 Found 42 hits with Last Name = 'barbeau' and Initial = 'x'
Found 42 hits with Last Name = 'barbeau' and Initial = 'x' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50307347

(CHEMBL1783614)Show SMILES COc1cc2nc(\C=C\c3cccnc3)nc(N3CCC(CCNS(N)(=O)=O)CC3)c2cc1OC Show InChI InChI=1S/C24H30N6O4S/c1-33-21-14-19-20(15-22(21)34-2)28-23(6-5-18-4-3-10-26-16-18)29-24(19)30-12-8-17(9-13-30)7-11-27-35(25,31)32/h3-6,10,14-17,27H,7-9,11-13H2,1-2H3,(H2,25,31,32)/b6-5+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Laval
Curated by ChEMBL
| Assay Description
Inhibition of human GFP-fused NPP1 expressed in COS7 cells using pnp-TMP as substrate after 60 mins |
Eur J Med Chem 147: 130-149 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.094
BindingDB Entry DOI: 10.7270/Q2280B4X |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50307126

(CHEMBL4160816)Show SMILES COc1cc2nc(\C=C\c3ccccn3)nc(N3CCC(CCNS(N)(=O)=O)CC3)c2cc1OC Show InChI InChI=1S/C24H30N6O4S/c1-33-21-15-19-20(16-22(21)34-2)28-23(7-6-18-5-3-4-11-26-18)29-24(19)30-13-9-17(10-14-30)8-12-27-35(25,31)32/h3-7,11,15-17,27H,8-10,12-14H2,1-2H3,(H2,25,31,32)/b7-6+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Laval
Curated by ChEMBL
| Assay Description
Inhibition of human GFP-fused NPP1 expressed in COS7 cells using pnp-TMP as substrate after 60 mins |
Eur J Med Chem 147: 130-149 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.094
BindingDB Entry DOI: 10.7270/Q2280B4X |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50306037
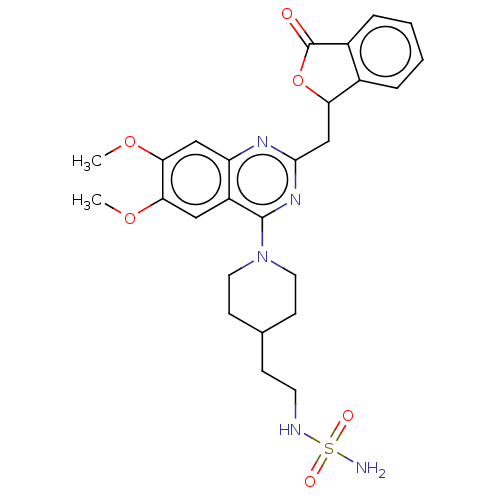
(CHEMBL4163119)Show SMILES COc1cc2nc(CC3OC(=O)c4ccccc34)nc(N3CCC(CCNS(N)(=O)=O)CC3)c2cc1OC Show InChI InChI=1S/C26H31N5O6S/c1-35-22-13-19-20(14-23(22)36-2)29-24(15-21-17-5-3-4-6-18(17)26(32)37-21)30-25(19)31-11-8-16(9-12-31)7-10-28-38(27,33)34/h3-6,13-14,16,21,28H,7-12,15H2,1-2H3,(H2,27,33,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Laval
Curated by ChEMBL
| Assay Description
Inhibition of human GFP-fused NPP1 expressed in COS7 cells using pnp-TMP as substrate after 60 mins |
Eur J Med Chem 147: 130-149 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.094
BindingDB Entry DOI: 10.7270/Q2280B4X |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50306044

(CHEMBL4163923)Show SMILES COc1cccc(\C=C\c2nc(N3CCC(CCNS(N)(=O)=O)CC3)c3cc(OC)c(OC)cc3n2)c1 Show InChI InChI=1S/C26H33N5O5S/c1-34-20-6-4-5-19(15-20)7-8-25-29-22-17-24(36-3)23(35-2)16-21(22)26(30-25)31-13-10-18(11-14-31)9-12-28-37(27,32)33/h4-8,15-18,28H,9-14H2,1-3H3,(H2,27,32,33)/b8-7+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 101 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Laval
Curated by ChEMBL
| Assay Description
Inhibition of human GFP-fused NPP1 expressed in COS7 cells using pnp-TMP as substrate after 60 mins |
Eur J Med Chem 147: 130-149 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.094
BindingDB Entry DOI: 10.7270/Q2280B4X |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50307248

