

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM39347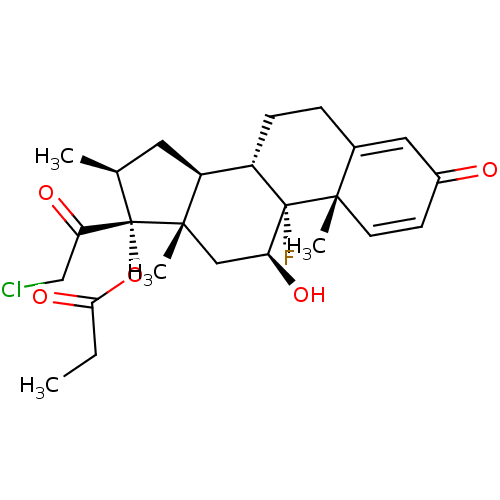 (CLOBETASOL PROPIONATE | MLS000028708 | SMR00005874...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of CYP3A5 in doxycycline-induced CYP3A5 overexpressing wild type human AsPC1 cells assessed as decrease in 1-hydroxymidazolam formation us... | J Med Chem 63: 1415-1433 (2020) Article DOI: 10.1021/acs.jmedchem.9b02067 BindingDB Entry DOI: 10.7270/Q2NS0Z6Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM429515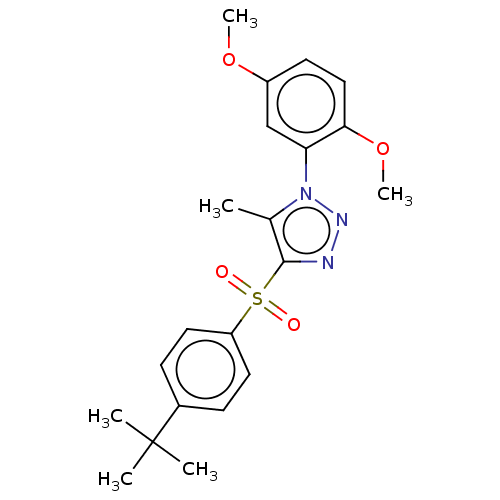 (US10550091, No. LC-1 | US10947203, No. LC-1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inverse agonist activity at human PXR expressed in HepG2 cells co-expressing luciferase gene under control of CYP3A4 promoter incubated for 24 hrs by... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02201 BindingDB Entry DOI: 10.7270/Q2KS6WBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM19993 (CHEMBL62136 | N-[4-(1,1,1,3,3,3-hexafluoro-2-hydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of BODIPY FL vindoline from GST-tagged human PXR LBD incubated for 60 mins by TR-FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02201 BindingDB Entry DOI: 10.7270/Q2KS6WBB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM39347 (CLOBETASOL PROPIONATE | MLS000028708 | SMR00005874...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of CYP3A5 in wild type human AsPC1 cells assessed as decrease in 1-hydroxymidazolam formation using midazolam as substrate after 24 hrs by... | J Med Chem 63: 1415-1433 (2020) Article DOI: 10.1021/acs.jmedchem.9b02067 BindingDB Entry DOI: 10.7270/Q2NS0Z6Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50575435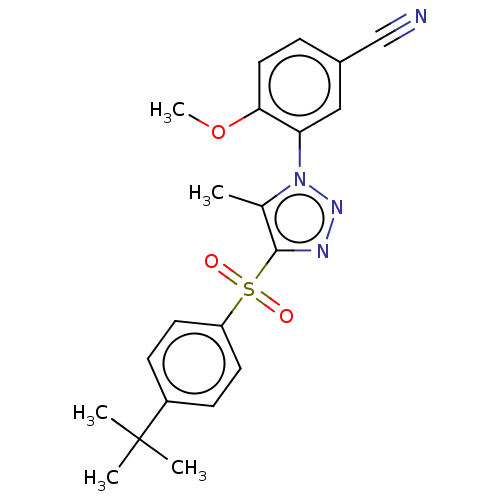 (CHEMBL4846228) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inverse agonist activity at human PXR expressed in HepG2 cells co-expressing luciferase gene under control of CYP3A4 promoter incubated for 24 hrs by... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02201 BindingDB Entry DOI: 10.7270/Q2KS6WBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50575432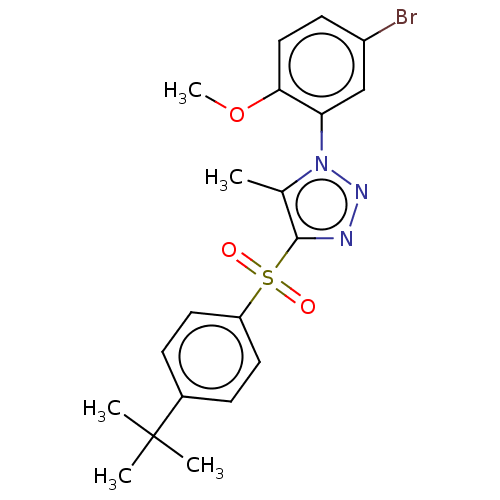 (CHEMBL4857525) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inverse agonist activity at human PXR expressed in HepG2 cells co-expressing luciferase gene under control of CYP3A4 promoter incubated for 24 hrs by... