

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine kinase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50524390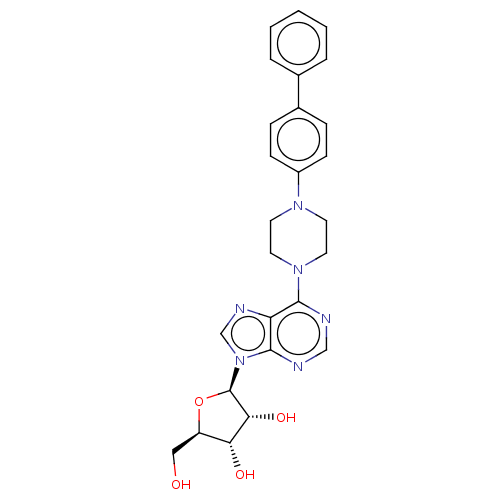 (CHEMBL4475619) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University Curated by ChEMBL | Assay Description Inhibition of wild-type N-terminal TEV cleavage site-fused/His-tagged Mycobacterium tuberculosis H37Rv adenosine kinase expressed in Escherichia coli... | J Med Chem 62: 4483-4499 (2019) Article DOI: 10.1021/acs.jmedchem.9b00020 BindingDB Entry DOI: 10.7270/Q2028VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50524393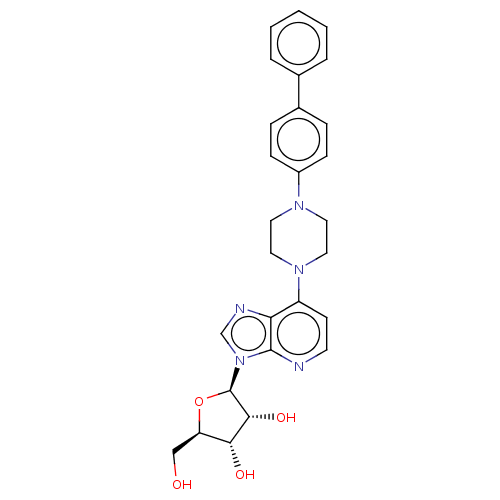 (CHEMBL4448092) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University Curated by ChEMBL | Assay Description Inhibition of wild-type N-terminal TEV cleavage site-fused/His-tagged Mycobacterium tuberculosis H37Rv adenosine kinase expressed in Escherichia coli... | J Med Chem 62: 4483-4499 (2019) Article DOI: 10.1021/acs.jmedchem.9b00020 BindingDB Entry DOI: 10.7270/Q2028VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50524392 (CHEMBL4475059) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University Curated by ChEMBL | Assay Description Inhibition of wild-type N-terminal TEV cleavage site-fused/His-tagged Mycobacterium tuberculosis H37Rv adenosine kinase expressed in Escherichia coli... | J Med Chem 62: 4483-4499 (2019) Article DOI: 10.1021/acs.jmedchem.9b00020 BindingDB Entry DOI: 10.7270/Q2028VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50524387 (CHEMBL4566815) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University Curated by ChEMBL | Assay Description Inhibition of wild-type N-terminal TEV cleavage site-fused/His-tagged Mycobacterium tuberculosis H37Rv adenosine kinase expressed in Escherichia coli... | J Med Chem 62: 4483-4499 (2019) Article DOI: 10.1021/acs.jmedchem.9b00020 BindingDB Entry DOI: 10.7270/Q2028VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50524388 (CHEMBL4449169) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University Curated by ChEMBL | Assay Description Inhibition of wild-type N-terminal TEV cleavage site-fused/His-tagged Mycobacterium tuberculosis H37Rv adenosine kinase expressed in Escherichia coli... | J Med Chem 62: 4483-4499 (2019) Article DOI: 10.1021/acs.jmedchem.9b00020 BindingDB Entry DOI: 10.7270/Q2028VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50524389 (CHEMBL4525944) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University Curated by ChEMBL | Assay Description Inhibition of wild-type N-terminal TEV cleavage site-fused/His-tagged Mycobacterium tuberculosis H37Rv adenosine kinase expressed in Escherichia coli... | J Med Chem 62: 4483-4499 (2019) Article DOI: 10.1021/acs.jmedchem.9b00020 BindingDB Entry DOI: 10.7270/Q2028VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50524394 (CHEMBL4539148) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University Curated by ChEMBL | Assay Description Inhibition of wild-type N-terminal TEV cleavage site-fused/His-tagged Mycobacterium tuberculosis H37Rv adenosine kinase