(CHEMBL4174875)Show SMILES COc1cc2nc(\C=C\c3ccccc3F)nc(N3CCC(CCNS(N)(=O)=O)CC3)c2cc1OC Show InChI InChI=1S/C25H30FN5O4S/c1-34-22-15-19-21(16-23(22)35-2)29-24(8-7-18-5-3-4-6-20(18)26)30-25(19)31-13-10-17(11-14-31)9-12-28-36(27,32)33/h3-8,15-17,28H,9-14H2,1-2H3,(H2,27,32,33)/b8-7+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 211 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Laval
Curated by ChEMBL
| Assay Description
Inhibition of human GFP-fused NPP1 expressed in COS7 cells using pnp-TMP as substrate after 60 mins |
Eur J Med Chem 147: 130-149 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.094
BindingDB Entry DOI: 10.7270/Q2280B4X |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50307128
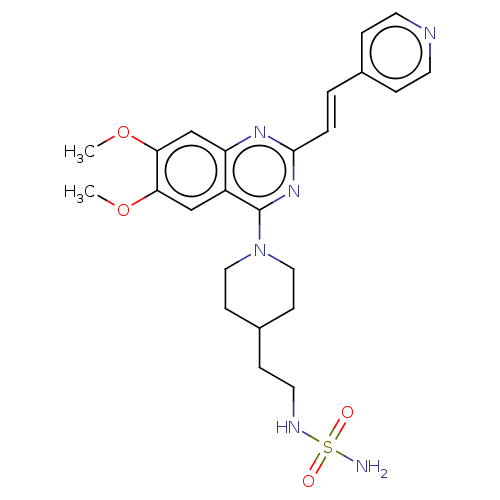
(CHEMBL4164216)Show SMILES COc1cc2nc(\C=C\c3ccncc3)nc(N3CCC(CCNS(N)(=O)=O)CC3)c2cc1OC Show InChI InChI=1S/C24H30N6O4S/c1-33-21-15-19-20(16-22(21)34-2)28-23(4-3-17-5-10-26-11-6-17)29-24(19)30-13-8-18(9-14-30)7-12-27-35(25,31)32/h3-6,10-11,15-16,18,27H,7-9,12-14H2,1-2H3,(H2,25,31,32)/b4-3+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 218 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Laval
Curated by ChEMBL
| Assay Description
Inhibition of human GFP-fused NPP1 expressed in COS7 cells using pnp-TMP as substrate after 60 mins |
Eur J Med Chem 147: 130-149 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.094
BindingDB Entry DOI: 10.7270/Q2280B4X |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50306578

(CHEMBL4166272)Show SMILES COc1ccc(\C=C\c2nc(N3CCC(CCNS(N)(=O)=O)CC3)c3cc(OC)c(OC)cc3n2)cc1 Show InChI InChI=1S/C26H33N5O5S/c1-34-20-7-4-18(5-8-20)6-9-25-29-22-17-24(36-3)23(35-2)16-21(22)26(30-25)31-14-11-19(12-15-31)10-13-28-37(27,32)33/h4-9,16-17,19,28H,10-15H2,1-3H3,(H2,27,32,33)/b9-6+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 231 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Laval
Curated by ChEMBL
| Assay Description
Inhibition of human GFP-fused NPP1 expressed in COS7 cells using pnp-TMP as substrate after 60 mins |
Eur J Med Chem 147: 130-149 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.094
BindingDB Entry DOI: 10.7270/Q2280B4X |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50307116
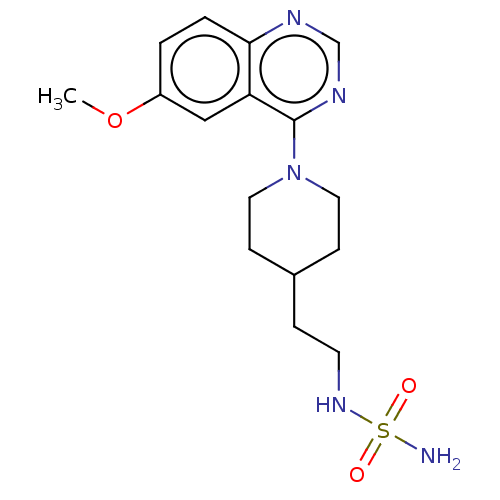
(CHEMBL4162412)Show InChI InChI=1S/C16H23N5O3S/c1-24-13-2-3-15-14(10-13)16(19-11-18-15)21-8-5-12(6-9-21)4-7-20-25(17,22)23/h2-3,10-12,20H,4-9H2,1H3,(H2,17,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 242 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Laval
Curated by ChEMBL
| Assay Description
Inhibition of human GFP-fused NPP1 expressed in COS7 cells using pnp-TMP as substrate after 60 mins |
Eur J Med Chem 147: 130-149 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.094
BindingDB Entry DOI: 10.7270/Q2280B4X |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50306038

(CHEMBL4159456)Show SMILES COC(=O)c1cccc(\C=C\c2nc(N3CCC(CCNS(N)(=O)=O)CC3)c3cc(OC)c(OC)cc3n2)c1 Show InChI InChI=1S/C27H33N5O6S/c1-36-23-16-21-22(17-24(23)37-2)30-25(8-7-19-5-4-6-20(15-19)27(33)38-3)31-26(21)32-13-10-18(11-14-32)9-12-29-39(28,34)35/h4-8,15-18,29H,9-14H2,1-3H3,(H2,28,34,35)/b8-7+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 297 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Laval
Curated by ChEMBL
| Assay Description
Inhibition of human GFP-fused NPP1 expressed in COS7 cells using pnp-TMP as substrate after 60 mins |
Eur J Med Chem 147: 130-149 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.094
BindingDB Entry DOI: 10.7270/Q2280B4X |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50306040

(CHEMBL4174575)Show SMILES COc1cc2nc(\C=C\c3ccccc3OC)nc(N3CCC(CCNS(N)(=O)=O)CC3)c2cc1OC Show InChI InChI=1S/C26H33N5O5S/c1-34-22-7-5-4-6-19(22)8-9-25-29-21-17-24(36-3)23(35-2)16-20(21)26(30-25)31-14-11-18(12-15-31)10-13-28-37(27,32)33/h4-9,16-18,28H,10-15H2,1-3H3,(H2,27,32,33)/b9-8+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 302 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Laval
Curated by ChEMBL
| Assay Description
Inhibition of human GFP-fused NPP1 expressed in COS7 cells using pnp-TMP as substrate after 60 mins |
Eur J Med Chem 147: 130-149 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.094
BindingDB Entry DOI: 10.7270/Q2280B4X |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50306036

