

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuraminidase (Influenza A virus) | BDBM50365366 (CHEMBL1232591) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/Indonesia/5/2005(H5N1)) X-31 recombinant neuraminidase using 2'-(4-methylumbelliferyl)-alpha-D-N-acetylneuraminic ... | Eur J Med Chem 54: 764-70 (2012) Article DOI: 10.1016/j.ejmech.2012.06.033 BindingDB Entry DOI: 10.7270/Q2FT8PVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50489423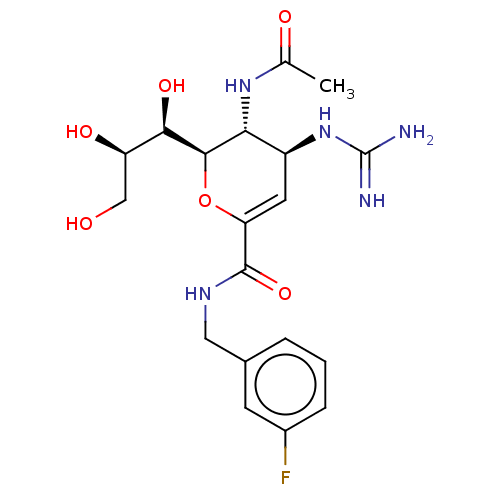 (CHEMBL2315323) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/Indonesia/5/2005(H5N1)) neuraminidase infected in chick embryo using MUNANA as substrate after 1 hr by spectrophot... | J Med Chem 56: 671-84 (2013) Article DOI: 10.1021/jm3009713 BindingDB Entry DOI: 10.7270/Q2RV0RM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50330326 ((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of influenza A virus (A/Guangdong/376/2001(H1N1)) neuraminidase infected in chick embryo using MUNANA as substrate after 1 hr by spectroph... | J Med Chem 56: 671-84 (2013) Article DOI: 10.1021/jm3009713 BindingDB Entry DOI: 10.7270/Q2RV0RM7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50330326 ((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/Sydney/5/97(H3N2)) recombinant neuraminidase using 2'-(4- methylumbelliferyl)-alpha-D-N-acetylneuraminic acid as s... | Eur J Med Chem 54: 764-70 (2012) Article DOI: 10.1016/j.ejmech.2012.06.033 BindingDB Entry DOI: 10.7270/Q2FT8PVF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50330326 ((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/Sydney/5/1997(H3N2)) neuraminidase infected in chick embryo using MUNANA as substrate after 1 hr by spectrophotome... | J Med Chem 56: 671-84 (2013) Article DOI: 10.1021/jm3009713 BindingDB Entry DOI: 10.7270/Q2RV0RM7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50330326 ((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/Indonesia/5/2005(H5N1)) X-31 recombinant neuraminidase using 2'-(4-methylumbelliferyl)-alpha-D-N-acetylneuraminic ... | Eur J Med Chem 54: 764-70 (2012) Article DOI: 10.1016/j.ejmech.2012.06.033 BindingDB Entry DOI: 10.7270/Q2FT8PVF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuraminidase (Influenza A virus) | BDBM4706 ((2R,3R,4S)-3-acetamido-4-hydroxy-2-[(1R,2R)-1,2,3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of Influenza A virus H5N1 neuraminidase | J Med Chem 56: 671-84 (2013) Article DOI: 10.1021/jm3009713 BindingDB Entry DOI: 10.7270/Q2RV0RM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50330326 ((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/Indonesia/5/2005(H5N1)) neuraminidase