 Found 157 hits with Last Name = 'bonnette' and Initial = 'wg'
Found 157 hits with Last Name = 'bonnette' and Initial = 'wg' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant COX-2 using arachidonic acid as substrate preincubated for 60 mins followed by substrate addition for 2 secs by ADPH ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00890
BindingDB Entry DOI: 10.7270/Q24B352D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50566910
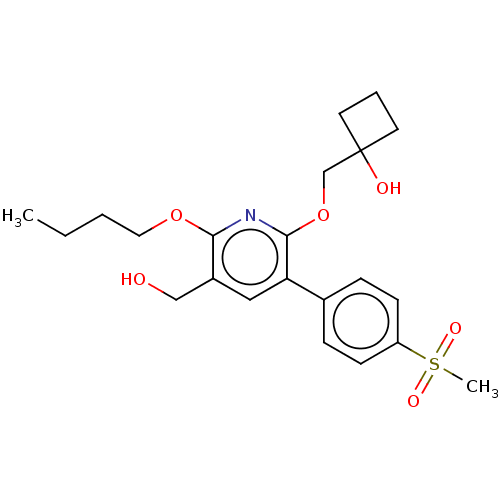
(CHEMBL4870569)Show SMILES CCCCOc1nc(OCC2(O)CCC2)c(cc1CO)-c1ccc(cc1)S(C)(=O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant COX-2 using arachidonic acid as substrate preincubated for 60 mins followed by substrate addition for 2 secs by ADPH ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00890
BindingDB Entry DOI: 10.7270/Q24B352D |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50566912
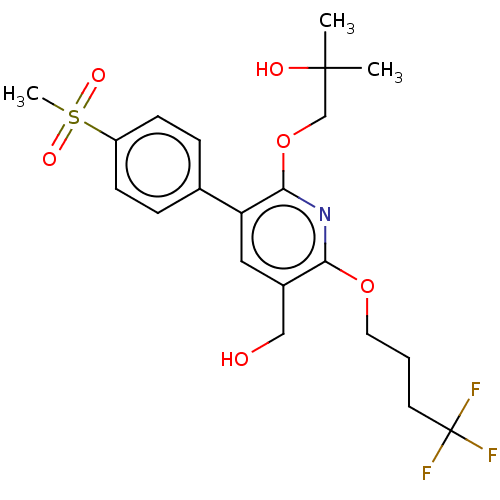
(CHEMBL4859571)Show SMILES CC(C)(O)COc1nc(OCCCC(F)(F)F)c(CO)cc1-c1ccc(cc1)S(C)(=O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant COX-2 using arachidonic acid as substrate preincubated for 60 mins followed by substrate addition for 2 secs by ADPH ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00890
BindingDB Entry DOI: 10.7270/Q24B352D |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50566901
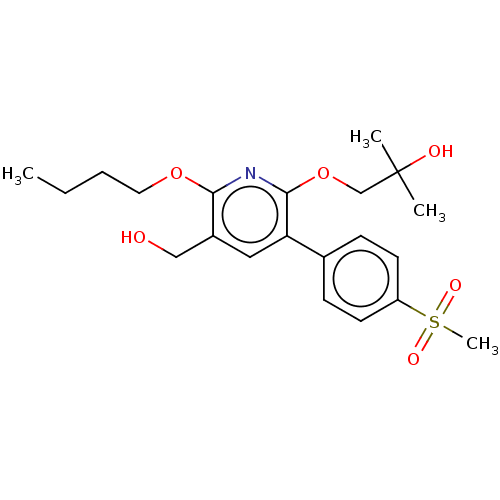
(CHEMBL4865464)Show SMILES CCCCOc1nc(OCC(C)(C)O)c(cc1CO)-c1ccc(cc1)S(C)(=O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant COX-2 using arachidonic acid as substrate preincubated for 60 mins followed by substrate addition for 2 secs by ADPH ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00890
BindingDB Entry DOI: 10.7270/Q24B352D |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50566913