(CHEMBL4171038)Show SMILES COc1cc2nc(CCc3ccc(F)cc3)nc(N3CCC(CCNS(N)(=O)=O)CC3)c2cc1OC Show InChI InChI=1S/C25H32FN5O4S/c1-34-22-15-20-21(16-23(22)35-2)29-24(8-5-17-3-6-19(26)7-4-17)30-25(20)31-13-10-18(11-14-31)9-12-28-36(27,32)33/h3-4,6-7,15-16,18,28H,5,8-14H2,1-2H3,(H2,27,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 366 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Laval
Curated by ChEMBL
| Assay Description
Inhibition of human GFP-fused NPP1 expressed in COS7 cells using pnp-TMP as substrate after 60 mins |
Eur J Med Chem 147: 130-149 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.094
BindingDB Entry DOI: 10.7270/Q2280B4X |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50306035
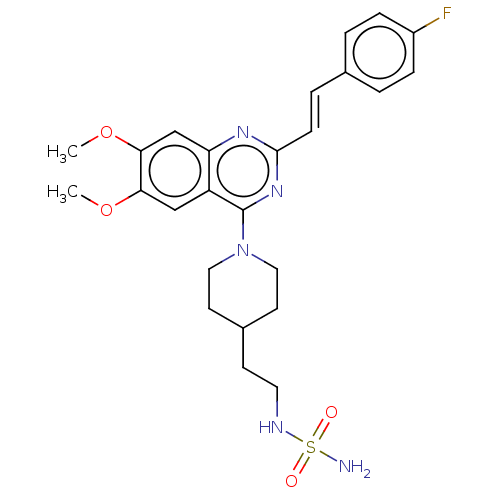
(CHEMBL4159758)Show SMILES COc1cc2nc(\C=C\c3ccc(F)cc3)nc(N3CCC(CCNS(N)(=O)=O)CC3)c2cc1OC Show InChI InChI=1S/C25H30FN5O4S/c1-34-22-15-20-21(16-23(22)35-2)29-24(8-5-17-3-6-19(26)7-4-17)30-25(20)31-13-10-18(11-14-31)9-12-28-36(27,32)33/h3-8,15-16,18,28H,9-14H2,1-2H3,(H2,27,32,33)/b8-5+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 465 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Laval
Curated by ChEMBL
| Assay Description
Inhibition of human GFP-fused NPP1 expressed in COS7 cells using pnp-TMP as substrate after 60 mins |
Eur J Med Chem 147: 130-149 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.094
BindingDB Entry DOI: 10.7270/Q2280B4X |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50307124
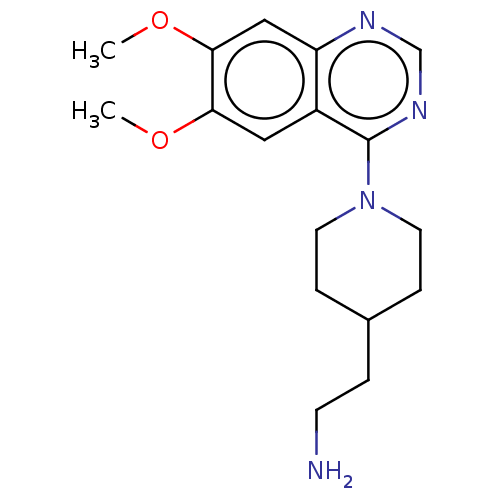
(CHEMBL4168465)Show InChI InChI=1S/C17H24N4O2/c1-22-15-9-13-14(10-16(15)23-2)19-11-20-17(13)21-7-4-12(3-6-18)5-8-21/h9-12H,3-8,18H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 699 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Laval
Curated by ChEMBL
| Assay Description
Inhibition of human GFP-fused NPP1 expressed in COS7 cells using pnp-TMP as substrate after 60 mins |
Eur J Med Chem 147: 130-149 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.094
BindingDB Entry DOI: 10.7270/Q2280B4X |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50306708
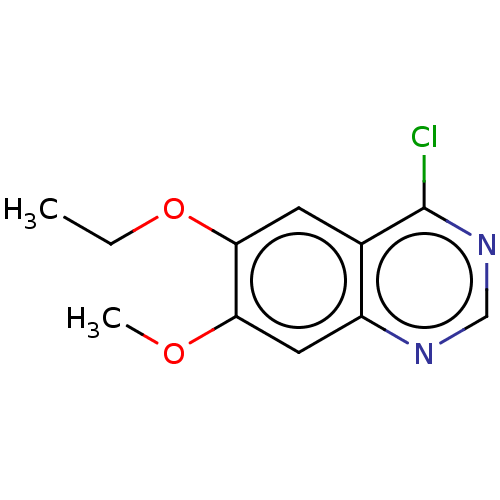
(CHEMBL4174184)Show InChI InChI=1S/C11H11ClN2O2/c1-3-16-10-4-7-8(5-9(10)15-2)13-6-14-11(7)12/h4-6H,3H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Laval
Curated by ChEMBL
| Assay Description
Inhibition of human GFP-fused NPP1 expressed in COS7 cells using pnp-TMP as substrate after 60 mins |
Eur J Med Chem 147: 130-149 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.094
BindingDB Entry DOI: 10.7270/Q2280B4X |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50306039

