511713
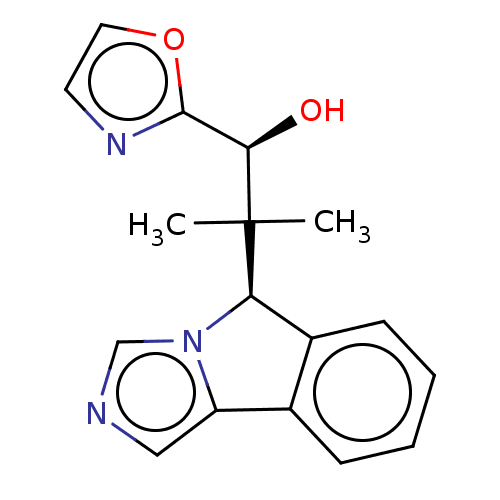
(CHEMBL4467673)Show SMILES [H][C@@]1(c2ccccc2-c2cncn12)C(C)(C)[C@H](O)c1ncco1 |r| Show InChI InChI=1S/C17H17N3O2/c1-17(2,15(21)16-19-7-8-22-16)14-12-6-4-3-5-11(12)13-9-18-10-20(13)14/h3-10,14-15,21H,1-2H3/t14-,15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 71 | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of TDO in human SW48 cells after 24 hrs by NFK green reagent based assay |
ACS Med Chem Lett 11: 541-549 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00004
BindingDB Entry DOI: 10.7270/Q2RR22JS |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50511714

(CHEMBL4520696)Show SMILES [H][C@@]1(c2ccccc2-c2cncn12)[C@]1([H])CCc2ccc(cc2[C@H]1O)S(C)(=O)=O |r| Show InChI InChI=1S/C21H20N2O3S/c1-27(25,26)14-8-6-13-7-9-17(21(24)18(13)10-14)20-16-5-3-2-4-15(16)19-11-22-12-23(19)20/h2-6,8,10-12,17,20-21,24H,7,9H2,1H3/t17-,20+,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 39 | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of TDO in human SW48 cells after 24 hrs by NFK green reagent based assay |
ACS Med Chem Lett 11: 541-549 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00004
BindingDB Entry DOI: 10.7270/Q2RR22JS |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50511715

(CHEMBL4540245)Show SMILES [H][C@@]1(c2ccccc2-c2cncn12)[C@]1([H])COCC[C@H]1O |r| Show InChI InChI=1S/C15H16N2O2/c18-14-5-6-19-8-12(14)15-11-4-2-1-3-10(11)13-7-16-9-17(13)15/h1-4,7,9,12,14-15,18H,5-6,8H2/t12-,14-,15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 210 | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of TDO in human SW48 cells after 24 hrs by NFK green reagent based assay |
ACS Med Chem Lett 11: 541-549 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00004
BindingDB Entry DOI: 10.7270/Q2RR22JS |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50511710

(CHEMBL4541824)Show SMILES [H][C@@]1(c2ccccc2-c2cncn12)[C@]1([H])CCc2ccc(cc2[C@H]1O)C#N |r| Show InChI InChI=1S/C21H17N3O/c22-10-13-5-6-14-7-8-17(21(25)18(14)9-13)20-16-4-2-1-3-15(16)19-11-23-12-24(19)20/h1-6,9,11-12,17,20-21,25H,7-8H2/t17-,20+,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 60 | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of TDO in human SW48 cells after 24 hrs by NFK green reagent based assay |
ACS Med Chem Lett 11: 541-549 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00004
BindingDB Entry DOI: 10.7270/Q2RR22JS |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50511716

(CHEMBL4539108)Show SMILES [H][C@@]1(c2ccccc2-c2cncn12)[C@]1([H])CCc2cc(ccc2[C@H]1O)S(C)(=O)=O |r| Show InChI InChI=1S/C21H20N2O3S/c1-27(25,26)14-7-9-15-13(10-14)6-8-18(21(15)24)20-17-5-3-2-4-16(17)19-11-22-12-23(19)20/h2-5,7,9-12,18,20-21,24H,6,8H2,1H3/t18-,20+,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 65 | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of TDO in human SW48 cells after 24 hrs by NFK green reagent based assay |
ACS Med Chem Lett 11: 541-549 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00004
BindingDB Entry DOI: 10.7270/Q2RR22JS |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50511717

