

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50327809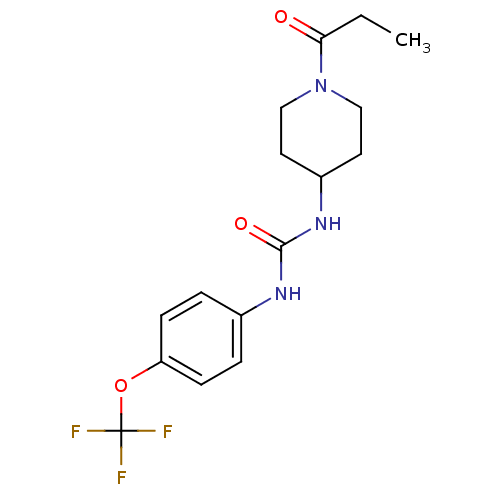 (1-(1-Propionylpiperidin-4-yl)-3-(4-(trifluorometho...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Curated by ChEMBL | Assay Description Inhibition of recombinant human full length sEH (1 to 555 residues) expressed in Escherichia coli BL21 DE3 cells assessed as reduction in 6-methoxyna... | ACS Med Chem Lett 11: 1244-1249 (2020) Article DOI: 10.1021/acsmedchemlett.0c00102 BindingDB Entry DOI: 10.7270/Q2FB56H6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM5025 (Oseltamivir | US10919856, POSITIVE CONTROL | ethyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/California/04/2009 (H1N1) neuraminidase expressed by HEK293 cells incubated for 1 hr using 4-MU-NANA substrate by ... | Bioorg Med Chem 27: 3846-3852 (2019) Article DOI: 10.1016/j.bmc.2019.07.013 BindingDB Entry DOI: 10.7270/Q2V40ZMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM23971 ((2S)-2-[(2S,3R)-3-amino-2-hydroxy-4-phenylbutanami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 266 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Curated by ChEMBL | Assay Description Inhibition of recombinant human full length C-terminal His6-tagged LTA4H expressed in Escherichia coli BL21 DE3 cells assessed as reduction in amino-... | ACS Med Chem Lett 11: 1244-1249 (2020) Article DOI: 10.1021/acsmedchemlett.0c00102 BindingDB Entry DOI: 10.7270/Q2FB56H6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50541959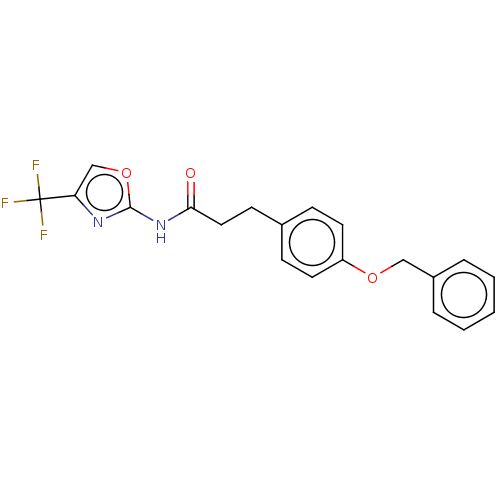 (CHEMBL4649188) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Curated by ChEMBL | Assay Description Inhibition of recombinant human full length sEH (1 to 555 residues) expressed in Escherichia coli BL21 DE3 cells assessed as reduction in 6-methoxyna... | ACS Med Chem Lett 11: 1244-1249 (2020) Article DOI: 10.1021/acsmedchemlett.0c00102 BindingDB Entry DOI: 10.7270/Q2FB56H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50541953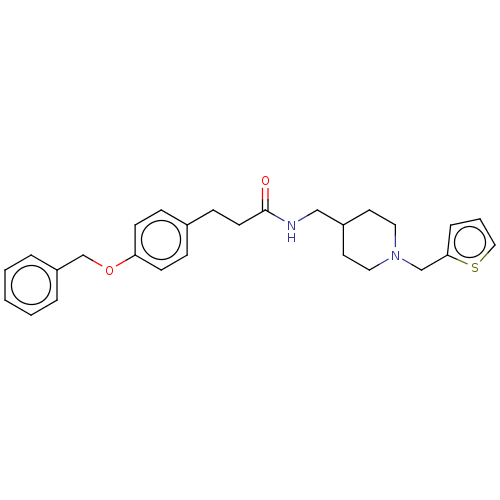 (CHEMBL4644372) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Curated by ChEMBL | Assay Description Inhibition of recombinant human full length sEH (1 to 555 residues) expressed in Escherichia coli BL21 DE3 cells assessed as reduction in 6-methoxyna... | ACS Med Chem Lett 11: 1244-1249 (2020) Article DOI: 10.1021/acsmedchemlett.0c00102 BindingDB Entry DOI: 10.7270/Q2FB56H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50541959 (CHEMBL4649188) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Curated by ChEMBL | Assay Description Inhibition of recombinant human full length C-terminal His6-tagged LTA4H expressed in Escherichia