infected in chick embryo using MUNANA as substrate after 1 hr by spectrophot... | J Med Chem 56: 671-84 (2013) Article DOI: 10.1021/jm3009713 BindingDB Entry DOI: 10.7270/Q2RV0RM7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50489423 (CHEMBL2315323) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/Sydney/5/1997(H3N2)) neuraminidase infected in chick embryo using MUNANA as substrate after 1 hr by spectrophotome... | J Med Chem 56: 671-84 (2013) Article DOI: 10.1021/jm3009713 BindingDB Entry DOI: 10.7270/Q2RV0RM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM479502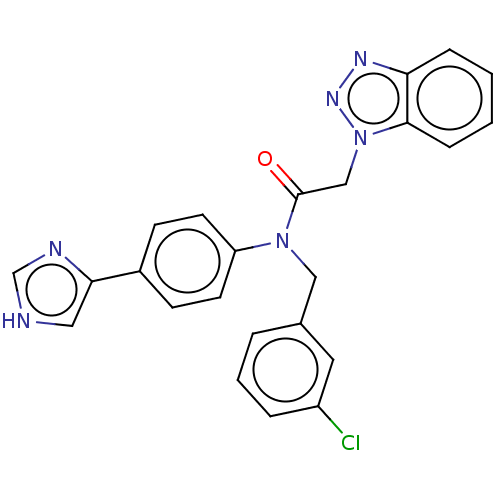 (ML300-based SC inhibitor 41) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Cleveland Clinic | Assay Description The protease activity and subsequent 10-point IC50 curves were spectroscopically determined using a scaled down, end point assay adapted from a previ... | J Med Chem 64: (2021) Article DOI: 10.1021/acs.jmedchem.1c00598 BindingDB Entry DOI: 10.7270/Q2NV9NCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM479482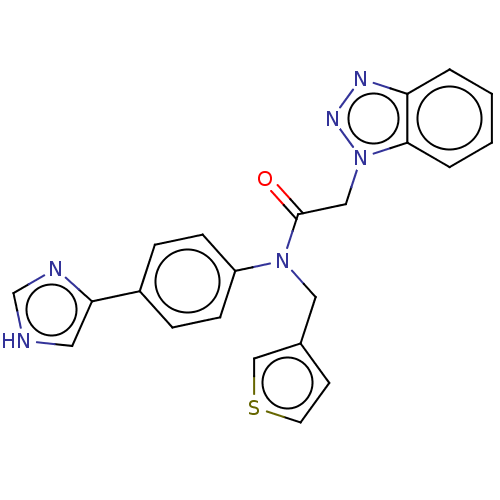 (ML300-based SC inhibitor 21) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Cleveland Clinic | Assay Description The protease activity and subsequent 10-point IC50 curves were spectroscopically determined using a scaled down, end point assay adapted from a previ... | J Med Chem 64: (2021) Article DOI: 10.1021/acs.jmedchem.1c00598 BindingDB Entry DOI: 10.7270/Q2NV9NCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50489411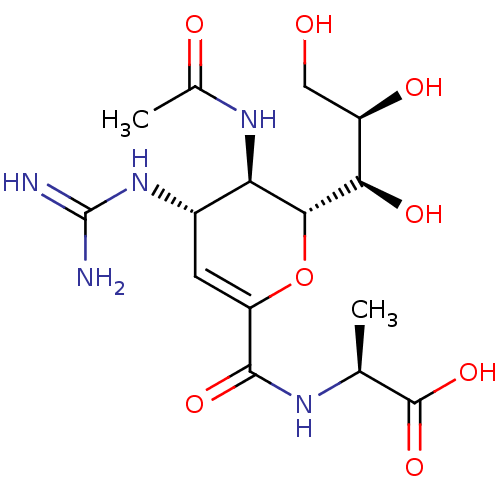 (CHEMBL2315321) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/Indonesia/5/2005(H5N1)) neuraminidase infected in chick embryo using MUNANA as substrate after 1 hr by spectrophot... | J Med Chem 56: 671-84 (2013) Article DOI: 10.1021/jm3009713 BindingDB Entry DOI: 10.7270/Q2RV0RM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM479502 (ML300-based SC inhibitor 41) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Cleveland Clinic | Assay Description The protease activity and subsequent 10-point IC50 curves were spectroscopically determined using a scaled down, end point assay adapted from a previ... | J Med Chem 64: (2021) Article DOI: 10.1021/acs.jmedchem.1c00598 BindingDB Entry DOI: 10.7270/Q2NV9NCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50489410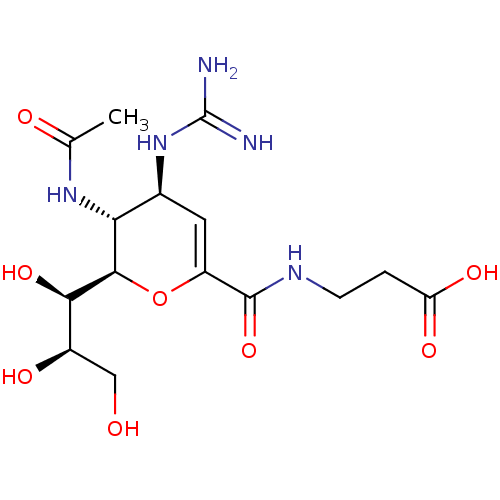 (CHEMBL2315322) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/Indonesia/5/2005(H5N1)) neuraminidase infected in chick embryo using MUNANA as substrate after 1 hr by spectrophot... | J Med Chem 56: 671-84 (2013) Article DOI: 10.1021/jm3009713 BindingDB