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02201 BindingDB Entry DOI: 10.7270/Q2KS6WBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM429642 (US10550091, No. LC-51 | US10947203, No. LC-51) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of BODIPY FL vindoline from GST-tagged human PXR LBD incubated for 60 mins by TR-FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02201 BindingDB Entry DOI: 10.7270/Q2KS6WBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM39347 (CLOBETASOL PROPIONATE | MLS000028708 | SMR00005874...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of CYP3A5 in lentiviral pLVX-TRE3G-ZsGreen1-CYP3A5 transduced wild type human AsPC1 cells overexpressing CYP3A5 assessed as decrease in 1-... | J Med Chem 63: 1415-1433 (2020) Article DOI: 10.1021/acs.jmedchem.9b02067 BindingDB Entry DOI: 10.7270/Q2NS0Z6Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM39347 (CLOBETASOL PROPIONATE | MLS000028708 | SMR00005874...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 103 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of CYP3A5 in CRISPR/Cas9-mediated CYP3A5 knock-out and doxycycline-induced CYP3A5 overexpressing human AsPC1 cells assessed as decrease in... | J Med Chem 63: 1415-1433 (2020) Article DOI: 10.1021/acs.jmedchem.9b02067 BindingDB Entry DOI: 10.7270/Q2NS0Z6Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM8610 (1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 117 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in CRISPR/Cas9-mediated CYP3A5 knock-out and doxycycline-induced CYP3A4 overexpressing human AsPC1 cells assessed as decrease in... | J Med Chem 63: 1415-1433 (2020) Article DOI: 10.1021/acs.jmedchem.9b02067 BindingDB Entry DOI: 10.7270/Q2NS0Z6Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM8610 (1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 141 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of CYP3A5 in doxycycline-induced CYP3A5 overexpressing wild type human AsPC1 cells assessed as decrease in 1-hydroxymidazolam formation us... | J Med Chem 63: 1415-1433 (2020) Article DOI: 10.1021/acs.jmedchem.9b02067 BindingDB Entry DOI: 10.7270/Q2NS0Z6Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM8610 (1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 162 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of CYP3A5 in CRISPR/Cas9-mediated CYP3A5 knock-out and doxycycline-induced CYP3A5 overexpressing