(CHEMBL4437257)Show SMILES [H][C@@]1(c2ccccc2-c2cncn12)[C@]1([H])CCc2cnccc2[C@H]1O |r| Show InChI InChI=1S/C19H17N3O/c23-19-13-7-8-20-9-12(13)5-6-16(19)18-15-4-2-1-3-14(15)17-10-21-11-22(17)18/h1-4,7-11,16,18-19,23H,5-6H2/t16-,18+,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 48 | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of TDO in human SW48 cells after 24 hrs by NFK green reagent based assay |
ACS Med Chem Lett 11: 541-549 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00004
BindingDB Entry DOI: 10.7270/Q2RR22JS |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50511718

(CHEMBL4542307)Show SMILES [H][C@@]1(c2ccccc2-c2cncn12)[C@]1([H])CCN(C[C@H]1O)S(C)(=O)=O |r| Show InChI InChI=1S/C16H19N3O3S/c1-23(21,22)18-7-6-13(15(20)9-18)16-12-5-3-2-4-11(12)14-8-17-10-19(14)16/h2-5,8,10,13,15-16,20H,6-7,9H2,1H3/t13-,15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 970 | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in human A172 cells after 24 hrs in presence of IFNgamma by NFK green reagent based assay |
ACS Med Chem Lett 11: 541-549 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00004
BindingDB Entry DOI: 10.7270/Q2RR22JS |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50511720
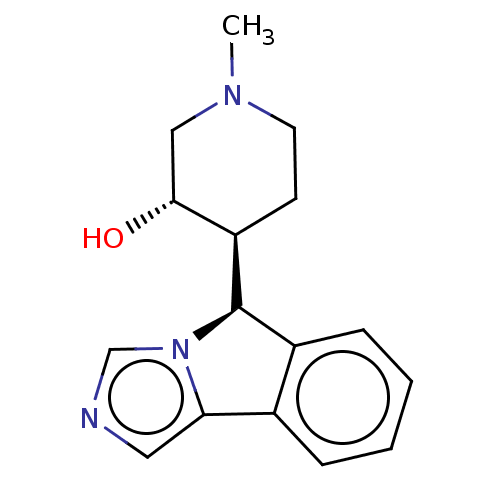
(CHEMBL4558670)Show SMILES [H][C@@]1(c2ccccc2-c2cncn12)[C@]1([H])CCN(C)C[C@H]1O |r| Show InChI InChI=1S/C16H19N3O/c1-18-7-6-13(15(20)9-18)16-12-5-3-2-4-11(12)14-8-17-10-19(14)16/h2-5,8,10,13,15-16,20H,6-7,9H2,1H3/t13-,15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in human A172 cells after 24 hrs in presence of IFNgamma by NFK green reagent based assay |
ACS Med Chem Lett 11: 541-549 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00004
BindingDB Entry DOI: 10.7270/Q2RR22JS |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50511715

(CHEMBL4540245)Show SMILES [H][C@@]1(c2ccccc2-c2cncn12)[C@]1([H])COCC[C@H]1O |r| Show InChI InChI=1S/C15H16N2O2/c18-14-5-6-19-8-12(14)15-11-4-2-1-3-10(11)13-7-16-9-17(13)15/h1-4,7,9,12,14-15,18H,5-6,8H2/t12-,14-,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in human A172 cells after 24 hrs in presence of IFNgamma by NFK green reagent based assay |
ACS Med Chem Lett 11: 541-549 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00004
BindingDB Entry DOI: 10.7270/Q2RR22JS |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50511721
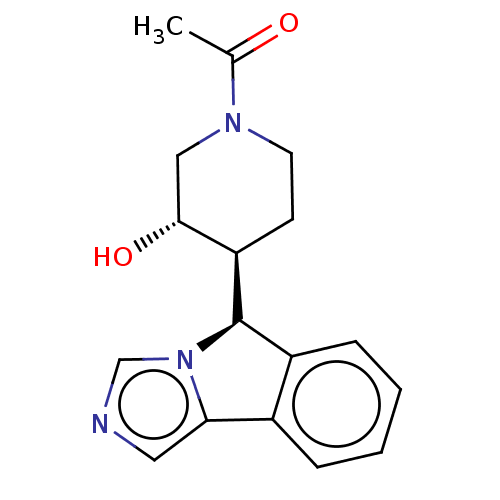
(CHEMBL4473543)Show SMILES [H][C@@]1(c2ccccc2-c2cncn12)[C@]1([H])CCN(C[C@H]1O)C(C)=O |r| Show InChI InChI=1S/C17H19N3O2/c1-11(21)19-7-6-14(16(22)9-19)17-13-5-3-2-4-12(13)15-8-18-10-20(15)17/h2-5,8,10,14,16-17,22H,6-7,9H2,1H3/t14-,16-,17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of TDO in human SW48 cells after 24 hrs by NFK green reagent based assay |
ACS Med Chem Lett 11: 541-549 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00004
BindingDB Entry DOI: 10.7270/Q2RR22JS |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50511720