(CHEMBL4167364)Show SMILES COC(=O)c1ccc(\C=C\c2nc(N3CCC(CCNS(N)(=O)=O)CC3)c3cc(OC)c(OC)cc3n2)cc1 Show InChI InChI=1S/C27H33N5O6S/c1-36-23-16-21-22(17-24(23)37-2)30-25(9-6-18-4-7-20(8-5-18)27(33)38-3)31-26(21)32-14-11-19(12-15-32)10-13-29-39(28,34)35/h4-9,16-17,19,29H,10-15H2,1-3H3,(H2,28,34,35)/b9-6+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Laval
Curated by ChEMBL
| Assay Description
Inhibition of human GFP-fused NPP1 expressed in COS7 cells using pnp-TMP as substrate after 60 mins |
Eur J Med Chem 147: 130-149 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.094
BindingDB Entry DOI: 10.7270/Q2280B4X |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50307125

(CHEMBL4168092)Show SMILES COc1cc2nc(\C=C\c3ccccc3)nc(N3CCC(CCNS(N)(=O)=O)CC3)c2cc1OC Show InChI InChI=1S/C25H31N5O4S/c1-33-22-16-20-21(17-23(22)34-2)28-24(9-8-18-6-4-3-5-7-18)29-25(20)30-14-11-19(12-15-30)10-13-27-35(26,31)32/h3-9,16-17,19,27H,10-15H2,1-2H3,(H2,26,31,32)/b9-8+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Laval
Curated by ChEMBL
| Assay Description
Inhibition of human GFP-fused NPP1 expressed in COS7 cells using pnp-TMP as substrate after 60 mins |
Eur J Med Chem 147: 130-149 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.094
BindingDB Entry DOI: 10.7270/Q2280B4X |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50306945

(CHEMBL4162819)Show InChI InChI=1S/C12H13ClN2O2/c1-3-4-17-11-5-8-9(6-10(11)16-2)14-7-15-12(8)13/h5-7H,3-4H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Laval
Curated by ChEMBL
| Assay Description
Inhibition of human GFP-fused NPP1 expressed in COS7 cells using pnp-TMP as substrate after 60 mins |
Eur J Med Chem 147: 130-149 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.094
BindingDB Entry DOI: 10.7270/Q2280B4X |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50307249

(CHEMBL4167667)Show SMILES COc1cc2nc(\C=C\c3cccc(F)c3)nc(N3CCC(CCNS(N)(=O)=O)CC3)c2cc1OC Show InChI InChI=1S/C25H30FN5O4S/c1-34-22-15-20-21(16-23(22)35-2)29-24(7-6-18-4-3-5-19(26)14-18)30-25(20)31-12-9-17(10-13-31)8-11-28-36(27,32)33/h3-7,14-17,28H,8-13H2,1-2H3,(H2,27,32,33)/b7-6+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Laval
Curated by ChEMBL
| Assay Description
Inhibition of human GFP-fused NPP1 expressed in COS7 cells using pnp-TMP as substrate after 60 mins |
Eur J Med Chem 147: 130-149 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.094
BindingDB Entry DOI: 10.7270/Q2280B4X |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50306946

(CHEMBL4173090)Show InChI InChI=1S/C12H10ClF5N2O2S/c1-21-10-6-9-8(12(13)20-7-19-9)5-11(10)22-3-2-4-23(14,15,16,17)18/h2,4-7H,3H2,1H3/b4-2+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.97E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Laval
Curated by ChEMBL
| Assay Description
Inhibition of human GFP-fused NPP1 expressed in COS7 cells using pnp-TMP as substrate after 60 mins |
Eur J Med Chem 147: 130-149 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.094
BindingDB Entry DOI: 10.7270/Q2280B4X |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50369432

(CHEMBL1627465)Show SMILES CC(C)(C)c1ccc(C[C@]2(O)CC[C@H]3[C@@H]4CCc5cc(OS(N)(=O)=O)ccc5[C@H]4CC[C@]23C)cc1 Show InChI InChI=1S/C29H39NO4S/c1-27(2,3)21-8-5-19(6-9-21)18-29(31)16-14-26-25-11-7-20-17-22(34-35(30,32)33)10-12-23(20)24(25)13-15-28(26,29)4/h5-6,8-10,12,17,24-26,31H,7,11,13-16,18H2,1-4H3,(H2,30,32,33)/t24-,25-,26+,28+,29-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
CHU de Qu£bec - Research Center
Curated by ChEMBL
| Assay Description
Irreversible inhibition of human steroid sulfatase expressed in HEK293 cells using [3H] E1S as substrate after 2 hrs by liquid scintillation counting... |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115368
BindingDB Entry DOI: 10.7270/Q2V98CNK |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50545255

(CHEMBL4643348)Show SMILES [H][C@@]12CC[C@@](O)(Cc3ccc(cc3)C(C)(C)C)C1(C)CC[C@]1([H])c3cc(OC)c(OS(N)(=O)=O)cc3CC[C@@]21[H] |r| Show InChI InChI=1S/C30H41NO5S/c1-28(2,3)21-9-6-19(7-10-21)18-30(32)15-13-25-23-11-8-20-16-27(36-37(31,33)34)26(35-5)17-24(20)22(23)12-14-29(25,30)4/h6-7,9-10,16-17,22-23,25,32H,8,11-15,18H2,1-5H3,(H2,31,33,34)/t22-,23+,25-,29?,30+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
CHU de Qu£bec - Research Center
Curated by ChEMBL
| Assay Description
Irreversible inhibition of human steroid sulfatase expressed in HEK293 cells using [3H] E1S as substrate after 2 hrs by liquid scintillation counting... |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115368
BindingDB Entry DOI: 10.7270/Q2V98CNK |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50168219