expressed in Escherichia coli... | J Med Chem 62: 4483-4499 (2019) Article DOI: 10.1021/acs.jmedchem.9b00020 BindingDB Entry DOI: 10.7270/Q2028VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50524383 (CHEMBL4442160) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University Curated by ChEMBL | Assay Description Inhibition of wild-type N-terminal TEV cleavage site-fused/His-tagged Mycobacterium tuberculosis H37Rv adenosine kinase expressed in Escherichia coli... | J Med Chem 62: 4483-4499 (2019) Article DOI: 10.1021/acs.jmedchem.9b00020 BindingDB Entry DOI: 10.7270/Q2028VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50524384 (CHEMBL4458246) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University Curated by ChEMBL | Assay Description Inhibition of wild-type N-terminal TEV cleavage site-fused/His-tagged Mycobacterium tuberculosis H37Rv adenosine kinase expressed in Escherichia coli... | J Med Chem 62: 4483-4499 (2019) Article DOI: 10.1021/acs.jmedchem.9b00020 BindingDB Entry DOI: 10.7270/Q2028VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Homo sapiens (Human)) | BDBM50375654 (CHEMBL99203 | US11633415, Compound 5-iodotubercidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University Curated by ChEMBL | Assay Description Inhibition of purified human adenosine kinase using varying levels of [3H]Ado as substrate in presence of adenosine deaminase inhibitor deoxycoformyc... | J Med Chem 62: 4483-4499 (2019) Article DOI: 10.1021/acs.jmedchem.9b00020 BindingDB Entry DOI: 10.7270/Q2028VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50524385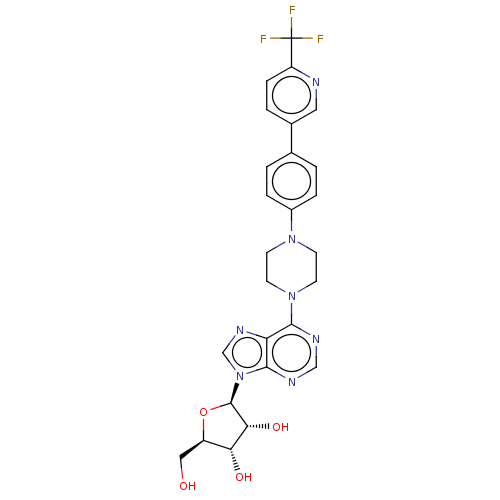 (CHEMBL4463459) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University Curated by ChEMBL | Assay Description Inhibition of wild-type N-terminal TEV cleavage site-fused/His-tagged Mycobacterium tuberculosis H37Rv adenosine kinase expressed in Escherichia coli... | J Med Chem 62: 4483-4499 (2019) Article DOI: 10.1021/acs.jmedchem.9b00020 BindingDB Entry DOI: 10.7270/Q2028VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50524386 (CHEMBL4474951) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University Curated by ChEMBL | Assay Description Inhibition of wild-type N-terminal TEV cleavage site-fused/His-tagged Mycobacterium tuberculosis H37Rv adenosine kinase expressed in Escherichia coli... | J Med Chem 62: 4483-4499 (2019) Article DOI: 10.1021/acs.jmedchem.9b00020 BindingDB Entry DOI: 10.7270/Q2028VZ7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine kinase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50524391 (CHEMBL2042164) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University Curated by ChEMBL | Assay Description Inhibition of wild-type N-terminal TEV cleavage site-fused/His-tagged Mycobacterium tuberculosis H37Rv adenosine kinase expressed in Escherichia coli... | J Med Chem 62: 4483-4499 (2019) Article DOI: 10.1021/acs.jmedchem.9b00020 BindingDB Entry DOI: 10.7270/Q2028VZ7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine kinase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50375654 (CHEMBL99203 | US11633415, Compound 5-iodotubercidi...