human AsPC1 cells assessed as decrease in... | J Med Chem 63: 1415-1433 (2020) Article DOI: 10.1021/acs.jmedchem.9b02067 BindingDB Entry DOI: 10.7270/Q2NS0Z6Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM429628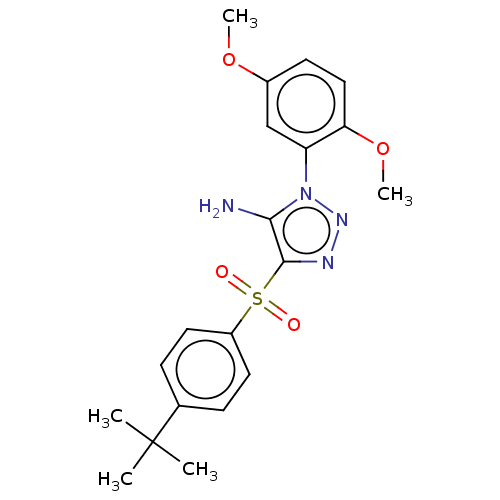 (US10550091, No. LC-37 | US10947203, No. LC-37) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of BODIPY FL vindoline from GST-tagged human PXR LBD incubated for 60 mins by TR-FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02201 BindingDB Entry DOI: 10.7270/Q2KS6WBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM429515 (US10550091, No. LC-1 | US10947203, No. LC-1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of BODIPY FL vindoline from GST-tagged human PXR LBD incubated for 60 mins by TR-FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02201 BindingDB Entry DOI: 10.7270/Q2KS6WBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM39347 (CLOBETASOL PROPIONATE | MLS000028708 | SMR00005874...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 206 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP3A4 expressed in supersomes assessed as decrease in formation of D-luciferin using luciferin-IPA as substrate incu... | J Med Chem 63: 1415-1433 (2020) Article DOI: 10.1021/acs.jmedchem.9b02067 BindingDB Entry DOI: 10.7270/Q2NS0Z6Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM429641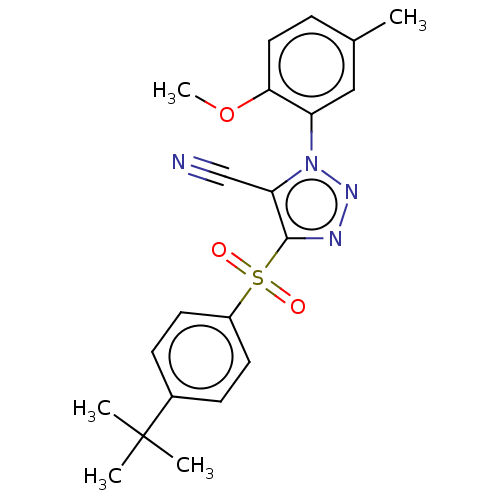 (US10550091, No. LC-50 | US10947203, No. LC-50) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of BODIPY FL vindoline from GST-tagged human PXR LBD incubated for 60 mins by TR-FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02201 BindingDB Entry DOI: 10.7270/Q2KS6WBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50575432 (CHEMBL4857525) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human PXR expressed in HepG2 cells co-expressing luciferase gene under control of CYP3A4 promoter incubated for 24 hrs in pres... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02201 BindingDB Entry DOI: 10.7270/Q2KS6WBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM429598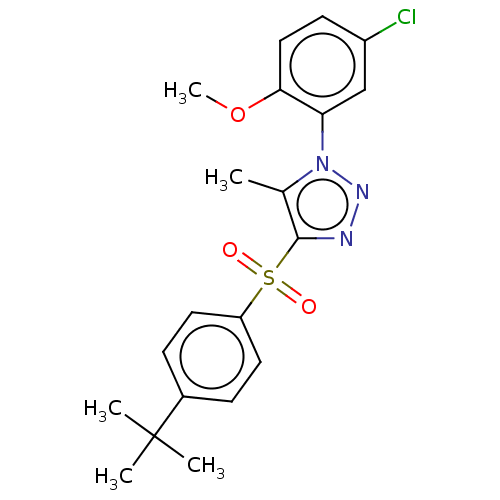 (US10550091, No. LC-7 | US10947203, No. LC-7) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of BODIPY FL vindoline from GST-tagged human PXR LBD incubated for 60 mins by TR-FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02201 BindingDB Entry DOI: 10.7270/Q2KS6WBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50575430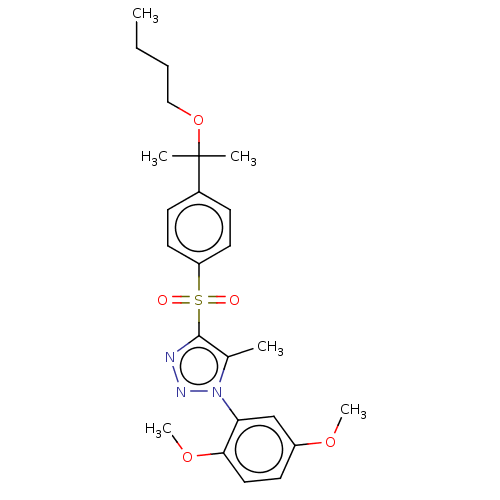 (CHEMBL4853694) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inverse agonist activity at human PXR expressed in HepG2 cells co-expressing luciferase gene under control of CYP3A4 promoter incubated for 24 hrs by... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02201 BindingDB Entry DOI: 10.7270/Q2KS6WBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM429515 (US10550091, No. LC-1 | US10947203, No. LC-1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human PXR expressed in HepG2 cells co-expressing luciferase gene under control of CYP3A4 promoter incubated for 24 hrs in pres... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02201 BindingDB Entry DOI: 10.7270/Q2KS6WBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM429644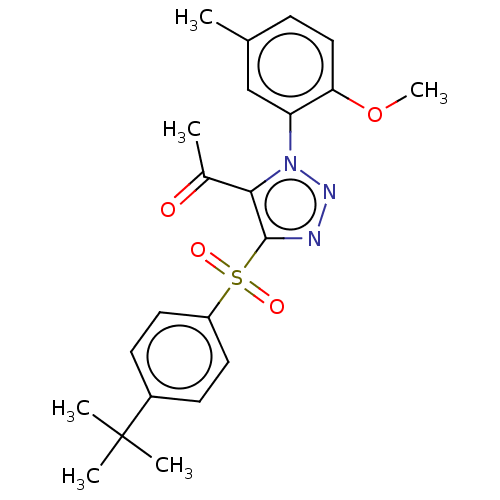 (US10550091, No. LC-53 | US10947203, No. LC-53) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of BODIPY FL vindoline from GST-tagged human PXR LBD incubated for 60 mins by TR-FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02201 BindingDB Entry DOI: 10.7270/Q2KS6WBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM429598 (US10550091, No. LC-7 | US10947203, No. LC-7) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inverse agonist activity at human PXR expressed in HepG2 cells co-expressing luciferase gene under control of CYP3A4 promoter incubated for 24 hrs by... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02201 BindingDB Entry DOI: 10.7270/Q2KS6WBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM429629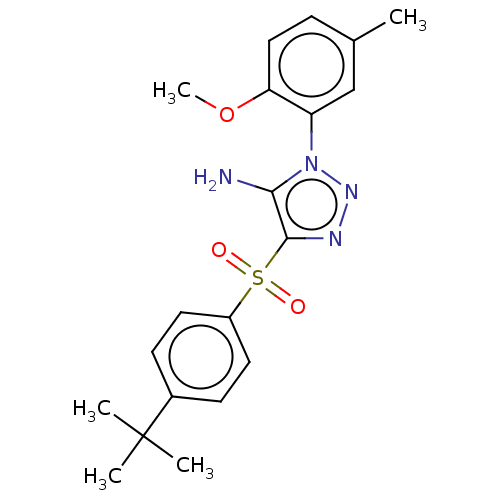 (US10550091, No. LC-38 | US10947203, No. LC-38) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of BODIPY FL vindoline from GST-tagged human PXR LBD incubated for 60 mins by TR-FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02201 BindingDB Entry DOI: 10.7270/Q2KS6WBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM429640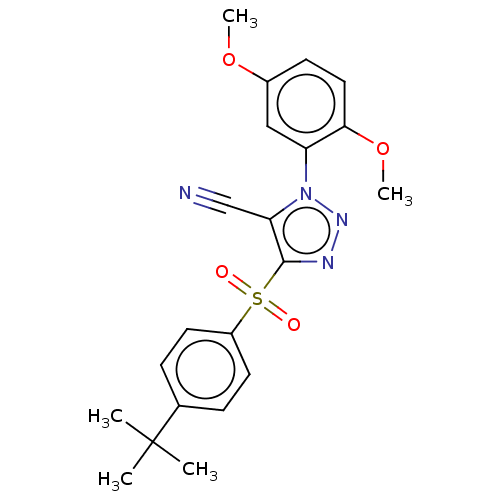 (US10550091, No. LC-49 | US10947203, No. LC-49) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of BODIPY FL vindoline from GST-tagged human PXR LBD incubated for 60 mins by TR-FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02201 BindingDB Entry DOI: 10.7270/Q2KS6WBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50575434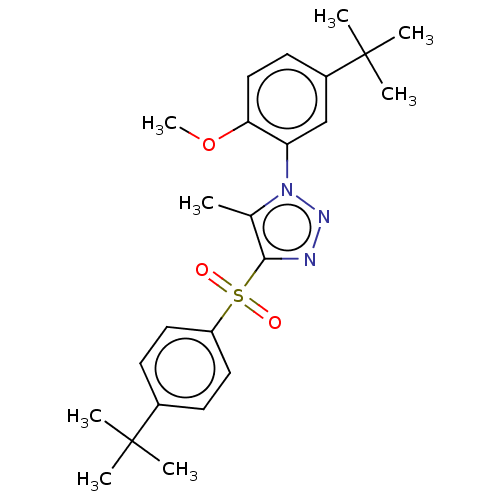 (CHEMBL4861132) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of BODIPY FL vindoline from GST-tagged human PXR LBD incubated for 60 mins by TR-FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02201 BindingDB Entry DOI: 10.7270/Q2KS6WBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM429598 (US10550091, No. LC-7 | US10947203, No. LC-7) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human PXR expressed in HepG2 cells co-expressing luciferase gene under control of CYP3A4 promoter incubated for 24 hrs in pres... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02201 BindingDB Entry DOI: 10.7270/Q2KS6WBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM429669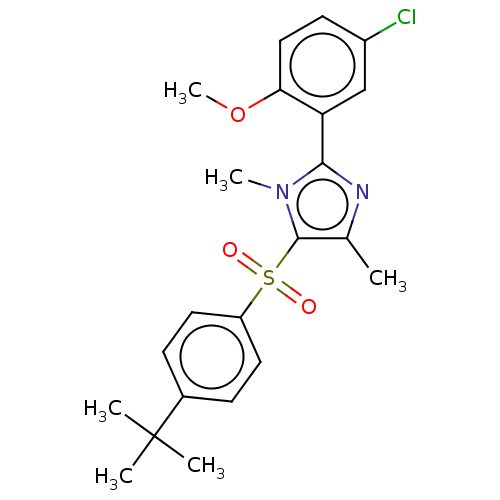 (US10550091, No. LC-78 | US10947203, No. LC-78) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of BODIPY FL vindoline from GST-tagged human PXR LBD incubated for 60 mins by TR-FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02201 BindingDB Entry DOI: 10.7270/Q2KS6WBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM429608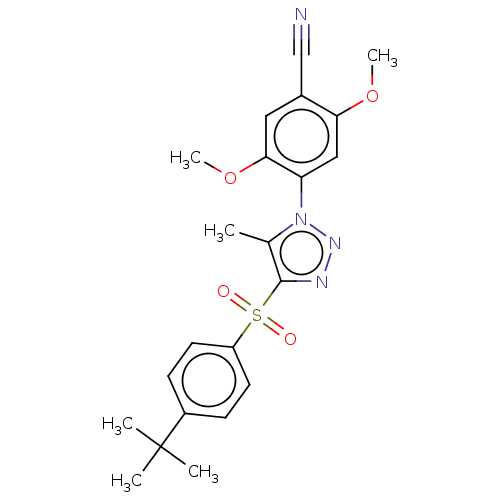 (US10550091, No. LC-17 | US10947203, No. LC-17) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of BODIPY FL vindoline from GST-tagged human PXR LBD incubated for 60 mins by TR-FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02201 BindingDB Entry DOI: 10.7270/Q2KS6WBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM429619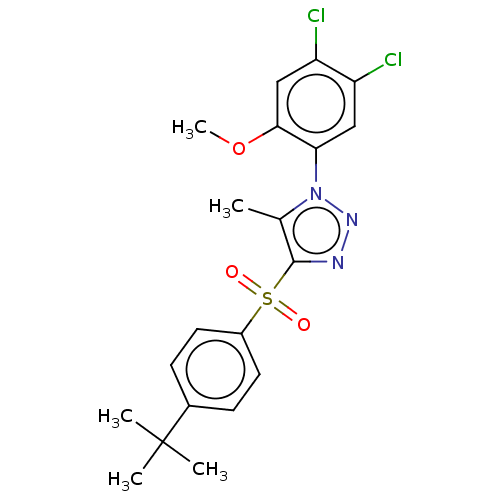 (US10550091, No. LC-28 | US10947203, No. LC-28) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of BODIPY FL vindoline from GST-tagged human PXR LBD incubated for 60 mins by TR-FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02201 BindingDB Entry DOI: 10.7270/Q2KS6WBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50575435 (CHEMBL4846228) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of BODIPY FL vindoline from GST-tagged human PXR LBD incubated for 60 mins by TR-FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02201 BindingDB Entry DOI: 10.7270/Q2KS6WBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50575432 (CHEMBL4857525) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of BODIPY FL vindoline from GST-tagged human PXR LBD incubated for 60 mins by TR-FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02201 BindingDB Entry DOI: 