(CHEMBL3805209)Show SMILES [H][C@@]12CC[C@@](O)(Cc3ccccc3)[C@@]1(C)CC[C@]1([H])c3cc(OC)c(OS(N)(=O)=O)cc3CC[C@@]21[H] |r| Show InChI InChI=1S/C26H33NO5S/c1-25-12-10-19-20(22(25)11-13-26(25,28)16-17-6-4-3-5-7-17)9-8-18-14-24(32-33(27,29)30)23(31-2)15-21(18)19/h3-7,14-15,19-20,22,28H,8-13,16H2,1-2H3,(H2,27,29,30)/t19-,20+,22-,25-,26+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
CHU de Qu£bec - Research Center (CHUL, T4)
Curated by ChEMBL
| Assay Description
Inhibition of steroid sulfatase (unknown origin) expressed in HEK293 cells assessed as reduction in transformation of [3H]-E1S to E1 after 2 hrs by l... |
Eur J Med Chem 119: 169-82 (2016)
Article DOI: 10.1016/j.ejmech.2016.04.044
BindingDB Entry DOI: 10.7270/Q2QR503Z |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50369432

(CHEMBL1627465)Show SMILES CC(C)(C)c1ccc(C[C@]2(O)CC[C@H]3[C@@H]4CCc5cc(OS(N)(=O)=O)ccc5[C@H]4CC[C@]23C)cc1 Show InChI InChI=1S/C29H39NO4S/c1-27(2,3)21-8-5-19(6-9-21)18-29(31)16-14-26-25-11-7-20-17-22(34-35(30,32)33)10-12-23(20)24(25)13-15-28(26,29)4/h5-6,8-10,12,17,24-26,31H,7,11,13-16,18H2,1-4H3,(H2,30,32,33)/t24-,25-,26+,28+,29-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
CHU de Qu£bec - Research Center
Curated by ChEMBL
| Assay Description
Inhibition of steroid sulfatase (unknown origin) expressed in HEK293 cells using [3H] E1S as substrate after 2 hrs by scintillation counting method |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115368
BindingDB Entry DOI: 10.7270/Q2V98CNK |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50545254
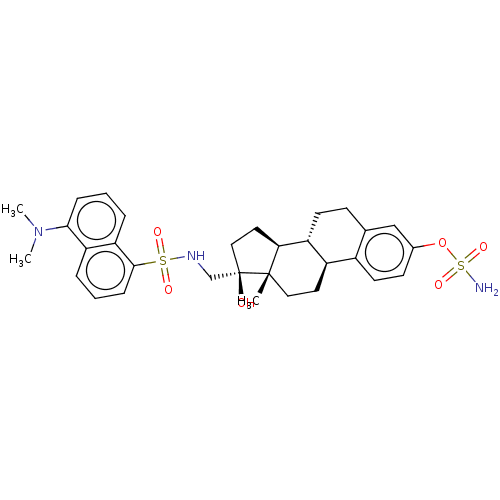
(CHEMBL4639657)Show SMILES [H][C@@]12CC[C@@](O)(CNS(=O)(=O)c3cccc4c(cccc34)N(C)C)[C@@]1(C)CC[C@]1([H])c3ccc(OS(N)(=O)=O)cc3CC[C@@]21[H] |r| Show InChI InChI=1S/C31H39N3O6S2/c1-30-16-14-23-22-13-11-21(40-42(32,38)39)18-20(22)10-12-24(23)27(30)15-17-31(30,35)19-33-41(36,37)29-9-5-6-25-26(29)7-4-8-28(25)34(2)3/h4-9,11,13,18,23-24,27,33,35H,10,12,14-17,19H2,1-3H3,(H2,32,38,39)/t23-,24-,27+,30+,31-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
CHU de Qu£bec - Research Center
Curated by ChEMBL
| Assay Description
Irreversible inhibition of steroid sulfatase (unknown origin) expressed in HEK293 cells using [3H] E1S as substrate after 2 hrs by scintillation coun... |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115368
BindingDB Entry DOI: 10.7270/Q2V98CNK |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50193084
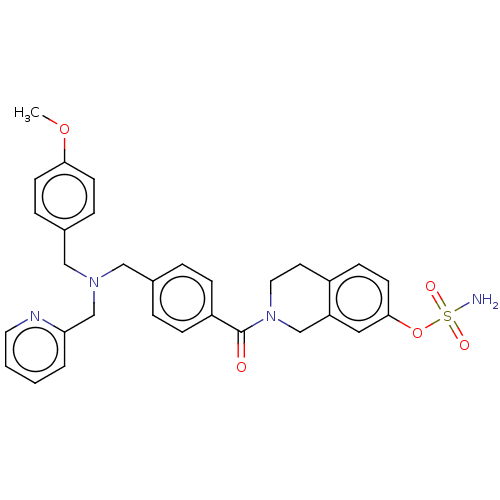
(CHEMBL3910182)Show SMILES COc1ccc(CN(Cc2ccc(cc2)C(=O)N2CCc3ccc(OS(N)(=O)=O)cc3C2)Cc2ccccn2)cc1 Show InChI InChI=1S/C31H32N4O5S/c1-39-29-12-7-24(8-13-29)20-34(22-28-4-2-3-16-33-28)19-23-5-9-26(10-6-23)31(36)35-17-15-25-11-14-30(18-27(25)21-35)40-41(32,37)38/h2-14,16,18H,15,17,19-22H2,1H3,(H2,32,37,38) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
CHU de Qu£bec - Research Center (CHUL, T4)
Curated by ChEMBL
| Assay Description
Inhibition of steroid sulfatase (unknown origin) expressed in HEK293 cells assessed as reduction in transformation of [3H]-E1S to E1 after 2 hrs by l... |
Eur J Med Chem 119: 169-82 (2016)
Article DOI: 10.1016/j.ejmech.2016.04.044
BindingDB Entry DOI: 10.7270/Q2QR503Z |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50193082
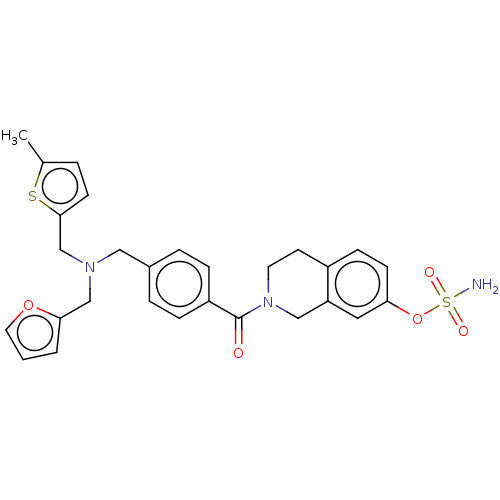
(CHEMBL3953180)Show SMILES Cc1ccc(CN(Cc2ccco2)Cc2ccc(cc2)C(=O)N2CCc3ccc(OS(N)(=O)=O)cc3C2)s1 Show InChI InChI=1S/C28H29N3O5S2/c1-20-4-11-27(37-20)19-30(18-26-3-2-14-35-26)16-21-5-7-23(8-6-21)28(32)31-13-12-22-9-10-25(15-24(22)17-31)36-38(29,33)34/h2-11,14-15H,12-13,16-19H2,1H3,(H2,29,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
CHU de Qu£bec - Research Center (CHUL, T4)
Curated by ChEMBL
| Assay Description
Inhibition of steroid sulfatase (unknown origin) expressed in HEK293 cells assessed as reduction in transformation of [3H]-E1S to E1 after 2 hrs by l... |
Eur J Med Chem 119: 169-82 (2016)
Article DOI: 10.1016/j.ejmech.2016.04.044
BindingDB Entry DOI: 10.7270/Q2QR503Z |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50193083