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis H37Ra ATCC 25177 adenosine kinase using varying levels of [3H]Ado as substrate in presence of adenosine deam... | J Med Chem 62: 4483-4499 (2019) Article DOI: 10.1021/acs.jmedchem.9b00020 BindingDB Entry DOI: 10.7270/Q2028VZ7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine kinase (Homo sapiens (Human)) | BDBM50524383 (CHEMBL4442160) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University Curated by ChEMBL | Assay Description Inhibition of recombinant human adenosine kinase expressed in Escherichia coli BL21 (DE3) using adenosine as substrate in presence of ATP and PEP by ... | J Med Chem 62: 4483-4499 (2019) Article DOI: 10.1021/acs.jmedchem.9b00020 BindingDB Entry DOI: 10.7270/Q2028VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Homo sapiens (Human)) | BDBM50524385 (CHEMBL4463459) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University Curated by ChEMBL | Assay Description Inhibition of recombinant human adenosine kinase expressed in Escherichia coli BL21 (DE3) using adenosine as substrate in presence of ATP and PEP by ... | J Med Chem 62: 4483-4499 (2019) Article DOI: 10.1021/acs.jmedchem.9b00020 BindingDB Entry DOI: 10.7270/Q2028VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketol-acid reductoisomerase (NADP(+)) (Escherichia coli) | BDBM82145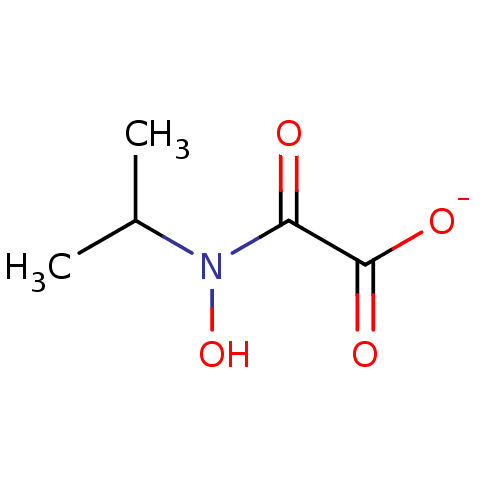 (N-hydroxy-N-isopropyloxamate, IpOHA) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 2.75E+3 | -32.3 | n/a | n/a | n/a | n/a | n/a | 8.0 | 30 |
Nankai University | Assay Description Inhibition of E. coli KARI (ketol-acid reductoisomerase) is time dependent, and activity was measured by the decrease in A340 at 30 C in solutions co... | Chem Biol Drug Des 75: 228-32 (2010) Article DOI: 10.1111/j.1747-0285.2009.00924.x BindingDB Entry DOI: 10.7270/Q2BV7F45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Homo sapiens (Human)) | BDBM50524391 (CHEMBL2042164) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University Curated by ChEMBL | Assay Description Inhibition of recombinant human adenosine kinase expressed in Escherichia coli BL21 (DE3) using adenosine as substrate in presence of ATP and PEP by ... | J Med Chem 62: 4483-4499 (2019) Article DOI: 10.1021/acs.jmedchem.9b00020 BindingDB Entry DOI: 10.7270/Q2028VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50174577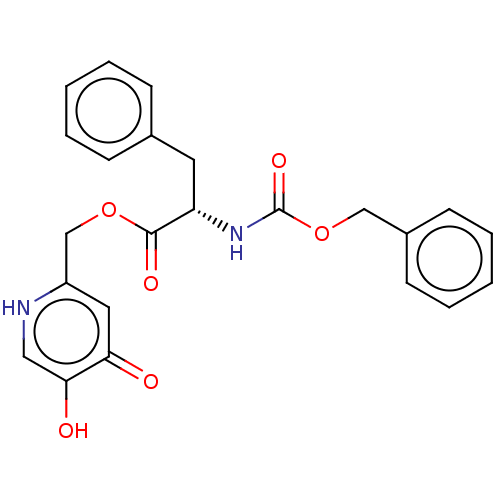 (CHEMBL3810334) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.72E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang Gongshang University Curated by ChEMBL | Assay Description Inhibition of diphenolase activity of mushroom tyrosinase using L-DOPA as substrate assessed as enzyme-inhibitor complex incubated for 10 mins by Lin... | Bioorg Med Chem Lett 26: 3103-8 (2016) Article DOI: 10.1016/j.bmcl.2016.05.006 BindingDB Entry DOI: 10.7270/Q2JM2CJ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50174577 (CHEMBL3810334) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang Gongshang University Curated by