(CHEMBL4857474)Show SMILES CCCCOc1nc(OCC(C)(C)O)c(cc1CO)-c1ccc(cn1)S(N)(=O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant COX-2 using arachidonic acid as substrate preincubated for 60 mins followed by substrate addition for 2 secs by ADPH ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00890
BindingDB Entry DOI: 10.7270/Q24B352D |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50566911
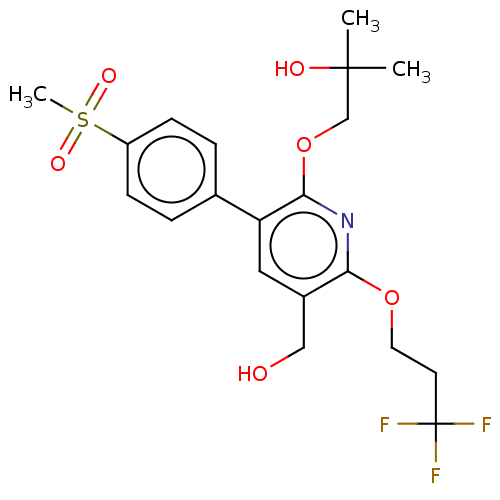
(CHEMBL4868446)Show SMILES CC(C)(O)COc1nc(OCCC(F)(F)F)c(CO)cc1-c1ccc(cc1)S(C)(=O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant COX-2 using arachidonic acid as substrate preincubated for 60 mins followed by substrate addition for 2 secs by ADPH ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00890
BindingDB Entry DOI: 10.7270/Q24B352D |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50072064

(5-Chloro-3-(4-methanesulfonyl-phenyl)-6''-methyl-[...)Show SMILES Cc1ccc(cn1)-c1ncc(Cl)cc1-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C18H15ClN2O2S/c1-12-3-4-14(10-20-12)18-17(9-15(19)11-21-18)13-5-7-16(8-6-13)24(2,22)23/h3-11H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant COX-2 using arachidonic acid as substrate preincubated for 60 mins followed by substrate addition for 2 secs by ADPH ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00890
BindingDB Entry DOI: 10.7270/Q24B352D |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50566900
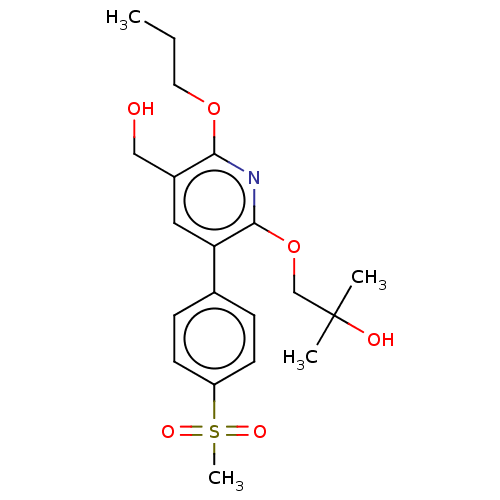
(CHEMBL4862802)Show SMILES CCCOc1nc(OCC(C)(C)O)c(cc1CO)-c1ccc(cc1)S(C)(=O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 114 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant COX-2 using arachidonic acid as substrate preincubated for 60 mins followed by substrate addition for 2 secs by ADPH ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00890
BindingDB Entry DOI: 10.7270/Q24B352D |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50566911

(CHEMBL4868446)Show SMILES CC(C)(O)COc1nc(OCCC(F)(F)F)c(CO)cc1-c1ccc(cc1)S(C)(=O)=O | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vivo inhibition of COX2 in po dosed C57BL/6J mouse model of spontaneous GI-tract tumor Apc-min assessed as chemopreventive effect by measuring red... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00890
BindingDB Entry DOI: 10.7270/Q24B352D |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50566912

(CHEMBL4859571)Show SMILES CC(C)(O)COc1nc(OCCCC(F)(F)F)c(CO)cc1-c1ccc(cc1)S(C)(=O)=O | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vivo inhibition of COX2 in po dosed C57BL/6J mouse model of spontaneous GI-tract tumor Apc-min assessed as chemopreventive effect by measuring red... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00890
BindingDB Entry DOI: 10.7270/Q24B352D |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vivo inhibition of COX2 in po dosed C57BL/6J mouse model of spontaneous GI-tract tumor Apc-min assessed as chemopreventive effect by measuring red... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00890
BindingDB Entry DOI: 10.7270/Q24B352D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vivo inhibition of COX2 in po dosed C57BL/6J mouse model of spontaneous GI-tract tumor Apc-min assessed as chemopreventive effect by measuring red... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00890
BindingDB Entry DOI: 10.7270/Q24B352D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant COX-2 assessed as reduction in peroxidase activity using arachidonic acid as substrate preincubated for 60 mins follo... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00890
BindingDB Entry DOI: 10.7270/Q24B352D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50566900