(CHEMBL3981927)Show SMILES NS(=O)(=O)Oc1ccc2CCN(Cc2c1)C(=O)c1ccc(CN(Cc2ccco2)Cc2ccccn2)cc1 Show InChI InChI=1S/C28H28N4O5S/c29-38(34,35)37-26-11-10-22-12-14-32(18-24(22)16-26)28(33)23-8-6-21(7-9-23)17-31(20-27-5-3-15-36-27)19-25-4-1-2-13-30-25/h1-11,13,15-16H,12,14,17-20H2,(H2,29,34,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
CHU de Qu£bec - Research Center (CHUL, T4)
Curated by ChEMBL
| Assay Description
Inhibition of steroid sulfatase (unknown origin) expressed in HEK293 cells assessed as reduction in transformation of [3H]-E1S to E1 after 2 hrs by l... |
Eur J Med Chem 119: 169-82 (2016)
Article DOI: 10.1016/j.ejmech.2016.04.044
BindingDB Entry DOI: 10.7270/Q2QR503Z |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM151585

(US11739089, Compound Ketoconazole | US8987315, Ket...)Show SMILES CC(=O)N1CCN(CC1)c1ccc(OCC2COC(Cn3ccnc3)(O2)c2ccc(Cl)cc2Cl)cc1 Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Laval University
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) by fluorescence assay |
J Med Chem 57: 204-22 (2014)
Article DOI: 10.1021/jm401639v
BindingDB Entry DOI: 10.7270/Q2V98C2Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50373700

(CHEMBL410242)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(O)ccc34)[C@@H]1C[C@H](Cc1cccc(c1)C(N)=O)[C@@H]2O |r| Show InChI InChI=1S/C26H31NO3/c1-26-10-9-21-20-8-6-19(28)13-16(20)5-7-22(21)23(26)14-18(24(26)29)12-15-3-2-4-17(11-15)25(27)30/h2-4,6,8,11,13,18,21-24,28-29H,5,7,9-10,12,14H2,1H3,(H2,27,30)/t18-,21+,22+,23-,24-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
| MMDB
PDB
Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Laval University
Curated by ChEMBL
| Assay Description
Inhibition of 17-beta HSD1 in human T47D cells assessed as transformation of [14C]E1 to [14C]E2 after 24 hrs by thin layer chromatography |
J Med Chem 57: 204-22 (2014)
Article DOI: 10.1021/jm401639v
BindingDB Entry DOI: 10.7270/Q2V98C2Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50366532

(CHEMBL518966)Show SMILES CC(C)(C)c1ccc(C[C@]2(O)CC[C@H]3[C@@H]4CCc5cc(O)ccc5[C@H]4CC[C@]23C)cc1 Show InChI InChI=1S/C29H38O2/c1-27(2,3)21-8-5-19(6-9-21)18-29(31)16-14-26-25-11-7-20-17-22(30)10-12-23(20)24(25)13-15-28(26,29)4/h5-6,8-10,12,17,24-26,30-31H,7,11,13-16,18H2,1-4H3/t24-,25-,26+,28+,29-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
CHU de Qu£bec - Research Center
Curated by ChEMBL
| Assay Description
Reversible inhibition of steroid sulfatase in human JEG3 cells using [3H] E1S as substrate by scintillation counting method |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115368
BindingDB Entry DOI: 10.7270/Q2V98CNK |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50366532