Entry DOI: 10.7270/Q2RV0RM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM479492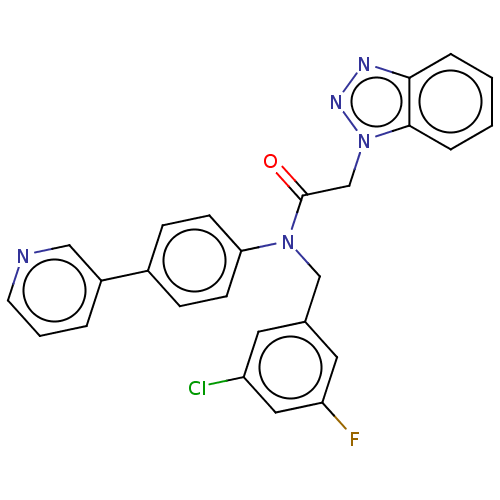 (ML300-based SC inhibitor 31) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Cleveland Clinic | Assay Description The protease activity and subsequent 10-point IC50 curves were spectroscopically determined using a scaled down, end point assay adapted from a previ... | J Med Chem 64: (2021) Article DOI: 10.1021/acs.jmedchem.1c00598 BindingDB Entry DOI: 10.7270/Q2NV9NCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50489423 (CHEMBL2315323) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of influenza A virus (A/Guangdong/376/2001(H1N1)) neuraminidase infected in chick embryo using MUNANA as substrate after 1 hr by spectroph... | J Med Chem 56: 671-84 (2013) Article DOI: 10.1021/jm3009713 BindingDB Entry DOI: 10.7270/Q2RV0RM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50489411 (CHEMBL2315321) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of influenza A virus (A/Guangdong/376/2001(H1N1)) neuraminidase infected in chick embryo using MUNANA as substrate after 1 hr by spectroph... | J Med Chem 56: 671-84 (2013) Article DOI: 10.1021/jm3009713 BindingDB Entry DOI: 10.7270/Q2RV0RM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50489418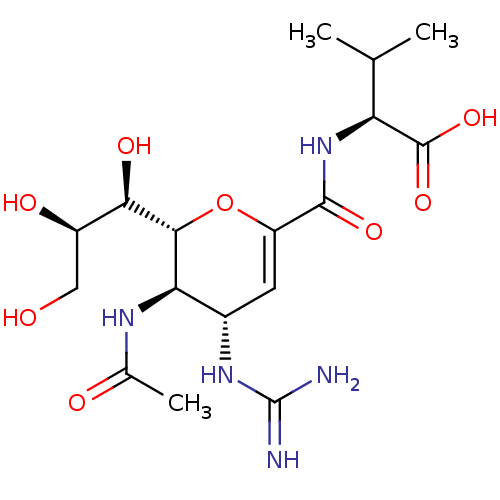 (CHEMBL2315320) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 107 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/Sydney/5/1997(H3N2)) neuraminidase infected in chick embryo using MUNANA as substrate after 1 hr by spectrophotome... | J Med Chem 56: 671-84 (2013) Article DOI: 10.1021/jm3009713 BindingDB Entry DOI: 10.7270/Q2RV0RM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM479480 (ML300-based SC inhibitor 19) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Cleveland Clinic | Assay Description The protease activity and subsequent 10-point IC50 curves were spectroscopically determined using a scaled down, end point assay adapted from a previ... | J Med Chem 64: (2021) Article DOI: 10.1021/acs.jmedchem.1c00598 BindingDB Entry DOI: 10.7270/Q2NV9NCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM479480 (ML300-based SC inhibitor 19) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Cleveland Clinic | Assay Description The protease activity and subsequent 10-point IC50 curves were spectroscopically determined using a scaled down, end point assay adapted from a previ... | J Med Chem 64: (2021) Article DOI: 10.1021/acs.jmedchem.1c00598 BindingDB Entry DOI: 10.7270/Q2NV9NCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM479493 (ML300-based SC inhibitor 32) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 111 | n/a | n/a | n/a | n/a | n/a | n/a |
