 Found 7 hits with Last Name = 'nikolaev' and Initial = 'vo'
Found 7 hits with Last Name = 'nikolaev' and Initial = 'vo' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM86258
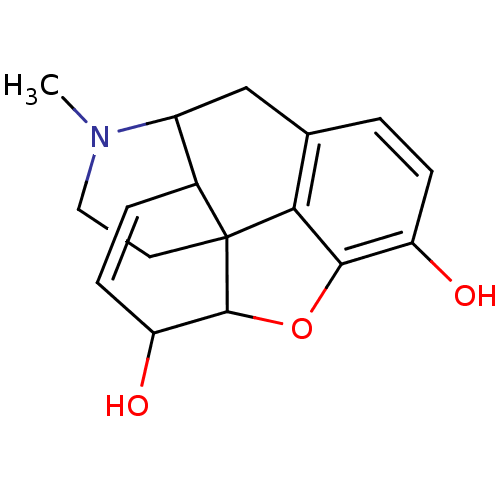
(CAS_23552-18-3 | Morphine | NSC_5980)Show SMILES CN1CCC23C4Oc5c2c(CC1C3C=CC4O)ccc5O |c:16,TLB:13:12:1.2.3:10.9.8| Show InChI InChI=1S/C17H19NO3/c1-18-7-6-17-10-3-5-13(20)16(17)21-15-12(19)4-2-9(14(15)17)8-11(10)18/h2-5,10-11,13,16,19-20H,6-8H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Würzburg
Curated by PDSP Ki Database
| |
J Biol Chem 282: 27126-32 (2007)
Article DOI: 10.1074/jbc.M703272200
BindingDB Entry DOI: 10.7270/Q2MP51W2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM86804
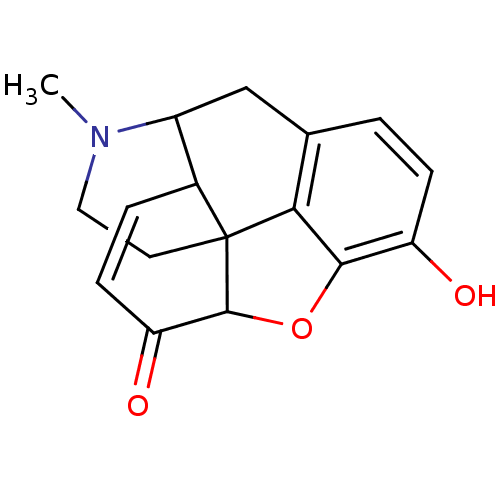
(CAS_5459823 | NSC_5459823 | morphinone)Show SMILES CN1CCC23C4Oc5c2c(CC1C3C=CC4=O)ccc5O |c:16,TLB:13:12:1.2.3:10.9.8| Show InChI InChI=1S/C17H17NO3/c1-18-7-6-17-10-3-5-13(20)16(17)21-15-12(19)4-2-9(14(15)17)8-11(10)18/h2-5,10-11,16,19H,6-8H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 146 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Würzburg
Curated by PDSP Ki Database
| |
J Biol Chem 282: 27126-32 (2007)
Article DOI: 10.1074/jbc.M703272200
BindingDB Entry DOI: 10.7270/Q2MP51W2 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM86800
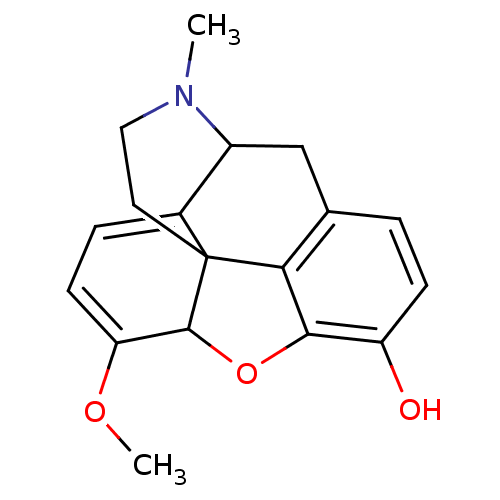
(CAS_5462306 | NSC_5462306 | oripavine)Show SMILES COC1=CC=C2C3Cc4ccc(O)c5OC1C2(CCN3C)c45 |t:2,4,TLB:4:5:19.18.17:7.8.21| Show InChI InChI=1S/C18H19NO3/c1-19-8-7-18-11-4-6-14(21-2)17(18)22-16-13(20)5-3-10(15(16)18)9-12(11)19/h3-6,12,17,20H,7-9H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 286 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Würzburg
Curated by PDSP Ki Database
| |
J Biol Chem 282: 27126-32 (2007)
Article DOI: 10.1074/jbc.M703272200
BindingDB Entry DOI: 10.7270/Q2MP51W2 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM86802
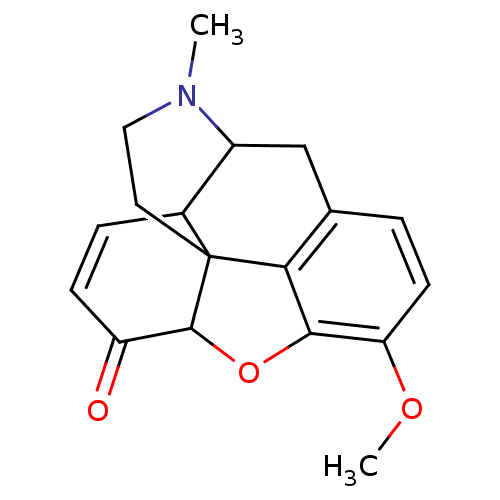
(CAS_5459910 | NSC_5459910 | codeinone)Show SMILES COc1ccc2CC3C4C=CC(=O)C5Oc1c2C45CCN3C |c:9,THB:9:8:20.19.18:6.5.16| Show InChI InChI=1S/C18H19NO3/c1-19-8-7-18-11-4-5-13(20)17(18)22-16-14(21-2)6-3-10(15(16)18)9-12(11)19/h3-6,11-12,17H,7-9H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 459 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Würzburg