ChEMBL | Assay Description Inhibition of diphenolase activity of mushroom tyrosinase using L-DOPA as substrate assessed as enzyme-substrate-inhibitor complex incubated for 10 m... | Bioorg Med Chem Lett 26: 3103-8 (2016) Article DOI: 10.1016/j.bmcl.2016.05.006 BindingDB Entry DOI: 10.7270/Q2JM2CJ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketol-acid reductoisomerase (NADP(+)) (Escherichia coli) | BDBM82144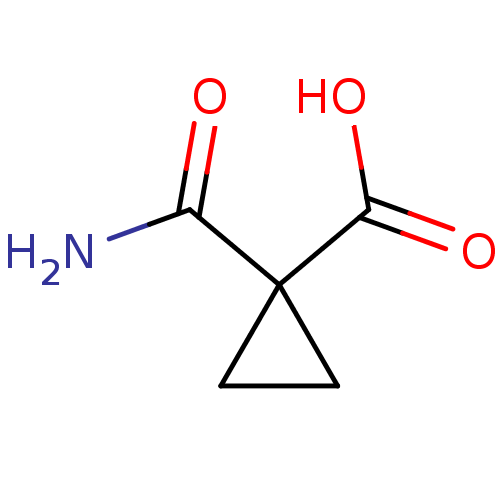 (Cyclopropane, 5) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents | Article PubMed | 3.12E+4 | -26.2 | n/a | n/a | n/a | n/a | n/a | 8.0 | 30 |
Nankai University | Assay Description Inhibition of E. coli KARI (ketol-acid reductoisomerase) is time dependent, and activity was measured by the decrease in A340 at 30 C in solutions co... | Chem Biol Drug Des 75: 228-32 (2010) Article DOI: 10.1111/j.1747-0285.2009.00924.x BindingDB Entry DOI: 10.7270/Q2BV7F45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50174582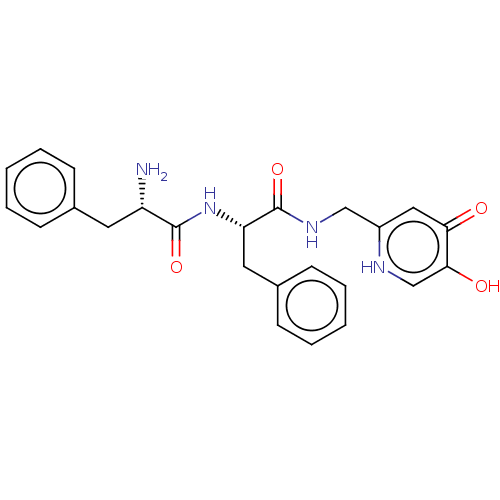 (CHEMBL3809975) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang Gongshang University Curated by ChEMBL | Assay Description Inhibition of diphenolase activity of mushroom tyrosinase using L-DOPA as substrate assessed as enzyme-inhibitor complex incubated for 10 mins by Lin... | Bioorg Med Chem Lett 26: 3103-8 (2016) Article DOI: 10.1016/j.bmcl.2016.05.006 BindingDB Entry DOI: 10.7270/Q2JM2CJ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Homo sapiens (Human)) | BDBM50524393 (CHEMBL4448092) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University Curated by ChEMBL | Assay Description Inhibition of recombinant human adenosine kinase expressed in Escherichia coli BL21 (DE3) using adenosine as substrate in presence of ATP and PEP by ... | J Med Chem 62: 4483-4499 (2019) Article DOI: 10.1021/acs.jmedchem.9b00020 BindingDB Entry DOI: 10.7270/Q2028VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Homo sapiens (Human)) | BDBM50524389 (CHEMBL4525944) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University Curated by ChEMBL | Assay Description Inhibition of recombinant human adenosine kinase expressed in Escherichia coli BL21 (DE3) using adenosine as substrate in presence of ATP and PEP by ... | J Med Chem 62: 4483-4499 (2019) Article DOI: 10.1021/acs.jmedchem.9b00020 BindingDB Entry DOI: 10.7270/Q2028VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Homo sapiens (Human)) | BDBM50524388 (CHEMBL4449169) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University Curated by ChEMBL | Assay Description Inhibition of recombinant human adenosine kinase expressed in Escherichia coli BL21 (DE3) using adenosine as substrate in presence of ATP and PEP by ... | J Med Chem 62: 4483-4499 (2019) Article DOI: 10.1021/acs.jmedchem.9b00020 BindingDB Entry DOI: 10.7270/Q2028VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Homo sapiens (Human)) | BDBM50524392 (CHEMBL4475059) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University Curated by ChEMBL | Assay Description Inhibition of recombinant human adenosine kinase