(CHEMBL4862802)Show SMILES CCCOc1nc(OCC(C)(C)O)c(cc1CO)-c1ccc(cc1)S(C)(=O)=O | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vivo inhibition of COX2 in po dosed C57BL/6J mouse model of spontaneous GI-tract tumor Apc-min assessed as chemopreventive effect by measuring red... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00890
BindingDB Entry DOI: 10.7270/Q24B352D |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50566907
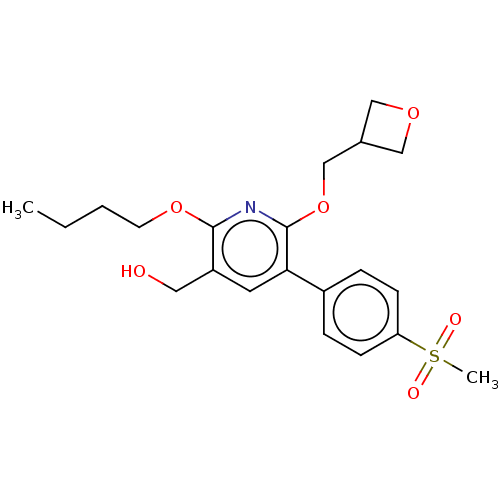
(CHEMBL4851236)Show SMILES CCCCOc1nc(OCC2COC2)c(cc1CO)-c1ccc(cc1)S(C)(=O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of COX2 (unknown origin) expressed in HEK293 TRex cells assessed as reduction in PGE2 production using arachidonic acid as substrate prein... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00890
BindingDB Entry DOI: 10.7270/Q24B352D |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50566910

(CHEMBL4870569)Show SMILES CCCCOc1nc(OCC2(O)CCC2)c(cc1CO)-c1ccc(cc1)S(C)(=O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of COX2 (unknown origin) expressed in HEK293 TRex cells assessed as reduction in PGE2 production using arachidonic acid as substrate prein... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00890
BindingDB Entry DOI: 10.7270/Q24B352D |
More data for this
Ligand-Target Pair | |
Kelch-like ECH-associated protein 1
(Homo sapiens (Human)) | BDBM50601802
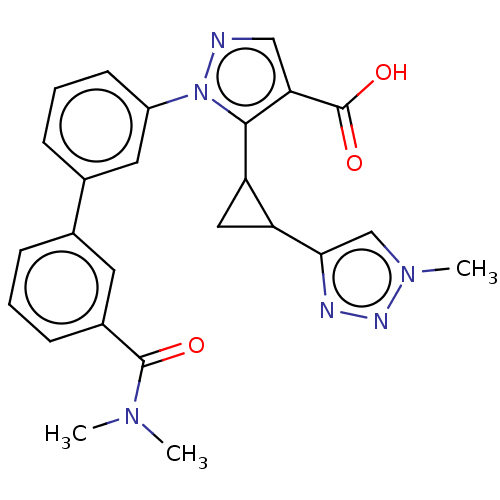
(CHEMBL5203783)Show SMILES CN(C)C(=O)c1cccc(c1)-c1cccc(c1)-n1ncc(C(O)=O)c1C1CC1c1cn(C)nn1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01351
BindingDB Entry DOI: 10.7270/Q2JS9VJF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Kelch-like ECH-associated protein 1
(Homo sapiens (Human)) | BDBM50601811
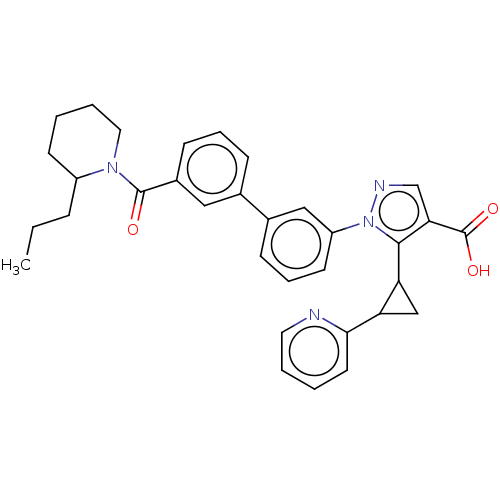
(CHEMBL5189627)Show SMILES CCCC1CCCCN1C(=O)c1cccc(c1)-c1cccc(c1)-n1ncc(C(O)=O)c1C1CC1c1ccccn1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01351
BindingDB Entry DOI: 10.7270/Q2JS9VJF |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50566901