coli BL21 DE3 cells assessed as reduction in amino-... | ACS Med Chem Lett 11: 1244-1249 (2020) Article DOI: 10.1021/acsmedchemlett.0c00102 BindingDB Entry DOI: 10.7270/Q2FB56H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50541960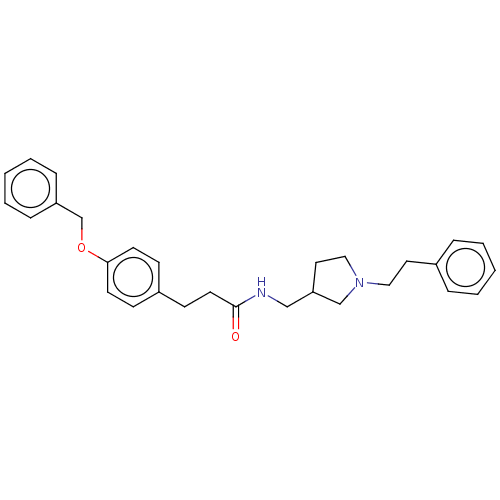 (CHEMBL4645053) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Curated by ChEMBL | Assay Description Inhibition of recombinant human full length C-terminal His6-tagged LTA4H expressed in Escherichia coli BL21 DE3 cells assessed as reduction in amino-... | ACS Med Chem Lett 11: 1244-1249 (2020) Article DOI: 10.1021/acsmedchemlett.0c00102 BindingDB Entry DOI: 10.7270/Q2FB56H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50541952 (CHEMBL4646891) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Curated by ChEMBL | Assay Description Inhibition of recombinant human full length C-terminal His6-tagged LTA4H expressed in Escherichia coli BL21 DE3 cells assessed as reduction in amino-... | ACS Med Chem Lett 11: 1244-1249 (2020) Article DOI: 10.1021/acsmedchemlett.0c00102 BindingDB Entry DOI: 10.7270/Q2FB56H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50541953 (CHEMBL4644372) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Curated by ChEMBL | Assay Description Inhibition of recombinant human full length C-terminal His6-tagged LTA4H expressed in Escherichia coli BL21 DE3 cells assessed as reduction in amino-... | ACS Med Chem Lett 11: 1244-1249 (2020) Article DOI: 10.1021/acsmedchemlett.0c00102 BindingDB Entry DOI: 10.7270/Q2FB56H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50541956 (CHEMBL4635127) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Curated by ChEMBL | Assay Description Inhibition of recombinant human full length sEH (1 to 555 residues) expressed in Escherichia coli BL21 DE3 cells assessed as reduction in 6-methoxyna... | ACS Med Chem Lett 11: 1244-1249 (2020) Article DOI: 10.1021/acsmedchemlett.0c00102 BindingDB Entry DOI: 10.7270/Q2FB56H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50521984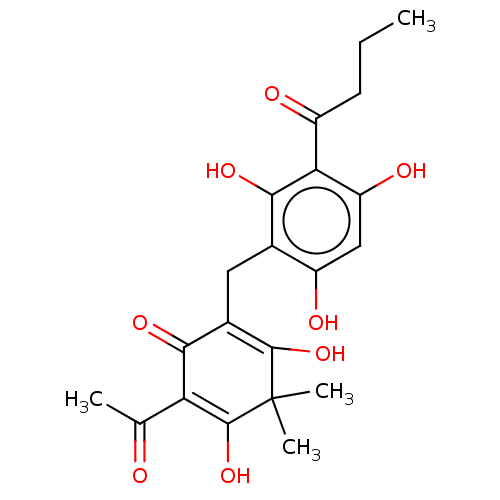 (CHEMBL4548268) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/Anhui/1/2013 (H7N9) neuraminidase expressed by HEK293 cells incubated for 1 hr using 4-MU-NANA substrate by spectr... | Bioorg Med Chem 27: 3846-3852 (2019) Article DOI: 10.1016/j.bmc.2019.07.013 BindingDB Entry DOI: 10.7270/Q2V40ZMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50521983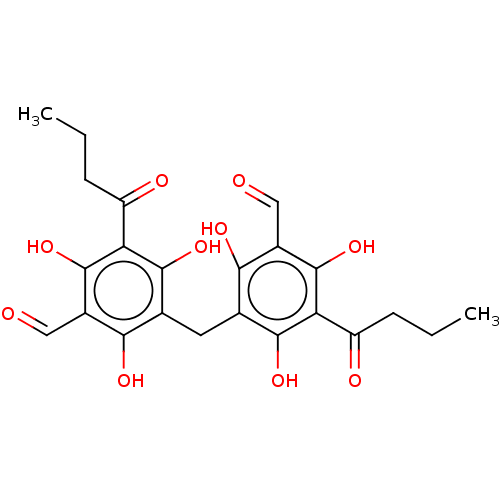 (CHEMBL4442860) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/Anhui/1/2013 (H7N9) neuraminidase expressed by HEK293 cells incubated for 1 hr using 4-MU-NANA substrate by