(CHEMBL518966)Show SMILES CC(C)(C)c1ccc(C[C@]2(O)CC[C@H]3[C@@H]4CCc5cc(O)ccc5[C@H]4CC[C@]23C)cc1 Show InChI InChI=1S/C29H38O2/c1-27(2,3)21-8-5-19(6-9-21)18-29(31)16-14-26-25-11-7-20-17-22(30)10-12-23(20)24(25)13-15-28(26,29)4/h5-6,8-10,12,17,24-26,30-31H,7,11,13-16,18H2,1-4H3/t24-,25-,26+,28+,29-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
CHU de Qu£bec - Research Center
Curated by ChEMBL
| Assay Description
Inhibition of steroid sulfatase (unknown origin) expressed in HEK293 cells using [3H] E1S as substrate after 2 hrs by scintillation counting method |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115368
BindingDB Entry DOI: 10.7270/Q2V98CNK |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50400509
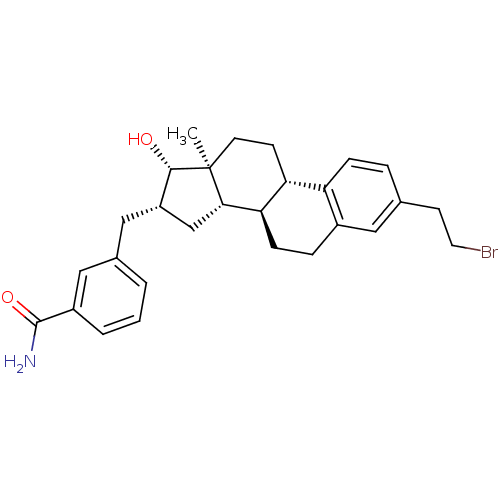
(CHEMBL2203397)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(CCBr)ccc34)[C@@H]1C[C@H](Cc1cccc(c1)C(N)=O)[C@@H]2O |r| Show InChI InChI=1S/C28H34BrNO2/c1-28-11-9-23-22-7-5-17(10-12-29)13-19(22)6-8-24(23)25(28)16-21(26(28)31)15-18-3-2-4-20(14-18)27(30)32/h2-5,7,13-14,21,23-26,31H,6,8-12,15-16H2,1H3,(H2,30,32)/t21-,23+,24+,25-,26-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Laval University
Curated by ChEMBL
| Assay Description
Inhibition of 17-beta HSD1 in human T47D cells assessed as transformation of [14C]E1 to [14C]E2 after 24 hrs by thin layer chromatography |
J Med Chem 57: 204-22 (2014)
Article DOI: 10.1021/jm401639v
BindingDB Entry DOI: 10.7270/Q2V98C2Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50545253

(CHEMBL4632735)Show SMILES [H][C@@]12CC[C@@](O)(CNS(=O)(=O)c3cccc4c(cccc34)N(C)C)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] |r| Show InChI InChI=1S/C31H38N2O4S/c1-30-16-14-23-22-13-11-21(34)18-20(22)10-12-24(23)27(30)15-17-31(30,35)19-32-38(36,37)29-9-5-6-25-26(29)7-4-8-28(25)33(2)3/h4-9,11,13,18,23-24,27,32,34-35H,10,12,14-17,19H2,1-3H3/t23-,24-,27+,30+,31-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
CHU de Qu£bec - Research Center
Curated by ChEMBL
| Assay Description
Reversible inhibition of steroid sulfatase (unknown origin) expressed in HEK293 cells using [3H] E1S as substrate after 2 hrs by scintillation counti... |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115368
BindingDB Entry DOI: 10.7270/Q2V98CNK |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50400509

(CHEMBL2203397)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(CCBr)ccc34)[C@@H]1C[C@H](Cc1cccc(c1)C(N)=O)[C@@H]2O |r| Show InChI InChI=1S/C28H34BrNO2/c1-28-11-9-23-22-7-5-17(10-12-29)13-19(22)6-8-24(23)25(28)16-21(26(28)31)15-18-3-2-4-20(14-18)27(30)32/h2-5,7,13-14,21,23-26,31H,6,8-12,15-16H2,1H3,(H2,30,32)/t21-,23+,24+,25-,26-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
Laval University
Curated by ChEMBL
| Assay Description
Inhibition of 17-beta HSD1 in human T47D cells |
J Med Chem 57: 204-22 (2014)
Article DOI: 10.1021/jm401639v
BindingDB Entry DOI: 10.7270/Q2V98C2Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50495475
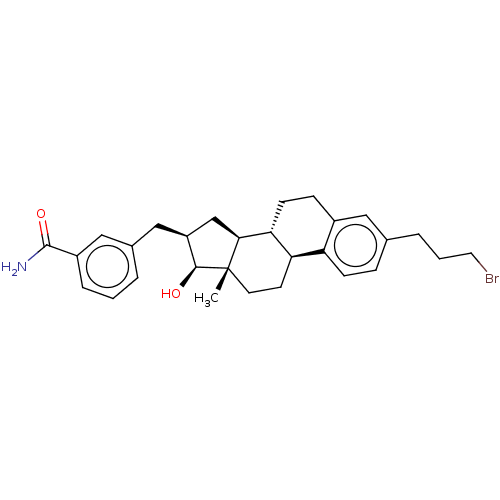
(CHEMBL3108979)Show SMILES [H][C@@]12C[C@H](Cc3cccc(c3)C(N)=O)[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(CCCBr)cc3CC[C@@]21[H] |r| Show InChI InChI=1S/C29H36BrNO2/c1-29-12-11-24-23-9-7-18(5-3-13-30)14-20(23)8-10-25(24)26(29)17-22(27(29)32)16-19-4-2-6-21(15-19)28(31)33/h2,4,6-7,9,14-15,22,24-27,32H,3,5,8,10-13,16-17H2,1H3,(H2,31,33)/t22-,24+,25+,26-,27-,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 153 | n/a | n/a | n/a | n/a | n/a | n/a |
Laval University
Curated by ChEMBL
| Assay Description
Inhibition of 17-beta HSD1 in human T47D cells assessed as transformation of [14C]E1 to [14C]E2 after 24 hrs by thin layer chromatography |
J Med Chem 57: 204-22 (2014)
Article DOI: 10.1021/jm401639v
BindingDB Entry DOI: 10.7270/Q2V98C2Z |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50495474