(CHEMBL4865464)Show SMILES CCCCOc1nc(OCC(C)(C)O)c(cc1CO)-c1ccc(cc1)S(C)(=O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of COX2 (unknown origin) expressed in HEK293 TRex cells assessed as reduction in PGE2 production using arachidonic acid as substrate prein... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00890
BindingDB Entry DOI: 10.7270/Q24B352D |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50566906

(CHEMBL4867038)Show SMILES CCCCOc1nc(-c2ccc(F)cc2)c(cc1CO)-c1ccc(cc1)S(C)(=O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of COX2 (unknown origin) expressed in HEK293 TRex cells assessed as reduction in PGE2 production using arachidonic acid as substrate prein... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00890
BindingDB Entry DOI: 10.7270/Q24B352D |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of COX2 (unknown origin) expressed in HEK293 TRex cells assessed as reduction in PGE2 production using arachidonic acid as substrate prein... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00890
BindingDB Entry DOI: 10.7270/Q24B352D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Kelch-like ECH-associated protein 1
(Homo sapiens (Human)) | BDBM50601805
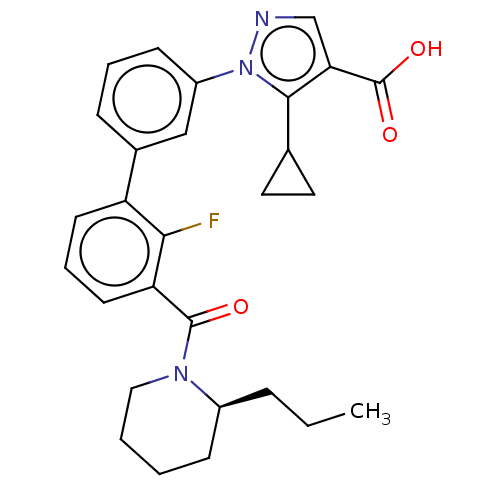
(CHEMBL5208399)Show SMILES CCC[C@H]1CCCCN1C(=O)c1cccc(c1F)-c1cccc(c1)-n1ncc(C(O)=O)c1C1CC1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01351
BindingDB Entry DOI: 10.7270/Q2JS9VJF |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50566912

(CHEMBL4859571)Show SMILES CC(C)(O)COc1nc(OCCCC(F)(F)F)c(CO)cc1-c1ccc(cc1)S(C)(=O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of COX2 (unknown origin) expressed in HEK293 TRex cells assessed as reduction in PGE2 production using arachidonic acid as substrate prein... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00890
BindingDB Entry DOI: 10.7270/Q24B352D |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50566910

(CHEMBL4870569)Show SMILES CCCCOc1nc(OCC2(O)CCC2)c(cc1CO)-c1ccc(cc1)S(C)(=O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant COX-2 assessed as reduction in peroxidase activity using arachidonic acid as substrate preincubated for 60 mins follo... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00890
BindingDB Entry DOI: 10.7270/Q24B352D |
More data for this
Ligand-Target Pair | |
Kelch-like ECH-associated protein 1
(Homo sapiens (Human)) | BDBM50601804
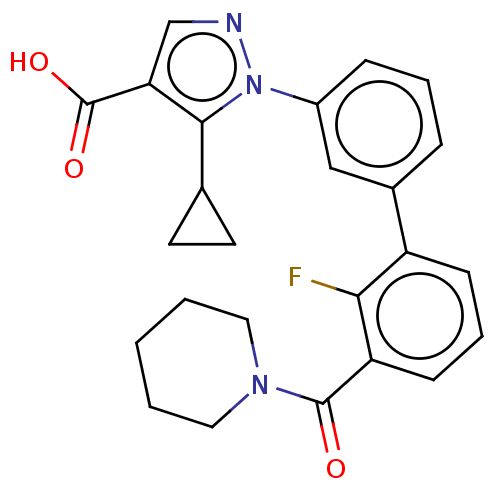
(CHEMBL5176162)Show SMILES OC(=O)c1cnn(c1C1CC1)-c1cccc(c1)-c1cccc(C(=O)N2CCCCC2)c1F | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01351
BindingDB Entry DOI: 10.7270/Q2JS9VJF |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50566911