Cleveland Clinic | Assay Description The protease activity and subsequent 10-point IC50 curves were spectroscopically determined using a scaled down, end point assay adapted from a previ... | J Med Chem 64: (2021) Article DOI: 10.1021/acs.jmedchem.1c00598 BindingDB Entry DOI: 10.7270/Q2NV9NCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM479492 (ML300-based SC inhibitor 31) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 119 | n/a | n/a | n/a | n/a | n/a | n/a |
Cleveland Clinic | Assay Description The protease activity and subsequent 10-point IC50 curves were spectroscopically determined using a scaled down, end point assay adapted from a previ... | J Med Chem 64: (2021) Article DOI: 10.1021/acs.jmedchem.1c00598 BindingDB Entry DOI: 10.7270/Q2NV9NCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM479482 (ML300-based SC inhibitor 21) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Cleveland Clinic | Assay Description The protease activity and subsequent 10-point IC50 curves were spectroscopically determined using a scaled down, end point assay adapted from a previ... | J Med Chem 64: (2021) Article DOI: 10.1021/acs.jmedchem.1c00598 BindingDB Entry DOI: 10.7270/Q2NV9NCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50489413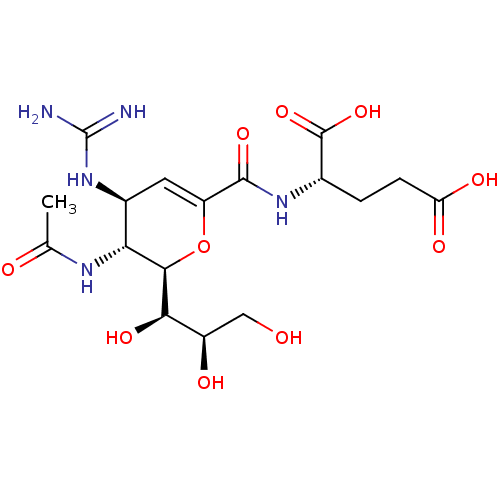 (CHEMBL2315318) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/Indonesia/5/2005(H5N1)) neuraminidase infected in chick embryo using MUNANA as substrate after 1 hr by spectrophot... | J Med Chem 56: 671-84 (2013) Article DOI: 10.1021/jm3009713 BindingDB Entry DOI: 10.7270/Q2RV0RM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM479501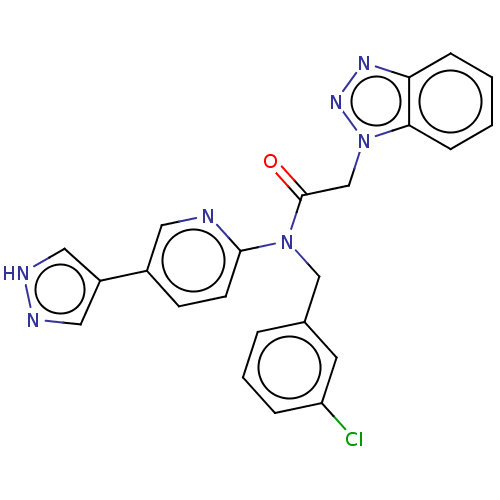 (ML300-based SC inhibitor 40) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 171 | n/a | n/a | n/a | n/a | n/a | n/a |
Cleveland Clinic | Assay Description The protease activity and subsequent 10-point IC50 curves were spectroscopically determined using a scaled down, end point assay adapted from a previ... | J Med Chem 64: (2021) Article DOI: 10.1021/acs.jmedchem.1c00598 BindingDB Entry DOI: 10.7270/Q2NV9NCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM479493 (ML300-based SC inhibitor 32) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 176 | n/a | n/a | n/a | n/a | n/a | n/a |
Cleveland Clinic | Assay Description The protease activity and subsequent 10-point IC50 curves were spectroscopically determined using a scaled down, end point assay adapted from a previ... | J Med Chem 64: (2021) Article DOI: 10.1021/acs.jmedchem.1c00598 BindingDB Entry DOI: 10.7270/Q2NV9NCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM479499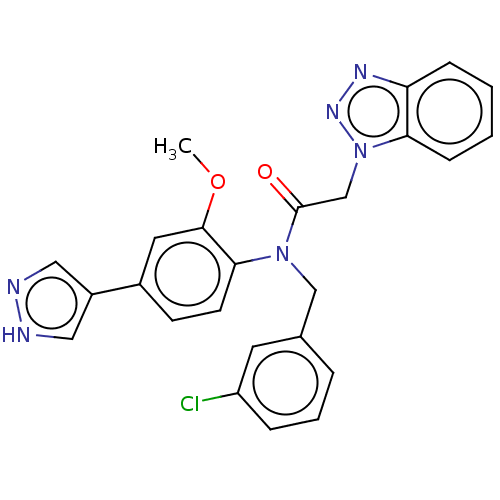 (ML300-based SC inhibitor 38) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 197 | n/a | n/a | n/a | n/a | n/a | n/a |