expressed in Escherichia coli BL21 (DE3) using adenosine as substrate in presence of ATP and PEP by ... | J Med Chem 62: 4483-4499 (2019) Article DOI: 10.1021/acs.jmedchem.9b00020 BindingDB Entry DOI: 10.7270/Q2028VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Homo sapiens (Human)) | BDBM50524386 (CHEMBL4474951) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University Curated by ChEMBL | Assay Description Inhibition of recombinant human adenosine kinase expressed in Escherichia coli BL21 (DE3) using adenosine as substrate in presence of ATP and PEP by ... | J Med Chem 62: 4483-4499 (2019) Article DOI: 10.1021/acs.jmedchem.9b00020 BindingDB Entry DOI: 10.7270/Q2028VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Homo sapiens (Human)) | BDBM50524384 (CHEMBL4458246) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University Curated by ChEMBL | Assay Description Inhibition of recombinant human adenosine kinase expressed in Escherichia coli BL21 (DE3) using adenosine as substrate in presence of ATP and PEP by ... | J Med Chem 62: 4483-4499 (2019) Article DOI: 10.1021/acs.jmedchem.9b00020 BindingDB Entry DOI: 10.7270/Q2028VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Homo sapiens (Human)) | BDBM50524390 (CHEMBL4475619) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University Curated by ChEMBL | Assay Description Inhibition of recombinant human adenosine kinase expressed in Escherichia coli BL21 (DE3) using adenosine as substrate in presence of ATP and PEP by ... | J Med Chem 62: 4483-4499 (2019) Article DOI: 10.1021/acs.jmedchem.9b00020 BindingDB Entry DOI: 10.7270/Q2028VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Homo sapiens (Human)) | BDBM50524387 (CHEMBL4566815) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University Curated by ChEMBL | Assay Description Inhibition of recombinant human adenosine kinase expressed in Escherichia coli BL21 (DE3) using adenosine as substrate in presence of ATP and PEP by ... | J Med Chem 62: 4483-4499 (2019) Article DOI: 10.1021/acs.jmedchem.9b00020 BindingDB Entry DOI: 10.7270/Q2028VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketol-acid reductoisomerase (NADP(+)) (Escherichia coli) | BDBM82142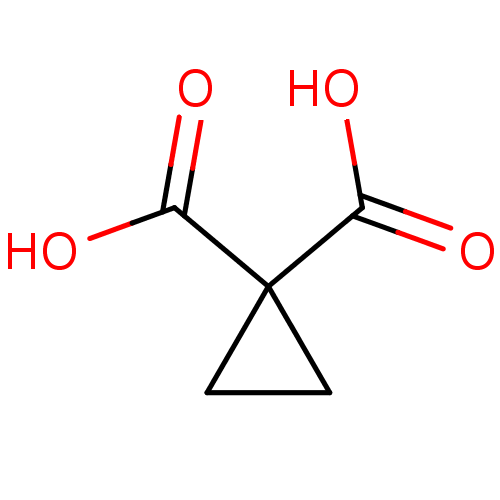 (Cyclopropane, 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents | Article PubMed | 7.66E+4 | -23.9 | n/a | n/a | n/a | n/a | n/a | 8.0 | 30 |
Nankai University | Assay Description Inhibition of E. coli KARI (ketol-acid reductoisomerase) is time dependent, and activity was measured by the decrease in A340 at 30 C in solutions co... | Chem Biol Drug Des 75: 228-32 (2010) Article DOI: 10.1111/j.1747-0285.2009.00924.x BindingDB Entry DOI: 10.7270/Q2BV7F45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50174582 (CHEMBL3809975) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang Gongshang University Curated by ChEMBL | Assay Description Inhibition of diphenolase activity of mushroom tyrosinase using L-DOPA as substrate assessed as enzyme-substrate-inhibitor complex incubated for 10 m... | Bioorg Med Chem Lett 26: 3103-8 (2016) Article DOI: 10.1016/j.bmcl.2016.05.006 BindingDB Entry DOI: 10.7270/Q2JM2CJ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketol-acid reductoisomerase (NADP(+)) (Escherichia coli) | BDBM82143 (Cyclopropane, 4) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents | Article PubMed | 9.53E+4 | -23.3 | n/a | n/a | n/a | n/a | n/a | 8.0 | 30 |