spectr... | Bioorg Med Chem 27: 3846-3852 (2019) Article DOI: 10.1016/j.bmc.2019.07.013 BindingDB Entry DOI: 10.7270/Q2V40ZMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM5025 (Oseltamivir | US10919856, POSITIVE CONTROL | ethyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/Anhui/1/2005(H5N1)) neuraminidase expressed by HEK293 cells incubated for 1 hr using 4-MU-NANA substrate by spectr... | Bioorg Med Chem 27: 3846-3852 (2019) Article DOI: 10.1016/j.bmc.2019.07.013 BindingDB Entry DOI: 10.7270/Q2V40ZMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50541954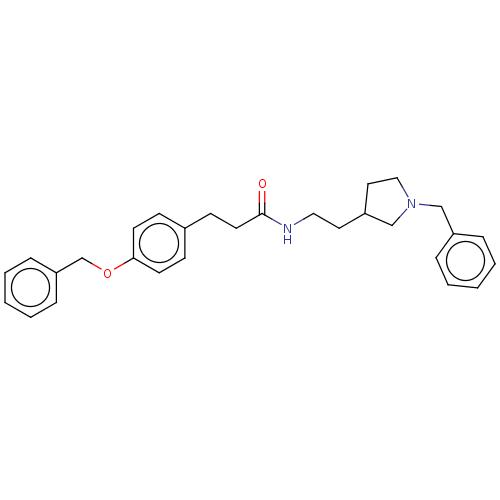 (CHEMBL4640292) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Curated by ChEMBL | Assay Description Inhibition of recombinant human full length C-terminal His6-tagged LTA4H expressed in Escherichia coli BL21 DE3 cells assessed as reduction in amino-... | ACS Med Chem Lett 11: 1244-1249 (2020) Article DOI: 10.1021/acsmedchemlett.0c00102 BindingDB Entry DOI: 10.7270/Q2FB56H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50521985 (CHEMBL4551878) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/Anhui/1/2013 (H7N9) neuraminidase expressed by HEK293 cells incubated for 1 hr using 4-MU-NANA substrate by spectr... | Bioorg Med Chem 27: 3846-3852 (2019) Article DOI: 10.1016/j.bmc.2019.07.013 BindingDB Entry DOI: 10.7270/Q2V40ZMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50541958 (CHEMBL4649795) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Curated by ChEMBL | Assay Description Inhibition of recombinant human full length C-terminal His6-tagged LTA4H expressed in Escherichia coli BL21 DE3 cells assessed as reduction in amino-... | ACS Med Chem Lett 11: 1244-1249 (2020) Article DOI: 10.1021/acsmedchemlett.0c00102 BindingDB Entry DOI: 10.7270/Q2FB56H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50541958 (CHEMBL4649795) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Curated by ChEMBL | Assay Description Inhibition of recombinant human full length sEH (1 to 555 residues) expressed in Escherichia coli BL21 DE3 cells assessed as reduction in 6-methoxyna... | ACS Med Chem Lett 11: 1244-1249 (2020) Article DOI: 10.1021/acsmedchemlett.0c00102 BindingDB Entry DOI: 10.7270/Q2FB56H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50068107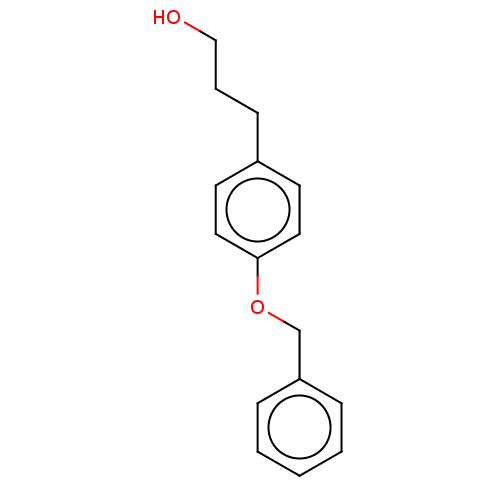 (CHEMBL3402237) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal His6-tagged LTA4H expressed in Escherichia coli BL21 DE3 cells using 7-amido-4-methylcoumarin as substrate... | ACS Med Chem Lett 11: 1244-1249 (2020) Article DOI: 10.1021/acsmedchemlett.0c00102 BindingDB Entry DOI: 10.7270/Q2FB56H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50521984 (CHEMBL4548268) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/California/04/2009 (H1N1) neuraminidase expressed by HEK293 cells incubated for 1 hr using 4-MU-NANA substrate by ... | Bioorg Med Chem 27: 3846-3852 (2019) Article DOI: 10.1016/j.bmc.2019.07.013 BindingDB Entry DOI: 10.7270/Q2V40ZMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM5025 (Oseltamivir | US10919856, POSITIVE CONTROL | ethyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/Anhui/1/2013 (H7N9) neuraminidase expressed by HEK293 cells incubated for 1 hr using 4-MU-NANA substrate by spectr... | Bioorg Med Chem 27: 3846-3852 (2019) Article DOI: 10.1016/j.bmc.2019.07.013 BindingDB Entry DOI: 10.7270/Q2V40ZMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50541955 (CHEMBL4644992) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Curated by ChEMBL | Assay Description Inhibition of recombinant human full length sEH (1 to 555 residues) expressed in Escherichia coli BL21 DE3 cells assessed as reduction in 6-methoxyna... | ACS Med Chem Lett 11: 1244-1249 (2020) Article DOI: 10.1021/acsmedchemlett.0c00102 BindingDB Entry DOI: 10.7270/Q2FB56H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50521983 (CHEMBL4442860) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/California/04/2009 (H1N1) neuraminidase expressed by HEK293 cells incubated for 1 hr using 4-MU-NANA substrate by ... | Bioorg Med Chem 27: 3846-3852 (2019) Article DOI: 10.1016/j.bmc.2019.07.013 BindingDB Entry DOI: 10.7270/Q2V40ZMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50521983 (CHEMBL4442860) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/Anhui/1/2005(H5N1)) neuraminidase expressed by HEK293 cells incubated for 1 hr using 4-MU-NANA substrate by spectr... | Bioorg Med Chem 27: 3846-3852 (2019) Article DOI: 10.1016/j.bmc.2019.07.013 BindingDB Entry DOI: 10.7270/Q2V40ZMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50541952 (CHEMBL4646891) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Curated by ChEMBL | Assay Description Inhibition of recombinant human full length sEH (1 to 555 residues) expressed in Escherichia coli BL21 DE3 cells assessed as reduction in 6-methoxyna... | ACS Med Chem Lett 11: 1244-1249 (2020) Article DOI: 10.1021/acsmedchemlett.0c00102 BindingDB Entry DOI: 10.7270/Q2FB56H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50541957 (CHEMBL4637146) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Curated by ChEMBL | Assay Description Inhibition of recombinant human full length sEH (1 to 555 residues) expressed in Escherichia coli BL21 DE3 cells assessed as reduction in 6-methoxyna... | ACS Med Chem Lett 11: 