Cleveland Clinic | Assay Description The protease activity and subsequent 10-point IC50 curves were spectroscopically determined using a scaled down, end point assay adapted from a previ... | J Med Chem 64: (2021) Article DOI: 10.1021/acs.jmedchem.1c00598 BindingDB Entry DOI: 10.7270/Q2NV9NCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM479498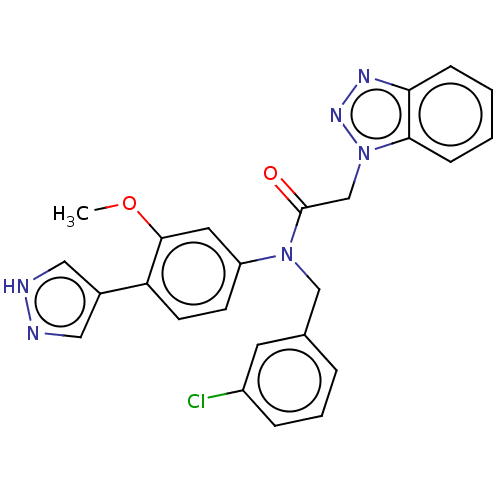 (ML300-based SC inhibitor 37) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 206 | n/a | n/a | n/a | n/a | n/a | n/a |
Cleveland Clinic | Assay Description The protease activity and subsequent 10-point IC50 curves were spectroscopically determined using a scaled down, end point assay adapted from a previ... | J Med Chem 64: (2021) Article DOI: 10.1021/acs.jmedchem.1c00598 BindingDB Entry DOI: 10.7270/Q2NV9NCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM479499 (ML300-based SC inhibitor 38) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 208 | n/a | n/a | n/a | n/a | n/a | n/a |
Cleveland Clinic | Assay Description The protease activity and subsequent 10-point IC50 curves were spectroscopically determined using a scaled down, end point assay adapted from a previ... | J Med Chem 64: (2021) Article DOI: 10.1021/acs.jmedchem.1c00598 BindingDB Entry DOI: 10.7270/Q2NV9NCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM479501 (ML300-based SC inhibitor 40) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 214 | n/a | n/a | n/a | n/a | n/a | n/a |
Cleveland Clinic | Assay Description The protease activity and subsequent 10-point IC50 curves were spectroscopically determined using a scaled down, end point assay adapted from a previ... | J Med Chem 64: (2021) Article DOI: 10.1021/acs.jmedchem.1c00598 BindingDB Entry DOI: 10.7270/Q2NV9NCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50489417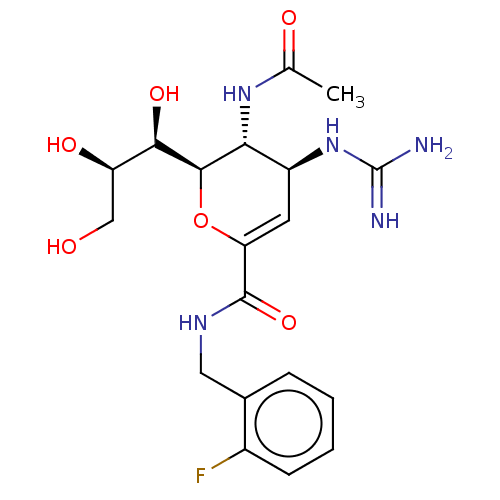 (CHEMBL2315324) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of influenza A virus (A/Guangdong/376/2001(H1N1)) neuraminidase infected in chick embryo using MUNANA as substrate after 1 hr by spectroph... | J Med Chem 56: 671-84 (2013) Article DOI: 10.1021/jm3009713 BindingDB Entry DOI: 10.7270/Q2RV0RM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM479489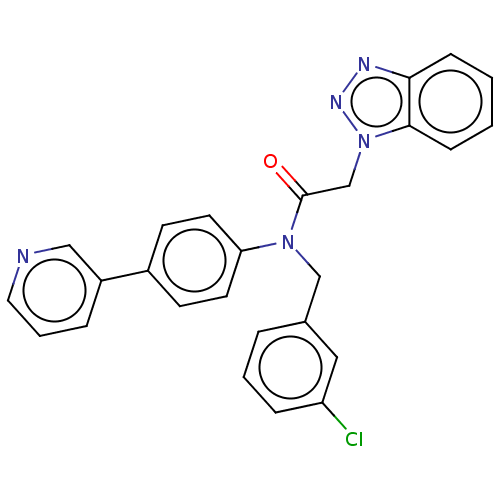 (ML300-based SC inhibitor 28) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Cleveland Clinic | Assay Description The protease activity and subsequent 10-point