Nankai University | Assay Description Inhibition of E. coli KARI (ketol-acid reductoisomerase) is time dependent, and activity was measured by the decrease in A340 at 30 C in solutions co... | Chem Biol Drug Des 75: 228-32 (2010) Article DOI: 10.1111/j.1747-0285.2009.00924.x BindingDB Entry DOI: 10.7270/Q2BV7F45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM185149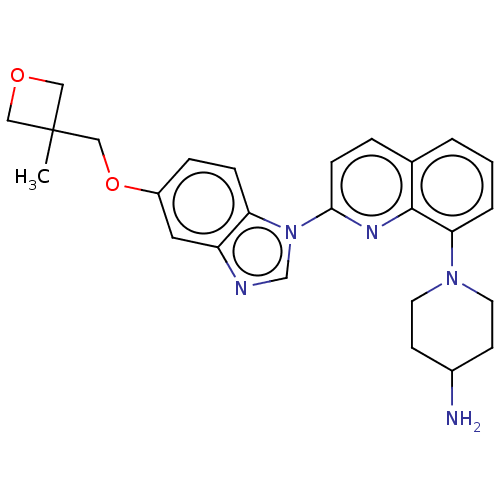 (1-[2-[5-[(3-methyloxetan-3-yl)methoxy]benzimidazol...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan Academy of Medical Science & Sichuan Provincial People's Hospital Curated by ChEMBL | Assay Description Inhibition of FLT3 D835Y mutant (unknown origin) | Eur J Med Chem 178: 468-483 (2019) Article DOI: 10.1016/j.ejmech.2019.06.002 BindingDB Entry DOI: 10.7270/Q22Z18W7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM144315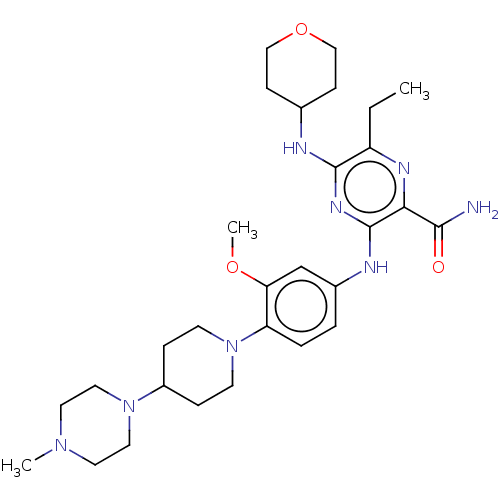 (Gilteritinib | US11512074, Example T-9 | US8969336...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan Academy of Medical Science & Sichuan Provincial People's Hospital Curated by ChEMBL | Assay Description Inhibition of N-terminal GST-tagged human FLT3 (564 to 993 residues) cytoplasmic domain expressed in baculovirus expression system by ELISA | Eur J Med Chem 178: 468-483 (2019) Article DOI: 10.1016/j.ejmech.2019.06.002 BindingDB Entry DOI: 10.7270/Q22Z18W7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM185149 (1-[2-[5-[(3-methyloxetan-3-yl)methoxy]benzimidazol...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan Academy of Medical Science & Sichuan Provincial People's Hospital Curated by ChEMBL | Assay Description Inhibition of FLT3 D835H mutant (unknown origin) | Eur J Med Chem 178: 468-483 (2019) Article DOI: 10.1016/j.ejmech.2019.06.002 BindingDB Entry DOI: 10.7270/Q22Z18W7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM144315 (Gilteritinib | US11512074, Example T-9 | US8969336...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan Academy of Medical Science & Sichuan Provincial People's Hospital Curated by ChEMBL | Assay Description Inhibition of N-terminal GST-tagged human AXL (464 to 885 residues) cytoplasmic domain expressed in baculovirus expression system by ELISA | Eur J Med Chem 178: 468-483 (2019) Article DOI: 10.1016/j.ejmech.2019.06.002 BindingDB Entry DOI: 10.7270/Q22Z18W7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50515042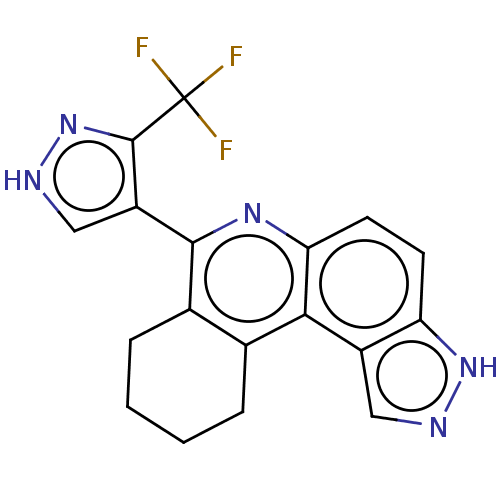 (CHEMBL4593398) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan Academy of Medical Science & Sichuan Provincial People's Hospital Curated by ChEMBL | Assay Description Inhibition of FLT3 D835Y mutant (unknown origin) using MBP as substrate after 3 hrs by ADP-Glo assay | Eur J Med Chem 178: 468-483 (2019) Article DOI: 10.1016/j.ejmech.2019.06.002 BindingDB Entry DOI: 10.7270/Q22Z18W7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| A5LHX3/O14818/P20618/P25786/P25787/P25788/P25789/P28062/P28065/P28066/P28070/P28072/P28074/P40306/P49720/P49721/P60900/Q8TAA3/Q99436 (Homo sapiens (Human)) | BDBM50531806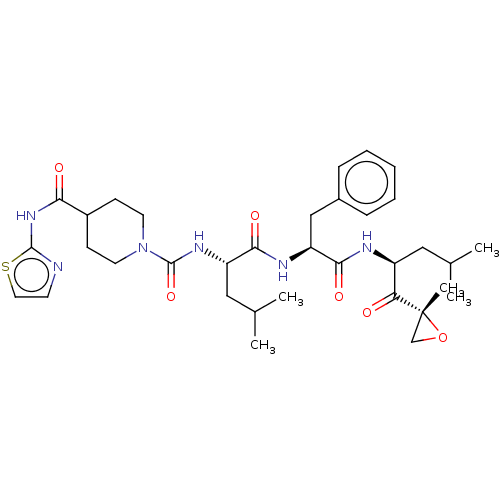 (CHEMBL4460323) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hangzhou Institute of Innovative Medicine Curated by ChEMBL | Assay Description Inhibition of human 20S proteasome preincubated for 15 mins followed by substrate addition | Eur J Med Chem 164: 602-614 (2019) Article DOI: 10.1016/j.ejmech.2018.12.064 BindingDB Entry DOI: 10.7270/Q2JM2F38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50531806 (CHEMBL4460323) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hangzhou Institute of Innovative Medicine Curated by ChEMBL | Assay Description Inhibition of human 20s constitutive proteasome beta5 chymotrypsin-like activity using Suc-LLVY-AMC as substrate preincubated for 15 mins followed by... | Eur J Med Chem 164: 602-614 (2019) Article DOI: 10.1016/j.ejmech.2018.12.064 BindingDB Entry DOI: 10.7270/Q2JM2F38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50515042 (CHEMBL4593398) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan Academy of Medical Science & Sichuan Provincial People's Hospital Curated by ChEMBL | Assay Description Inhibition of FLT3 ITD mutant (unknown origin) using MBP as substrate after 3 hrs by ADP-Glo assay | Eur J Med Chem 178: 468-483 (2019) Article DOI: 10.1016/j.ejmech.2019.06.002 BindingDB Entry DOI: 10.7270/Q22Z18W7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| A5LHX3/O14818/P20618/P25786/P25787/P25788/P25789/P28062/P28065/P28066/P28070/P28072/P28074/P40306/P49720/P49721/P60900/Q8TAA3/Q99436 (Homo sapiens (Human)) | BDBM50531814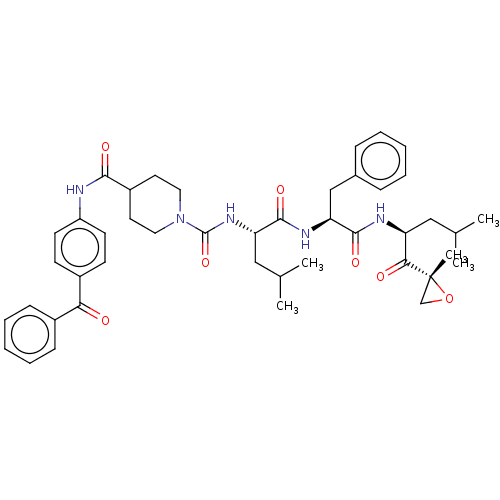 (CHEMBL4517600) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Hangzhou Institute of Innovative Medicine Curated by ChEMBL | Assay Description Inhibition of human 20S proteasome preincubated for 15 mins followed by substrate addition | Eur J Med Chem 164: 602-614 (2019) Article DOI: 10.1016/j.ejmech.2018.12.064 BindingDB Entry DOI: 10.7270/Q2JM2F38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50311926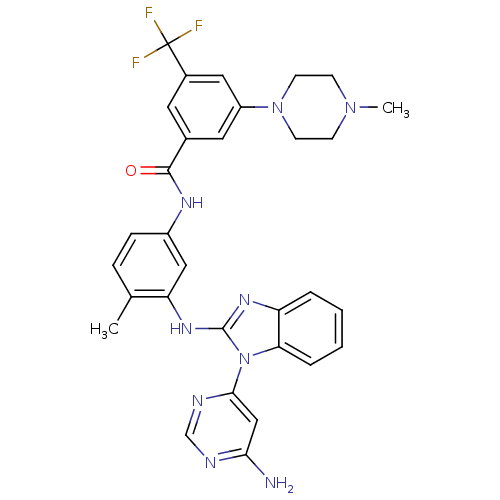 (CHEMBL1076436 | N-(3-(1-(6-aminopyrimidin-4-yl)-1H...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF) Curated by ChEMBL | Assay Description Inhibition of Tel-fused LCK expressed in mouse BAF3 cells | Bioorg Med Chem Lett 19: 6691-5 (2009) Article DOI: 10.1016/j.bmcl.2009.09.123 BindingDB Entry DOI: 10.7270/Q2Q52PRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| A5LHX3/O14818/P20618/P25786/P25787/P25788/P25789/P28062/P28065/P28066/P28070/P28072/P28074/P40306/P49720/P49721/P60900/Q8TAA3/Q99436 (Homo sapiens (Human)) | BDBM50531815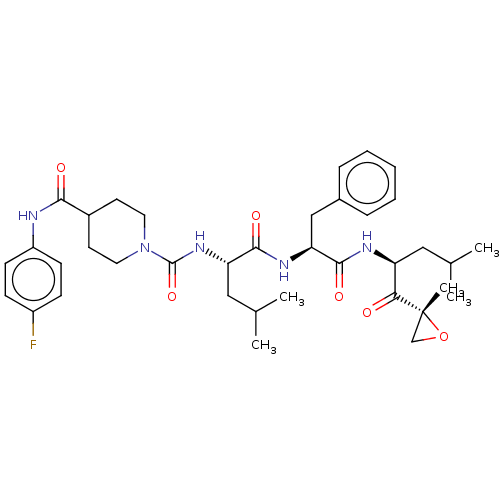 (CHEMBL4444107) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Hangzhou Institute of Innovative Medicine Curated by ChEMBL | Assay Description Inhibition