10.7270/Q2KS6WBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50575431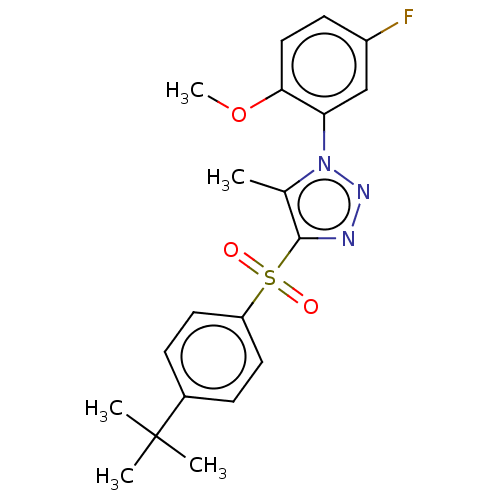 (CHEMBL4868841) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of BODIPY FL vindoline from GST-tagged human PXR LBD incubated for 60 mins by TR-FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02201 BindingDB Entry DOI: 10.7270/Q2KS6WBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50575429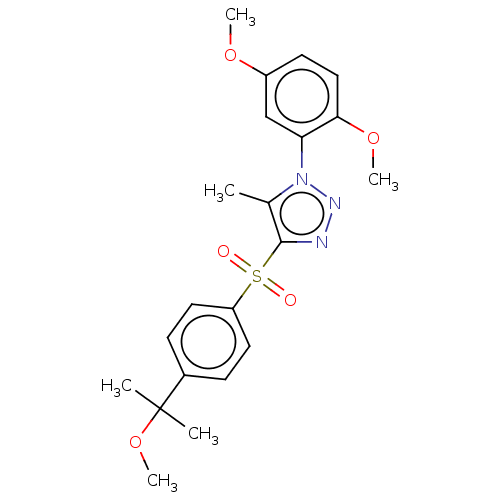 (CHEMBL4859314) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inverse agonist activity at human PXR expressed in HepG2 cells co-expressing luciferase gene under control of CYP3A4 promoter incubated for 24 hrs by... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02201 BindingDB Entry DOI: 10.7270/Q2KS6WBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM429668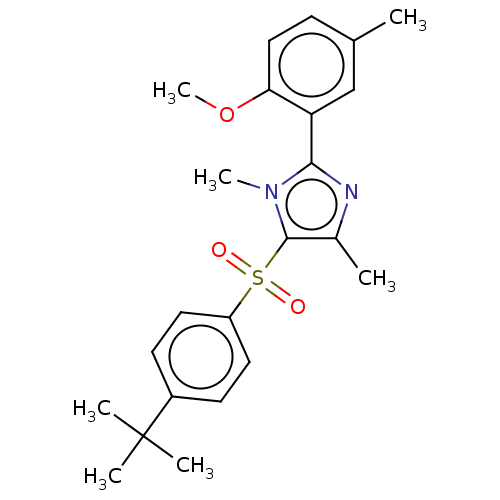 (US10550091, No. LC-77 | US10947203, No. LC-77) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of BODIPY FL vindoline from GST-tagged human PXR LBD incubated for 60 mins by TR-FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02201 BindingDB Entry DOI: 10.7270/Q2KS6WBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM429645 (US10550091, No. LC-54 | US10947203, No. LC-54) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of BODIPY FL vindoline from GST-tagged human PXR LBD incubated for 60 mins by TR-FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02201 BindingDB Entry DOI: 10.7270/Q2KS6WBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50575433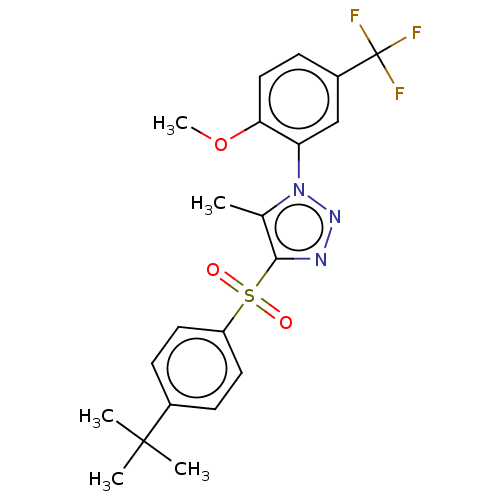 (CHEMBL4850074) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of BODIPY FL vindoline from GST-tagged human PXR LBD incubated for 60 mins by TR-FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02201 BindingDB Entry DOI: 10.7270/Q2KS6WBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM8610 (1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 439 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of CYP3A5 in lentiviral pLVX-TRE3G-ZsGreen1-CYP3A5 transduced wild type human AsPC1 cells overexpressing CYP3A5 assessed as decrease in 1-... | J Med Chem 63: 1415-1433 (2020) Article DOI: 10.1021/acs.jmedchem.9b02067 BindingDB Entry DOI: 10.7270/Q2NS0Z6Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50575435 (CHEMBL4846228) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human PXR expressed in HepG2 cells co-expressing luciferase gene under control of CYP3A4 promoter incubated for 24 hrs in pres... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02201 BindingDB Entry DOI: 10.7270/Q2KS6WBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM429624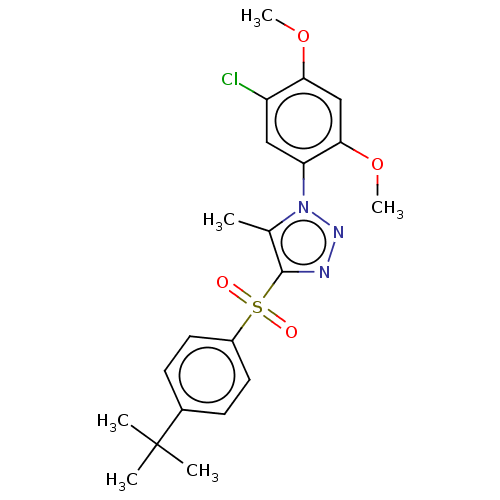 (US10550091, No. LC-33 | US10947203, No. LC-33) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of BODIPY FL vindoline from GST-tagged human PXR LBD incubated for 60 mins by TR-FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02201 BindingDB Entry DOI: 10.7270/Q2KS6WBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM429651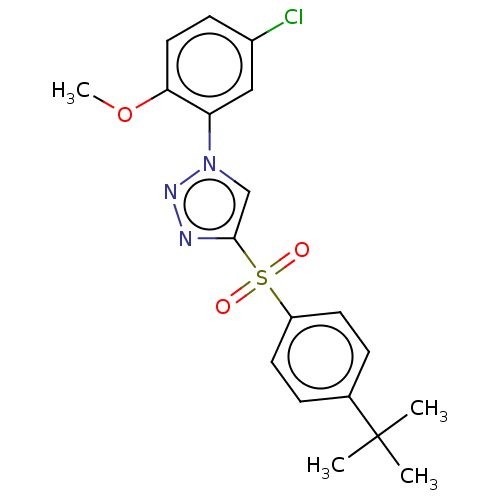 (US10550091, No. LC-60 | US10947203, No. LC-60) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of BODIPY FL vindoline from GST-tagged human PXR LBD incubated for 60 mins by TR-FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02201 BindingDB Entry DOI: 10.7270/Q2KS6WBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM429646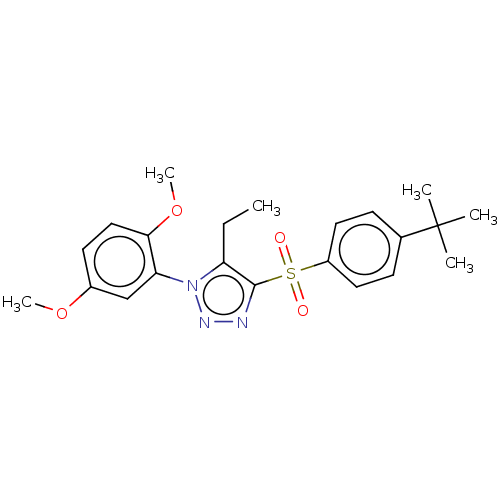 (US10550091, No. LC-55 | US10947203, No. LC-55) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human PXR expressed in HepG2 cells co-expressing luciferase gene under control of CYP3A4 promoter incubated for 24 hrs in pres... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02201 BindingDB Entry DOI: 10.7270/Q2KS6WBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM429602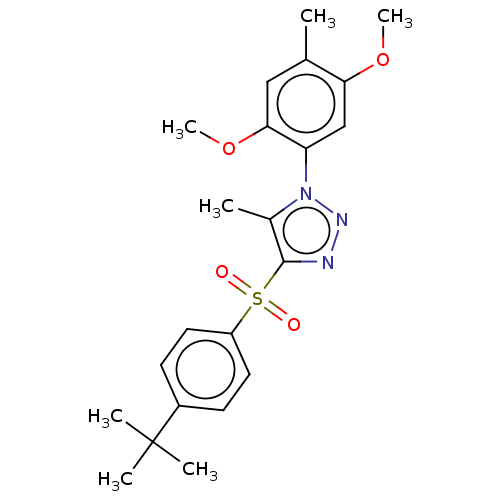 (US10550091, No. LC-11 | US10947203, No. LC-11) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of BODIPY FL vindoline from GST-tagged human PXR LBD incubated for 60 mins by TR-FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02201 BindingDB Entry DOI: 10.7270/Q2KS6WBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM429630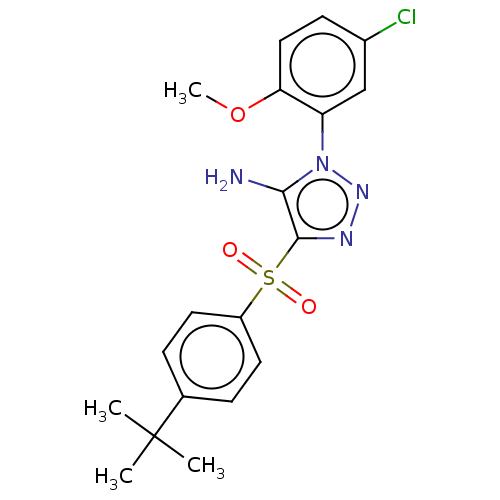 (US10550091, No. LC-39 | US10947203, No. LC-39) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of BODIPY FL vindoline from GST-tagged human PXR LBD incubated for 60 mins by TR-FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02201 BindingDB Entry DOI: 10.7270/Q2KS6WBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM429650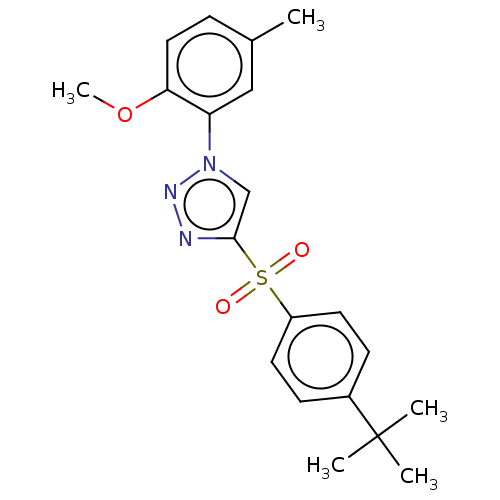 (US10550091, No. LC-59 | US10947203, No. LC-59) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of BODIPY FL vindoline from GST-tagged human PXR LBD incubated for 60 mins by TR-FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02201 