(CHEMBL4868446)Show SMILES CC(C)(O)COc1nc(OCCC(F)(F)F)c(CO)cc1-c1ccc(cc1)S(C)(=O)=O | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of COX1 (unknown origin) expressed in HEK293 TRex cells assessed as reduction in PGE2 production using arachidonic acid as substrate prein... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00890
BindingDB Entry DOI: 10.7270/Q24B352D |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50566911

(CHEMBL4868446)Show SMILES CC(C)(O)COc1nc(OCCC(F)(F)F)c(CO)cc1-c1ccc(cc1)S(C)(=O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of COX2 (unknown origin) expressed in HEK293 TRex cells assessed as reduction in PGE2 production using arachidonic acid as substrate prein... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00890
BindingDB Entry DOI: 10.7270/Q24B352D |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50566910

(CHEMBL4870569)Show SMILES CCCCOc1nc(OCC2(O)CCC2)c(cc1CO)-c1ccc(cc1)S(C)(=O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 146 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of COX2 in human whole blood assessed as reduction in LPS stimulated PGE2 production incubated for 1 hr by ELISA |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00890
BindingDB Entry DOI: 10.7270/Q24B352D |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50566912

(CHEMBL4859571)Show SMILES CC(C)(O)COc1nc(OCCCC(F)(F)F)c(CO)cc1-c1ccc(cc1)S(C)(=O)=O | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vivo inhibition of COX2 in po dosed C57BL/6J mouse model of spontaneous GI-tract tumor Apc-min assessed as chemopreventive effect by measuring red... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00890
BindingDB Entry DOI: 10.7270/Q24B352D |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50566909
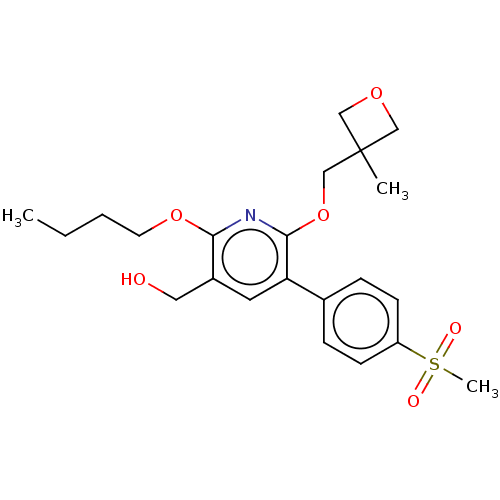
(CHEMBL4873184)Show SMILES CCCCOc1nc(OCC2(C)COC2)c(cc1CO)-c1ccc(cc1)S(C)(=O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of COX2 (unknown origin) expressed in HEK293 TRex cells assessed as reduction in PGE2 production using arachidonic acid as substrate prein... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00890
BindingDB Entry DOI: 10.7270/Q24B352D |
More data for this
Ligand-Target Pair | |
Kelch-like ECH-associated protein 1
(Homo sapiens (Human)) | BDBM50601809
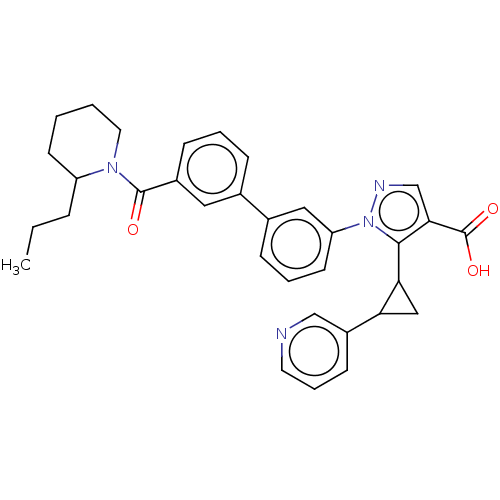
(CHEMBL5175068)Show SMILES CCCC1CCCCN1C(=O)c1cccc(c1)-c1cccc(c1)-n1ncc(C(O)=O)c1C1CC1c1cccnc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 221 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01351
BindingDB Entry DOI: 10.7270/Q2JS9VJF |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50566901