of human 20S proteasome preincubated for 15 mins followed by substrate addition | Eur J Med Chem 164: 602-614 (2019) Article DOI: 10.1016/j.ejmech.2018.12.064 BindingDB Entry DOI: 10.7270/Q2JM2F38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| A5LHX3/O14818/P20618/P25786/P25787/P25788/P25789/P28062/P28065/P28066/P28070/P28072/P28074/P40306/P49720/P49721/P60900/Q8TAA3/Q99436 (Homo sapiens (Human)) | BDBM50531807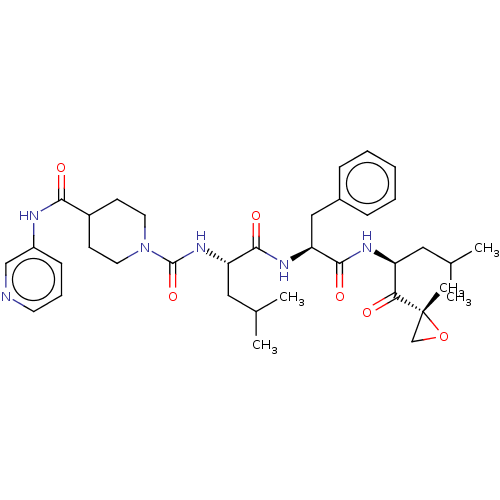 (CHEMBL4541038) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Hangzhou Institute of Innovative Medicine Curated by ChEMBL | Assay Description Inhibition of human 20S proteasome preincubated for 15 mins followed by substrate addition | Eur J Med Chem 164: 602-614 (2019) Article DOI: 10.1016/j.ejmech.2018.12.064 BindingDB Entry DOI: 10.7270/Q2JM2F38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| A5LHX3/O14818/P20618/P25786/P25787/P25788/P25789/P28062/P28065/P28066/P28070/P28072/P28074/P40306/P49720/P49721/P60900/Q8TAA3/Q99436 (Homo sapiens (Human)) | BDBM50531812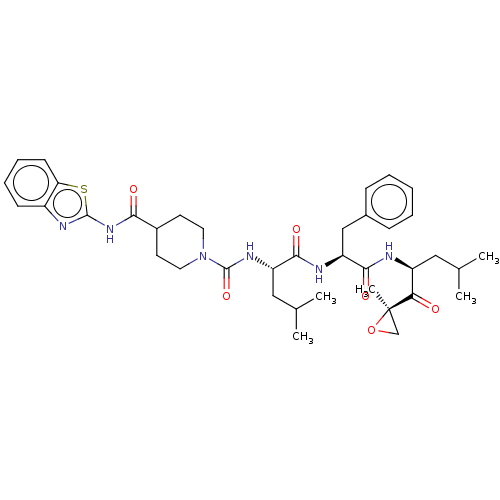 (CHEMBL4547405) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Hangzhou Institute of Innovative Medicine Curated by ChEMBL | Assay Description Inhibition of human 20S proteasome preincubated for 15 mins followed by substrate addition | Eur J Med Chem 164: 602-614 (2019) Article DOI: 10.1016/j.ejmech.2018.12.064 BindingDB Entry DOI: 10.7270/Q2JM2F38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50311921 (CHEMBL1086731 | N-(3-(1-(6-aminopyrimidin-4-yl)-1H...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF) Curated by ChEMBL | Assay Description Inhibition of Tel-fused SRC expressed in mouse BAF3 cells | Bioorg Med Chem Lett 19: 6691-5 (2009) Article DOI: 10.1016/j.bmcl.2009.09.123 BindingDB Entry DOI: 10.7270/Q2Q52PRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50311921 (CHEMBL1086731 | N-(3-(1-(6-aminopyrimidin-4-yl)-1H...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF) Curated by ChEMBL | Assay Description Inhibition of Tel-fused InsR expressed in mouse BAF3 cells | Bioorg Med Chem Lett 19: 6691-5 (2009) Article DOI: 10.1016/j.bmcl.2009.09.123 BindingDB Entry DOI: 10.7270/Q2Q52PRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50311927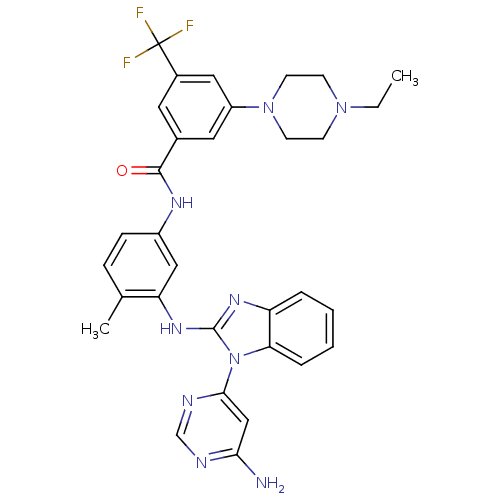 (CHEMBL1076437 | N-(3-(1-(6-aminopyrimidin-4-yl)-1H...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF) Curated by ChEMBL | Assay Description Inhibition of Tel-fused LCK expressed in mouse BAF3 cells | Bioorg Med Chem Lett 19: 6691-5 (2009) Article DOI: 10.1016/j.bmcl.2009.09.123 BindingDB Entry DOI: 10.7270/Q2Q52PRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50311921 (CHEMBL1086731 | N-(3-(1-(6-aminopyrimidin-4-yl)-1H...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF) Curated by ChEMBL | Assay Description Inhibition of Tel-fused LCK expressed in mouse BAF3 cells | Bioorg Med Chem Lett 19: 6691-5 (2009) Article DOI: 10.1016/j.bmcl.2009.09.123 BindingDB Entry DOI: 10.7270/Q2Q52PRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 626 total ) | Next | Last >> |