IC50 curves were spectroscopically determined using a scaled down, end point assay adapted from a previ... | J Med Chem 64: (2021) Article DOI: 10.1021/acs.jmedchem.1c00598 BindingDB Entry DOI: 10.7270/Q2NV9NCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50489405 (CHEMBL2315596) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of influenza A virus (A/Guangdong/376/2001(H1N1)) neuraminidase infected in chick embryo using MUNANA as substrate after 1 hr by spectroph... | J Med Chem 56: 671-84 (2013) Article DOI: 10.1021/jm3009713 BindingDB Entry DOI: 10.7270/Q2RV0RM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50489417 (CHEMBL2315324) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/Indonesia/5/2005(H5N1)) neuraminidase infected in chick embryo using MUNANA as substrate after 1 hr by spectrophot... | J Med Chem 56: 671-84 (2013) Article DOI: 10.1021/jm3009713 BindingDB Entry DOI: 10.7270/Q2RV0RM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM479497 (ML300-based SC inhibitor 36) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Cleveland Clinic | Assay Description The protease activity and subsequent 10-point IC50 curves were spectroscopically determined using a scaled down, end point assay adapted from a previ... | J Med Chem 64: (2021) Article DOI: 10.1021/acs.jmedchem.1c00598 BindingDB Entry DOI: 10.7270/Q2NV9NCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50489405 (CHEMBL2315596) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/Indonesia/5/2005(H5N1)) neuraminidase infected in chick embryo using MUNANA as substrate after 1 hr by spectrophot... | J Med Chem 56: 671-84 (2013) Article DOI: 10.1021/jm3009713 BindingDB Entry DOI: 10.7270/Q2RV0RM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM479494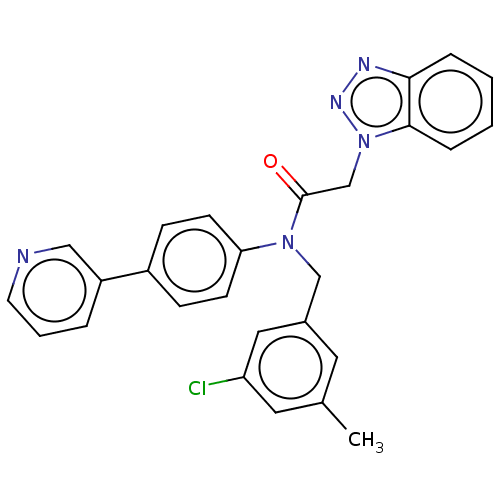 (ML300-based SC inhibitor 33) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 312 | n/a | n/a | n/a | n/a | n/a | n/a |
Cleveland Clinic | Assay Description The protease activity and subsequent 10-point IC50 curves were spectroscopically determined using a scaled down, end point assay adapted from a previ... | J Med Chem 64: (2021) Article DOI: 10.1021/acs.jmedchem.1c00598 BindingDB Entry DOI: 10.7270/Q2NV9NCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM479498 (ML300-based SC inhibitor 37) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 328 | n/a | n/a | n/a | n/a | n/a | n/a |