1244-1249 (2020) Article DOI: 10.1021/acsmedchemlett.0c00102 BindingDB Entry DOI: 10.7270/Q2FB56H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50541961 (CHEMBL4632833) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.83E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Curated by ChEMBL | Assay Description Inhibition of recombinant human full length C-terminal His6-tagged LTA4H expressed in Escherichia coli BL21 DE3 cells assessed as reduction in amino-... | ACS Med Chem Lett 11: 1244-1249 (2020) Article DOI: 10.1021/acsmedchemlett.0c00102 BindingDB Entry DOI: 10.7270/Q2FB56H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50521985 (CHEMBL4551878) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/California/04/2009 (H1N1) neuraminidase expressed by HEK293 cells incubated for 1 hr using 4-MU-NANA substrate by ... | Bioorg Med Chem 27: 3846-3852 (2019) Article DOI: 10.1016/j.bmc.2019.07.013 BindingDB Entry DOI: 10.7270/Q2V40ZMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50541954 (CHEMBL4640292) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Curated by ChEMBL | Assay Description Inhibition of recombinant human full length sEH (1 to 555 residues) expressed in Escherichia coli BL21 DE3 cells assessed as reduction in 6-methoxyna... | ACS Med Chem Lett 11: 1244-1249 (2020) Article DOI: 10.1021/acsmedchemlett.0c00102 BindingDB Entry DOI: 10.7270/Q2FB56H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50521984 (CHEMBL4548268) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/Anhui/1/2005(H5N1)) neuraminidase expressed by HEK293 cells incubated for 1 hr using 4-MU-NANA substrate by spectr... | Bioorg Med Chem 27: 3846-3852 (2019) Article DOI: 10.1016/j.bmc.2019.07.013 BindingDB Entry DOI: 10.7270/Q2V40ZMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50521985 (CHEMBL4551878) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/Anhui/1/2005(H5N1)) neuraminidase expressed by HEK293 cells incubated for 1 hr using 4-MU-NANA substrate by spectr... | Bioorg Med Chem 27: 3846-3852 (2019) Article DOI: 10.1016/j.bmc.2019.07.013 BindingDB Entry DOI: 10.7270/Q2V40ZMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50068107 (CHEMBL3402237) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 7.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Curated by ChEMBL | Assay Description Inhibition of recombinant human sEH using PHOME as substrate preincubated with enzyme for 10 mins prior to substrate addition by fluorescence based a... | ACS Med Chem Lett 11: 1244-1249 (2020) Article DOI: 10.1021/acsmedchemlett.0c00102 BindingDB Entry DOI: 10.7270/Q2FB56H6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||