BindingDB Entry DOI: 10.7270/Q2KS6WBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM8610 (1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 513 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of CYP3A5 in wild type human AsPC1 cells assessed as decrease in 1-hydroxymidazolam formation using midazolam as substrate after 24 hrs by... | J Med Chem 63: 1415-1433 (2020) Article DOI: 10.1021/acs.jmedchem.9b02067 BindingDB Entry DOI: 10.7270/Q2NS0Z6Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM429597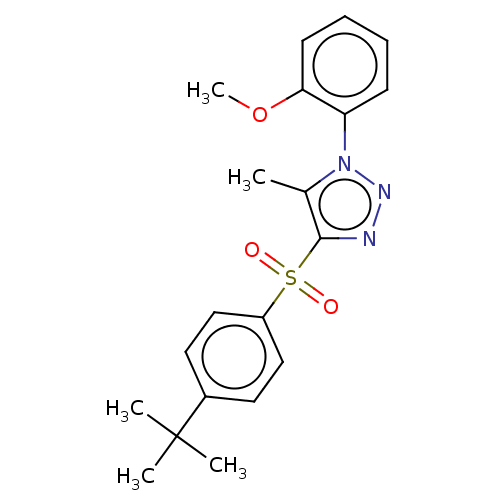 (US10550091, No. LC-6 | US10947203, No. LC-6) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of BODIPY FL vindoline from GST-tagged human PXR LBD incubated for 60 mins by TR-FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02201 BindingDB Entry DOI: 10.7270/Q2KS6WBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM429663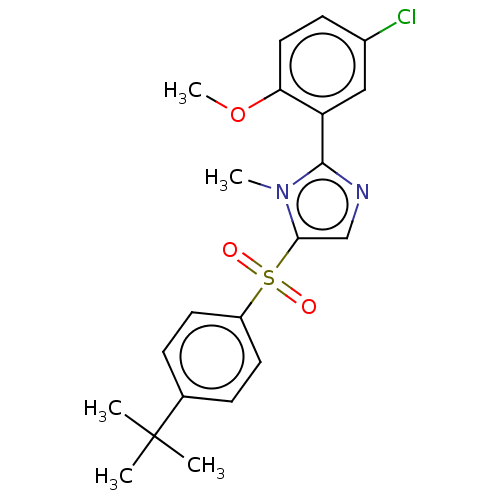 (US10550091, No. LC-72 | US10947203, No. LC-72) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of BODIPY FL vindoline from GST-tagged human PXR LBD incubated for 60 mins by TR-FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02201 BindingDB Entry DOI: 10.7270/Q2KS6WBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM429620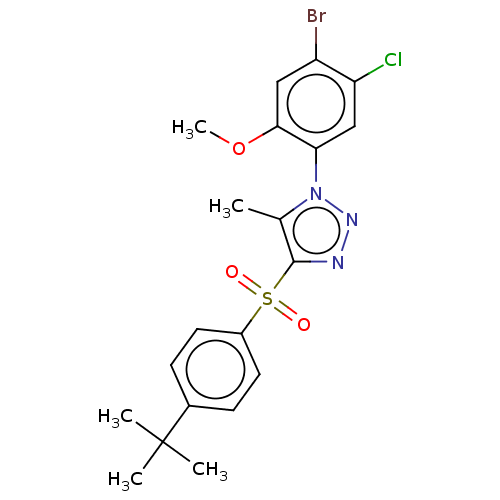 (US10550091, No. LC-29 | US10947203, No. LC-29) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of BODIPY FL vindoline from GST-tagged human PXR LBD incubated for 60 mins by TR-FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02201 BindingDB Entry DOI: 10.7270/Q2KS6WBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM429609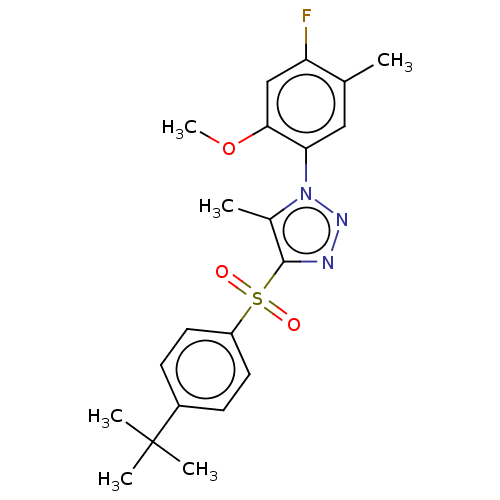 (US10550091, No. LC-18 | US10947203, No. LC-18) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of BODIPY FL vindoline from GST-tagged human PXR LBD incubated for 60 mins by TR-FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02201 BindingDB Entry DOI: 10.7270/Q2KS6WBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM429627 (US10550091, No. LC-36 | US10947203, No. LC-36) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of BODIPY FL vindoline from GST-tagged human PXR LBD incubated for 60 mins by TR-FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02201 BindingDB Entry DOI: 10.7270/Q2KS6WBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 197 total ) | Next | Last >> |