Cleveland Clinic | Assay Description The protease activity and subsequent 10-point IC50 curves were spectroscopically determined using a scaled down, end point assay adapted from a previ... | J Med Chem 64: (2021) Article DOI: 10.1021/acs.jmedchem.1c00598 BindingDB Entry DOI: 10.7270/Q2NV9NCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM479500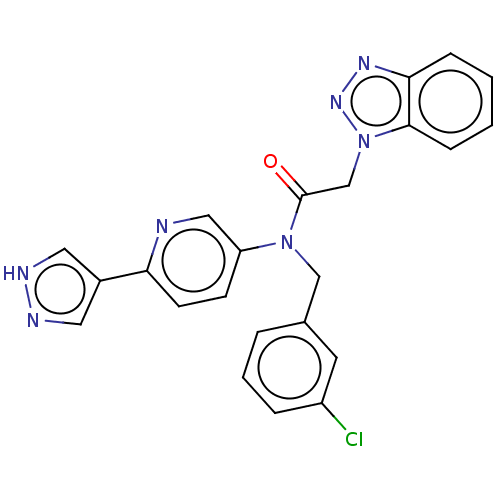 (ML300-based SC inhibitor 39) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 333 | n/a | n/a | n/a | n/a | n/a | n/a |
Cleveland Clinic | Assay Description The protease activity and subsequent 10-point IC50 curves were spectroscopically determined using a scaled down, end point assay adapted from a previ... | J Med Chem 64: (2021) Article DOI: 10.1021/acs.jmedchem.1c00598 BindingDB Entry DOI: 10.7270/Q2NV9NCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM479497 (ML300-based SC inhibitor 36) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 334 | n/a | n/a | n/a | n/a | n/a | n/a |
Cleveland Clinic | Assay Description The protease activity and subsequent 10-point IC50 curves were spectroscopically determined using a scaled down, end point assay adapted from a previ... | J Med Chem 64: (2021) Article DOI: 10.1021/acs.jmedchem.1c00598 BindingDB Entry DOI: 10.7270/Q2NV9NCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM479500 (ML300-based SC inhibitor 39) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 336 | n/a | n/a | n/a | n/a | n/a | n/a |
Cleveland Clinic | Assay Description The protease activity and subsequent 10-point IC50 curves were spectroscopically determined using a scaled down, end point assay adapted from a previ... | J Med Chem 64: (2021) Article DOI: 10.1021/acs.jmedchem.1c00598 BindingDB Entry DOI: 10.7270/Q2NV9NCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50489410 (CHEMBL2315322) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of influenza A virus (A/Guangdong/376/2001(H1N1)) neuraminidase infected in chick embryo using MUNANA as substrate after 1 hr by spectroph... | J Med Chem 56: 671-84 (2013) Article DOI: 10.1021/jm3009713 BindingDB Entry DOI: 10.7270/Q2RV0RM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM479495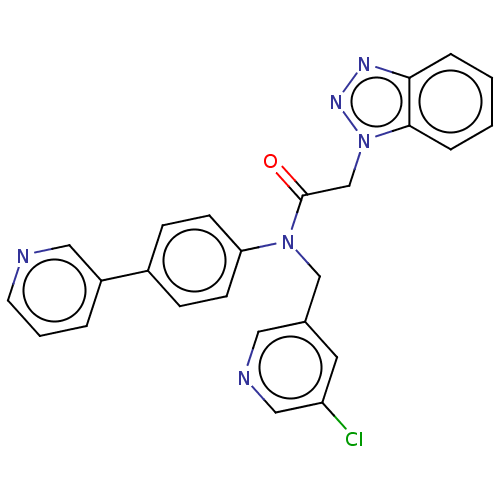 (ML300-based SC inhibitor 34) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Cleveland Clinic | Assay Description The protease activity and subsequent 10-point IC50 curves were spectroscopically determined using a scaled down, end point assay adapted from a previ... | J Med Chem 64: (2021) Article DOI: 10.1021/acs.jmedchem.1c00598 BindingDB Entry DOI: 10.7270/Q2NV9NCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM479471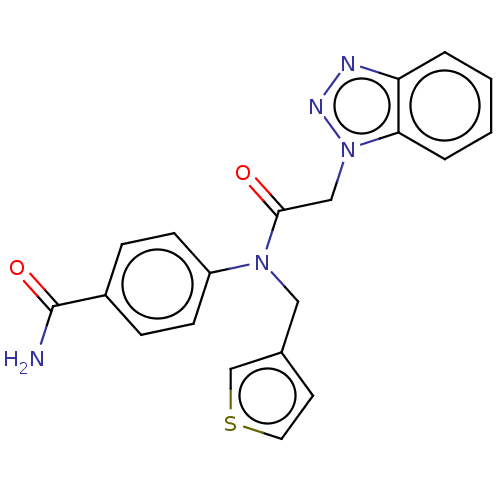 (ML300-based SC inhibitor 10) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Cleveland Clinic | Assay Description The protease activity and subsequent 10-point IC50 curves were spectroscopically