(CHEMBL4558670)Show SMILES [H][C@@]1(c2ccccc2-c2cncn12)[C@]1([H])CCN(C)C[C@H]1O |r| Show InChI InChI=1S/C16H19N3O/c1-18-7-6-13(15(20)9-18)16-12-5-3-2-4-11(12)14-8-17-10-19(14)16/h2-5,8,10,13,15-16,20H,6-7,9H2,1H3/t13-,15-,16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of TDO in human SW48 cells after 24 hrs by NFK green reagent based assay |
ACS Med Chem Lett 11: 541-549 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00004
BindingDB Entry DOI: 10.7270/Q2RR22JS |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50511722
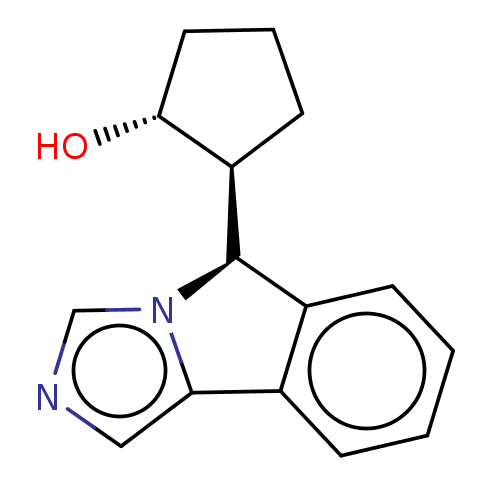
(CHEMBL4531114)Show SMILES [H][C@]1(CCC[C@H]1O)[C@@]1([H])c2ccccc2-c2cncn12 |r| Show InChI InChI=1S/C15H16N2O/c18-14-7-3-6-12(14)15-11-5-2-1-4-10(11)13-8-16-9-17(13)15/h1-2,4-5,8-9,12,14-15,18H,3,6-7H2/t12-,14-,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in human A172 cells after 24 hrs in presence of IFNgamma by NFK green reagent based assay |
ACS Med Chem Lett 11: 541-549 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00004
BindingDB Entry DOI: 10.7270/Q2RR22JS |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50511723
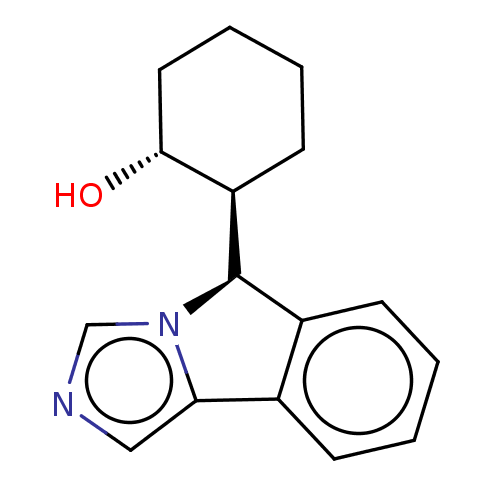
(CHEMBL4445816)Show SMILES [H][C@@]1(c2ccccc2-c2cncn12)[C@]1([H])CCCC[C@H]1O |r| Show InChI InChI=1S/C16H18N2O/c19-15-8-4-3-7-13(15)16-12-6-2-1-5-11(12)14-9-17-10-18(14)16/h1-2,5-6,9-10,13,15-16,19H,3-4,7-8H2/t13-,15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 720 | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in human A172 cells after 24 hrs in presence of IFNgamma by NFK green reagent based assay |
ACS Med Chem Lett 11: 541-549 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00004
BindingDB Entry DOI: 10.7270/Q2RR22JS |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50138828
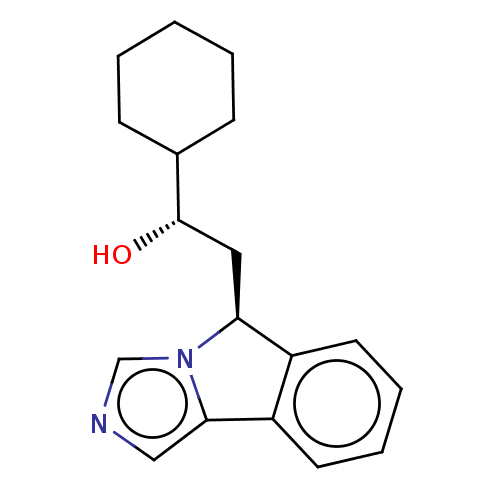
(CHEMBL3752060 | US10233190, Example 1417)Show SMILES O[C@@H](C[C@H]1c2ccccc2-c2cncn12)C1CCCCC1 |r| Show InChI InChI=1S/C18H22N2O/c21-18(13-6-2-1-3-7-13)10-16-14-8-4-5-9-15(14)17-11-19-12-20(16)17/h4-5,8-9,11-13,16,18,21H,1-3,6-7,10H2/t16-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 130 | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in human A172 cells after 24 hrs in presence of IFNgamma by NFK green reagent based assay |
ACS Med Chem Lett 11: 541-549 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00004
BindingDB Entry DOI: 10.7270/Q2RR22JS |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50511722