Curated by PDSP Ki Database
| |
J Biol Chem 282: 27126-32 (2007)
Article DOI: 10.1074/jbc.M703272200
BindingDB Entry DOI: 10.7270/Q2MP51W2 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM86803
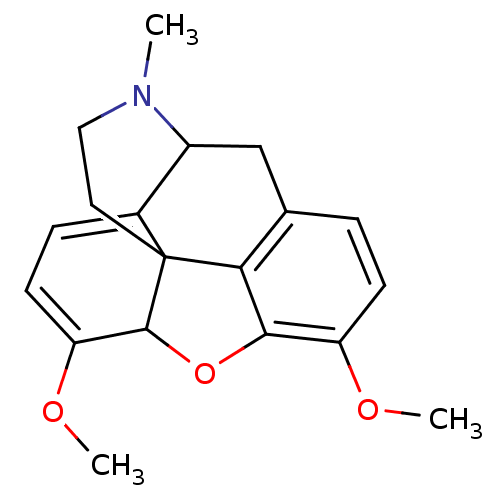
(CAS_5324289 | NSC_5324289 | thebaine)Show SMILES COC1=CC=C2C3Cc4ccc(OC)c5OC1C2(CCN3C)c45 |t:2,4,TLB:4:5:20.19.18:7.8.22| Show InChI InChI=1S/C19H21NO3/c1-20-9-8-19-12-5-7-15(22-3)18(19)23-17-14(21-2)6-4-11(16(17)19)10-13(12)20/h4-7,13,18H,8-10H2,1-3H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Würzburg
Curated by PDSP Ki Database
| |
J Biol Chem 282: 27126-32 (2007)
Article DOI: 10.1074/jbc.M703272200
BindingDB Entry DOI: 10.7270/Q2MP51W2 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM86801
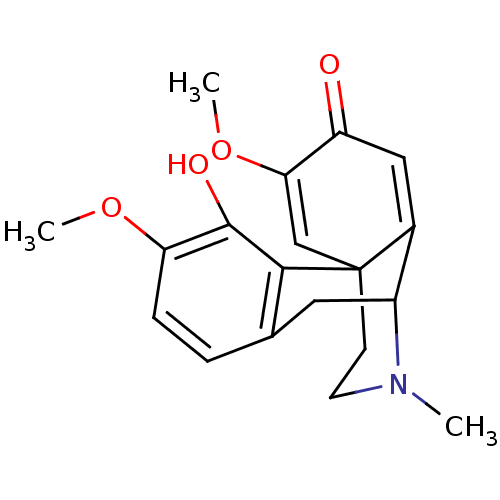
(CAS_5408233 | NSC_5408233 | sinoacutine)Show SMILES COC1=CC23CCN(C)C(Cc4ccc(OC)c(O)c24)C3=CC1=O |c:23,t:2,THB:8:7:20:10.11.19,21:20:7.6.5:10.11.19,17:19:7.6.5:20,(8.22,-2.59,;6.78,-3.13,;5.59,-2.16,;4.15,-2.7,;2.95,-1.73,;1.16,-1.75,;.22,-2.54,;.46,-1.04,;-.54,.13,;2.17,-1.04,;2.29,-2.82,;1.52,-3.9,;1.05,-5.37,;2.09,-6.51,;3.59,-6.18,;4.63,-7.32,;6.14,-6.98,;4.06,-4.71,;5.56,-4.38,;3.02,-3.57,;3.2,-.21,;4.64,.34,;5.83,-.64,;7.27,-.09,)| Show InChI InChI=1S/C19H21NO4/c1-20-7-6-19-10-16(24-3)14(21)9-12(19)13(20)8-11-4-5-15(23-2)18(22)17(11)19/h4-5,9-10,13,22H,6-8H2,1-3H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Würzburg
Curated by PDSP Ki Database
| |
J Biol Chem 282: 27126-32 (2007)
Article DOI: 10.1074/jbc.M703272200
BindingDB Entry DOI: 10.7270/Q2MP51W2 |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic/Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50000092

((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...)Show SMILES CN1CC[C@@]23[C@H]4Oc5c2c(C[C@@H]1[C@@H]3C=C[C@@H]4O)ccc5O |r,c:16,TLB:13:12:8.9.10:3.2.1| Show InChI InChI=1S/C17H19NO3/c1-18-7-6-17-10-3-5-13(20)16(17)21-15-12(19)4-2-9(14(15)17)8-11(10)18/h2-5,10-11,13,16,19-20H,6-8H2,1H3/t10-,11+,13-,16-,17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Inhibition of MOR/alpha2A adrenergic receptor heterodimer expressed in HEK293 cells assessed as stimulation of ERK1/2 phosphorylation |
Nat Chem Biol 4: 126-31 (2008)
Article DOI: 10.1038/nchembio.64
BindingDB Entry DOI: 10.7270/Q2MP563T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data