(CHEMBL4865464)Show SMILES CCCCOc1nc(OCC(C)(C)O)c(cc1CO)-c1ccc(cc1)S(C)(=O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 226 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant COX-2 assessed as reduction in peroxidase activity using arachidonic acid as substrate preincubated for 60 mins follo... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00890
BindingDB Entry DOI: 10.7270/Q24B352D |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50566913

(CHEMBL4857474)Show SMILES CCCCOc1nc(OCC(C)(C)O)c(cc1CO)-c1ccc(cn1)S(N)(=O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of COX2 (unknown origin) expressed in HEK293 TRex cells assessed as reduction in PGE2 production using arachidonic acid as substrate prein... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00890
BindingDB Entry DOI: 10.7270/Q24B352D |
More data for this
Ligand-Target Pair | |
Kelch-like ECH-associated protein 1
(Homo sapiens (Human)) | BDBM50601808
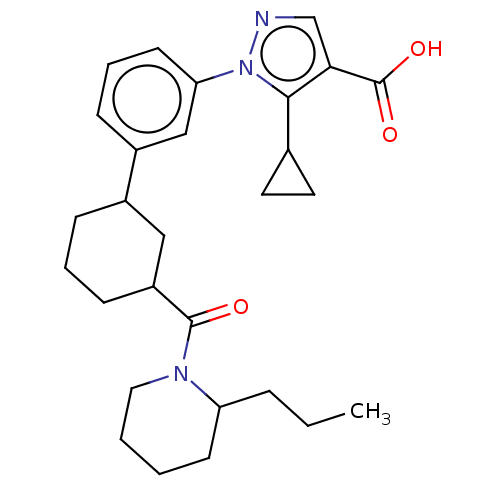
(CHEMBL5207900)Show SMILES CCCC1CCCCN1C(=O)C1CCCC(C1)c1cccc(c1)-n1ncc(C(O)=O)c1C1CC1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01351
BindingDB Entry DOI: 10.7270/Q2JS9VJF |
More data for this
Ligand-Target Pair | |
Kelch-like ECH-associated protein 1
(Homo sapiens (Human)) | BDBM50601806
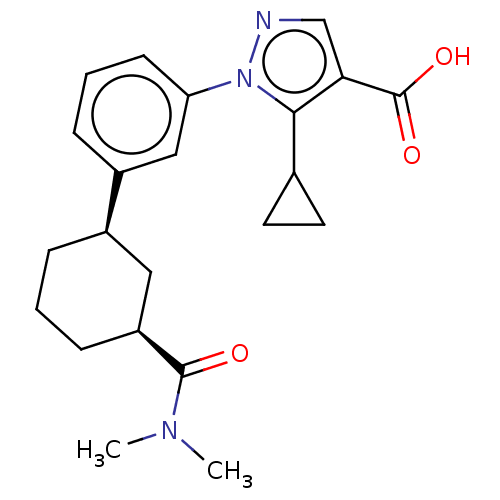
(CHEMBL5204388)Show SMILES CN(C)C(=O)[C@H]1CCC[C@H](C1)c1cccc(c1)-n1ncc(C(O)=O)c1C1CC1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01351
BindingDB Entry DOI: 10.7270/Q2JS9VJF |
More data for this
Ligand-Target Pair | |
Kelch-like ECH-associated protein 1
(Homo sapiens (Human)) | BDBM50601807
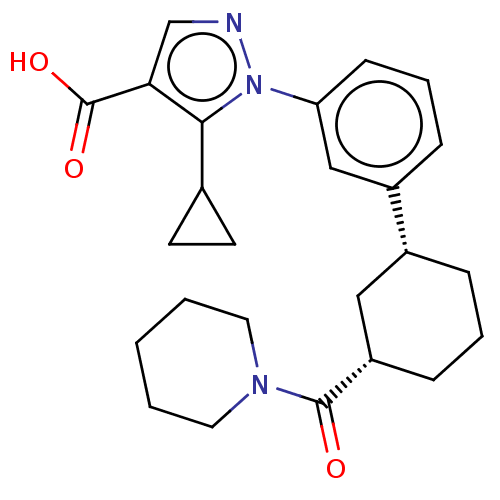
(CHEMBL5206413)Show SMILES OC(=O)c1cnn(c1C1CC1)-c1cccc(c1)[C@@H]1CCC[C@@H](C1)C(=O)N1CCCCC1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01351
BindingDB Entry DOI: 10.7270/Q2JS9VJF |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50566912