(CHEMBL4531114)Show SMILES [H][C@]1(CCC[C@H]1O)[C@@]1([H])c2ccccc2-c2cncn12 |r| Show InChI InChI=1S/C15H16N2O/c18-14-7-3-6-12(14)15-11-5-2-1-4-10(11)13-8-16-9-17(13)15/h1-2,4-5,8-9,12,14-15,18H,3,6-7H2/t12-,14-,15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 70 | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of TDO in human SW48 cells after 24 hrs by NFK green reagent based assay |
ACS Med Chem Lett 11: 541-549 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00004
BindingDB Entry DOI: 10.7270/Q2RR22JS |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50511724
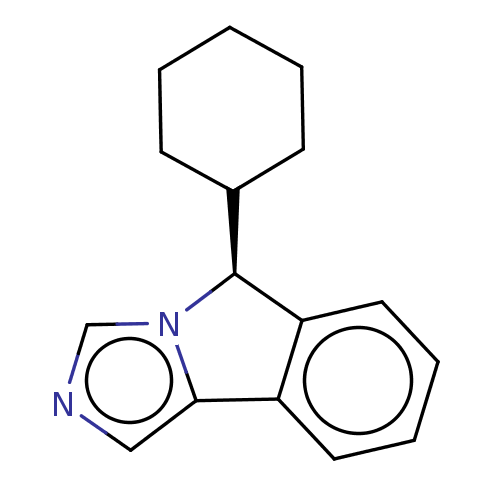
(CHEMBL4468203)Show InChI InChI=1S/C16H18N2/c1-2-6-12(7-3-1)16-14-9-5-4-8-13(14)15-10-17-11-18(15)16/h4-5,8-12,16H,1-3,6-7H2/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 33 | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of TDO in human SW48 cells after 24 hrs by NFK green reagent based assay |
ACS Med Chem Lett 11: 541-549 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00004
BindingDB Entry DOI: 10.7270/Q2RR22JS |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50511725
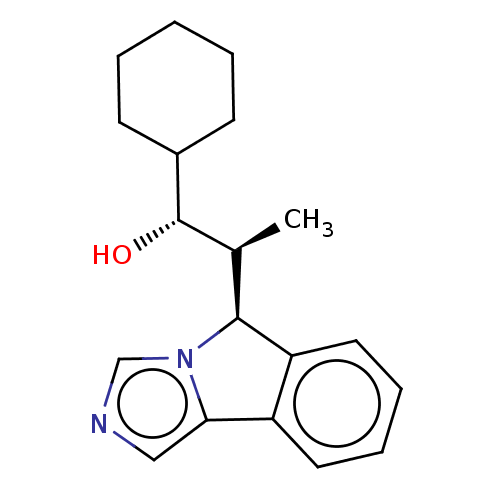
(CHEMBL4593111)Show SMILES [H][C@]1([C@H](C)[C@H](O)C2CCCCC2)c2ccccc2-c2cncn12 |r| Show InChI InChI=1S/C19H24N2O/c1-13(19(22)14-7-3-2-4-8-14)18-16-10-6-5-9-15(16)17-11-20-12-21(17)18/h5-6,9-14,18-19,22H,2-4,7-8H2,1H3/t13-,18-,19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 62 | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of TDO in human SW48 cells after 24 hrs by NFK green reagent based assay |
ACS Med Chem Lett 11: 541-549 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00004
BindingDB Entry DOI: 10.7270/Q2RR22JS |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50138828

(CHEMBL3752060 | US10233190, Example 1417)Show SMILES O[C@@H](C[C@H]1c2ccccc2-c2cncn12)C1CCCCC1 |r| Show InChI InChI=1S/C18H22N2O/c21-18(13-6-2-1-3-7-13)10-16-14-8-4-5-9-15(14)17-11-19-12-20(16)17/h4-5,8-9,11-13,16,18,21H,1-3,6-7,10H2/t16-,18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 250 | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of TDO in human SW48 cells after 24 hrs by NFK green reagent based assay |
ACS Med Chem Lett 11: 541-549 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00004
BindingDB Entry DOI: 10.7270/Q2RR22JS |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50511726