determined using a scaled down, end point assay adapted from a previ... | J Med Chem 64: (2021) Article DOI: 10.1021/acs.jmedchem.1c00598 BindingDB Entry DOI: 10.7270/Q2NV9NCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM479489 (ML300-based SC inhibitor 28) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Cleveland Clinic | Assay Description The protease activity and subsequent 10-point IC50 curves were spectroscopically determined using a scaled down, end point assay adapted from a previ... | J Med Chem 64: (2021) Article DOI: 10.1021/acs.jmedchem.1c00598 BindingDB Entry DOI: 10.7270/Q2NV9NCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50489411 (CHEMBL2315321) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/Sydney/5/1997(H3N2)) neuraminidase infected in chick embryo using MUNANA as substrate after 1 hr by spectrophotome... | J Med Chem 56: 671-84 (2013) Article DOI: 10.1021/jm3009713 BindingDB Entry DOI: 10.7270/Q2RV0RM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM479494 (ML300-based SC inhibitor 33) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 418 | n/a | n/a | n/a | n/a | n/a | n/a |
Cleveland Clinic | Assay Description The protease activity and subsequent 10-point IC50 curves were spectroscopically determined using a scaled down, end point assay adapted from a previ... | J Med Chem 64: (2021) Article DOI: 10.1021/acs.jmedchem.1c00598 BindingDB Entry DOI: 10.7270/Q2NV9NCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM479471 (ML300-based SC inhibitor 10) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Cleveland Clinic | Assay Description The protease activity and subsequent 10-point IC50 curves were spectroscopically determined using a scaled down, end point assay adapted from a previ... | J Med Chem 64: (2021) Article DOI: 10.1021/acs.jmedchem.1c00598 BindingDB Entry DOI: 10.7270/Q2NV9NCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50489413 (CHEMBL2315318) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of influenza A virus (A/Guangdong/376/2001(H1N1)) neuraminidase infected in chick embryo using MUNANA as substrate after 1 hr by spectroph... | J Med Chem 56: 671-84 (2013) Article DOI: 10.1021/jm3009713 BindingDB Entry DOI: 10.7270/Q2RV0RM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50489410 (CHEMBL2315322) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/Sydney/5/1997(H3N2)) neuraminidase infected in chick embryo using MUNANA as substrate after 1 hr by spectrophotome... | J Med Chem 56: 671-84 (2013) Article DOI: 10.1021/jm3009713 BindingDB Entry DOI: 10.7270/Q2RV0RM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 237 total ) | Next | Last >> |