(CHEMBL4859571)Show SMILES CC(C)(O)COc1nc(OCCCC(F)(F)F)c(CO)cc1-c1ccc(cc1)S(C)(=O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 345 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of COX2 in human whole blood assessed as reduction in LPS stimulated PGE2 production incubated for 1 hr by ELISA |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00890
BindingDB Entry DOI: 10.7270/Q24B352D |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50566901

(CHEMBL4865464)Show SMILES CCCCOc1nc(OCC(C)(C)O)c(cc1CO)-c1ccc(cc1)S(C)(=O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 346 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of COX2 in human whole blood assessed as reduction in LPS stimulated PGE2 production incubated for 1 hr by ELISA |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00890
BindingDB Entry DOI: 10.7270/Q24B352D |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50566912

(CHEMBL4859571)Show SMILES CC(C)(O)COc1nc(OCCCC(F)(F)F)c(CO)cc1-c1ccc(cc1)S(C)(=O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant COX-2 assessed as reduction in peroxidase activity using arachidonic acid as substrate preincubated for 60 mins follo... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00890
BindingDB Entry DOI: 10.7270/Q24B352D |
More data for this
Ligand-Target Pair | |
Kelch-like ECH-associated protein 1
(Homo sapiens (Human)) | BDBM50601801

(CHEMBL5169432)Show SMILES CN(C)C(=O)c1cccc(c1)-c1cccc(c1)-n1ncc(C(O)=O)c1C1CC1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01351
BindingDB Entry DOI: 10.7270/Q2JS9VJF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50078590
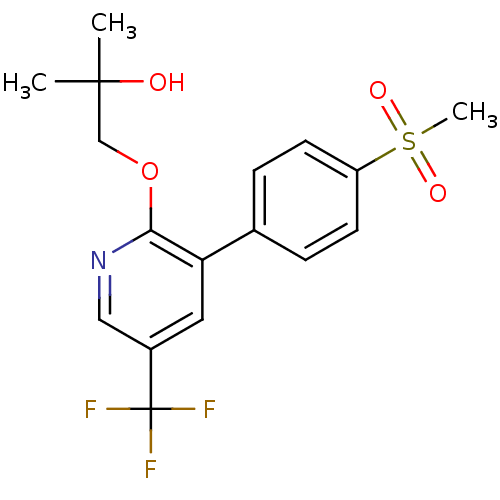
(1-[3-(4-Methanesulfonyl-phenyl)-5-trifluoromethyl-...)Show SMILES CC(C)(O)COc1ncc(cc1-c1ccc(cc1)S(C)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H18F3NO4S/c1-16(2,22)10-25-15-14(8-12(9-21-15)17(18,19)20)11-4-6-13(7-5-11)26(3,23)24/h4-9,22H,10H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant COX-2 assessed as reduction in peroxidase activity using arachidonic acid as substrate preincubated for 60 mins follo... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00890
BindingDB Entry DOI: 10.7270/Q24B352D |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of COX1 (unknown origin) expressed in HEK293 TRex cells assessed as reduction in PGE2 production using arachidonic acid as substrate prein... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00890
BindingDB Entry DOI: 10.7270/Q24B352D |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50072064

(5-Chloro-3-(4-methanesulfonyl-phenyl)-6''-methyl-[...)Show SMILES Cc1ccc(cn1)-c1ncc(Cl)cc1-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C18H15ClN2O2S/c1-12-3-4-14(10-20-12)18-17(9-15(19)11-21-18)13-5-7-16(8-6-13)24(2,22)23/h3-11H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant COX-2 assessed as reduction in peroxidase activity using arachidonic acid as substrate preincubated for 60 mins follo... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00890
BindingDB Entry DOI: 10.7270/Q24B352D |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of COX2 in human whole blood assessed as reduction in LPS stimulated PGE2 production incubated for 1 hr by ELISA |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00890
BindingDB Entry DOI: 10.7270/Q24B352D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50566913