(CHEMBL4440730)Show SMILES [H][C@@]1(c2ccccc2-c2cncn12)[C@]1([H])CCc2c(cnn2C)[C@H]1O |r| Show InChI InChI=1S/C18H18N4O/c1-21-15-7-6-13(18(23)14(15)8-20-21)17-12-5-3-2-4-11(12)16-9-19-10-22(16)17/h2-5,8-10,13,17-18,23H,6-7H2,1H3/t13-,17+,18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 380 | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of TDO in human SW48 cells after 24 hrs by NFK green reagent based assay |
ACS Med Chem Lett 11: 541-549 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00004
BindingDB Entry DOI: 10.7270/Q2RR22JS |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50511727

(CHEMBL4447185)Show SMILES [H][C@@]1(c2ccccc2-c2cncn12)[C@]1([H])CCc2ccncc2[C@H]1O |r| Show InChI InChI=1S/C19H17N3O/c23-19-15(6-5-12-7-8-20-9-16(12)19)18-14-4-2-1-3-13(14)17-10-21-11-22(17)18/h1-4,7-11,15,18-19,23H,5-6H2/t15-,18+,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in human A172 cells after 24 hrs in presence of IFNgamma by NFK green reagent based assay |
ACS Med Chem Lett 11: 541-549 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00004
BindingDB Entry DOI: 10.7270/Q2RR22JS |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50511728

(CHEMBL4577396)Show SMILES [H][C@@]1(c2ccccc2-c2cncn12)[C@]1([H])CCc2ccccc2[C@H]1O |r| Show InChI InChI=1S/C20H18N2O/c23-20-14-6-2-1-5-13(14)9-10-17(20)19-16-8-4-3-7-15(16)18-11-21-12-22(18)19/h1-8,11-12,17,19-20,23H,9-10H2/t17-,19+,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 240 | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in human A172 cells after 24 hrs in presence of IFNgamma by NFK green reagent based assay |
ACS Med Chem Lett 11: 541-549 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00004
BindingDB Entry DOI: 10.7270/Q2RR22JS |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50511714

(CHEMBL4520696)Show SMILES [H][C@@]1(c2ccccc2-c2cncn12)[C@]1([H])CCc2ccc(cc2[C@H]1O)S(C)(=O)=O |r| Show InChI InChI=1S/C21H20N2O3S/c1-27(25,26)14-8-6-13-7-9-17(21(24)18(13)10-14)20-16-5-3-2-4-15(16)19-11-22-12-23(19)20/h2-6,8,10-12,17,20-21,24H,7,9H2,1H3/t17-,20+,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 880 | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in human A172 cells after 24 hrs in presence of IFNgamma by NFK green reagent based assay |
ACS Med Chem Lett 11: 541-549 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00004
BindingDB Entry DOI: 10.7270/Q2RR22JS |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50511716

(CHEMBL4539108)Show SMILES [H][C@@]1(c2ccccc2-c2cncn12)[C@]1([H])CCc2cc(ccc2[C@H]1O)S(C)(=O)=O |r| Show InChI InChI=1S/C21H20N2O3S/c1-27(25,26)14-7-9-15-13(10-14)6-8-18(21(15)24)20-17-5-3-2-4-16(17)19-11-22-12-23(19)20/h2-5,7,9-12,18,20-21,24H,6,8H2,1H3/t18-,20+,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in human A172 cells after 24 hrs in presence of IFNgamma by NFK green reagent based assay |
ACS Med Chem Lett 11: 541-549 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00004
BindingDB Entry DOI: 10.7270/Q2RR22JS |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50511729

(CHEMBL4589215)Show SMILES [H][C@@]1(c2ccccc2-c2cncn12)[C@]1([H])CCc2ccc(cc2[C@H]1O)P(C)(C)=O |r| Show InChI InChI=1S/C22H23N2O2P/c1-27(2,26)15-9-7-14-8-10-18(22(25)19(14)11-15)21-17-6-4-3-5-16(17)20-12-23-13-24(20)21/h3-7,9,11-13,18,21-22,25H,8,10H2,1-2H3/t18-,21+,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in human A172 cells after 24 hrs in presence of IFNgamma by NFK green reagent based assay |
ACS Med Chem Lett 11: 541-549 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00004
BindingDB Entry DOI: 10.7270/Q2RR22JS |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data