(CHEMBL4857474)Show SMILES CCCCOc1nc(OCC(C)(C)O)c(cc1CO)-c1ccc(cn1)S(N)(=O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant COX-2 assessed as reduction in peroxidase activity using arachidonic acid as substrate preincubated for 60 mins follo... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00890
BindingDB Entry DOI: 10.7270/Q24B352D |
More data for this
Ligand-Target Pair | |
Kelch-like ECH-associated protein 1
(Homo sapiens (Human)) | BDBM50601793
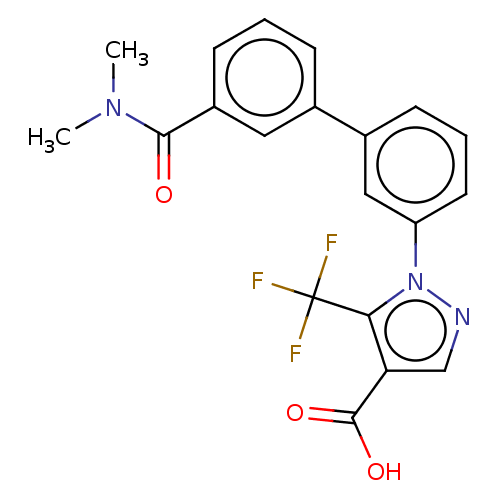
(CHEMBL5178836)Show SMILES CN(C)C(=O)c1cccc(c1)-c1cccc(c1)-n1ncc(C(O)=O)c1C(F)(F)F | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01351
BindingDB Entry DOI: 10.7270/Q2JS9VJF |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50566911

(CHEMBL4868446)Show SMILES CC(C)(O)COc1nc(OCCC(F)(F)F)c(CO)cc1-c1ccc(cc1)S(C)(=O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant COX-2 assessed as reduction in peroxidase activity using arachidonic acid as substrate preincubated for 60 mins follo... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00890
BindingDB Entry DOI: 10.7270/Q24B352D |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50566906

(CHEMBL4867038)Show SMILES CCCCOc1nc(-c2ccc(F)cc2)c(cc1CO)-c1ccc(cc1)S(C)(=O)=O | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 511 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of COX1 (unknown origin) expressed in HEK293 TRex cells assessed as reduction in PGE2 production using arachidonic acid as substrate prein... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00890
BindingDB Entry DOI: 10.7270/Q24B352D |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50566905
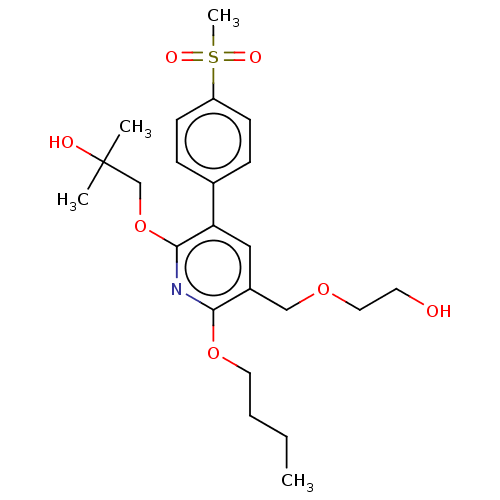
(CHEMBL4877517)Show SMILES CCCCOc1nc(OCC(C)(C)O)c(cc1COCCO)-c1ccc(cc1)S(C)(=O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of COX2 (unknown origin) expressed in HEK293 TRex cells assessed as reduction in PGE2 production using arachidonic acid as substrate prein... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00890
BindingDB Entry DOI: 10.7270/Q24B352D |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50072064

(5-Chloro-3-(4-methanesulfonyl-phenyl)-6''-methyl-[...)Show SMILES Cc1ccc(cn1)-c1ncc(Cl)cc1-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C18H15ClN2O2S/c1-12-3-4-14(10-20-12)18-17(9-15(19)11-21-18)13-5-7-16(8-6-13)24(2,22)23/h3-11H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of COX2 (unknown origin) expressed in HEK293 TRex cells assessed as reduction in PGE2 production using arachidonic acid as substrate prein... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00890
BindingDB Entry DOI: 10.7270/Q24B352D |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data