(CHEMBL3108980)Show SMILES [H][C@@]12C[C@H](Cc3cccc(c3)C(N)=O)[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(OCCBr)cc3CC[C@@]21[H] |r| Show InChI InChI=1S/C28H34BrNO3/c1-28-10-9-23-22-8-6-21(33-12-11-29)15-18(22)5-7-24(23)25(28)16-20(26(28)31)14-17-3-2-4-19(13-17)27(30)32/h2-4,6,8,13,15,20,23-26,31H,5,7,9-12,14,16H2,1H3,(H2,30,32)/t20-,23+,24+,25-,26-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 172 | n/a | n/a | n/a | n/a | n/a | n/a |
Laval University
Curated by ChEMBL
| Assay Description
Inhibition of 17-beta HSD1 in human T47D cells assessed as transformation of [14C]E1 to [14C]E2 after 24 hrs by thin layer chromatography |
J Med Chem 57: 204-22 (2014)
Article DOI: 10.1021/jm401639v
BindingDB Entry DOI: 10.7270/Q2V98C2Z |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50400510
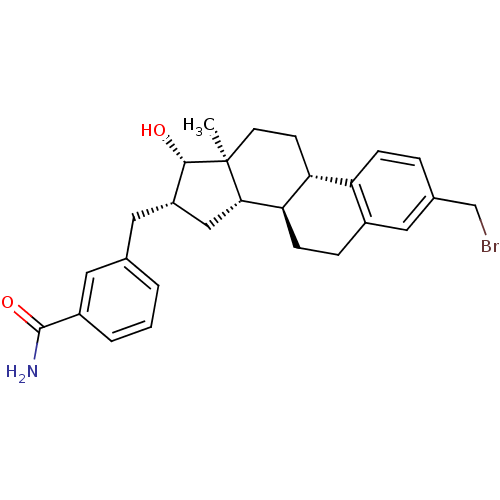
(CHEMBL2203399)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(CBr)ccc34)[C@@H]1C[C@H](Cc1cccc(c1)C(N)=O)[C@@H]2O |r| Show InChI InChI=1S/C27H32BrNO2/c1-27-10-9-22-21-7-5-17(15-28)13-18(21)6-8-23(22)24(27)14-20(25(27)30)12-16-3-2-4-19(11-16)26(29)31/h2-5,7,11,13,20,22-25,30H,6,8-10,12,14-15H2,1H3,(H2,29,31)/t20-,22+,23+,24-,25-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Laval University
Curated by ChEMBL
| Assay Description
Inhibition of 17-beta HSD1 in human T47D cells assessed as transformation of [14C]E1 to [14C]E2 after 24 hrs by thin layer chromatography |
J Med Chem 57: 204-22 (2014)
Article DOI: 10.1021/jm401639v
BindingDB Entry DOI: 10.7270/Q2V98C2Z |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50078673
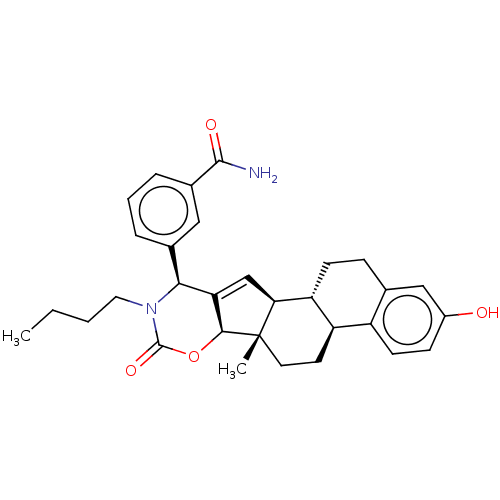
(CHEMBL3415436)Show SMILES [H][C@]12OC(=O)N(CCCC)[C@H](C1=C[C@@]1([H])[C@]3([H])CCc4cc(O)ccc4[C@@]3([H])CC[C@]21C)c1cccc(c1)C(N)=O |r,c:12| Show InChI InChI=1S/C31H36N2O4/c1-3-4-14-33-27(19-6-5-7-20(15-19)29(32)35)25-17-26-24-10-8-18-16-21(34)9-11-22(18)23(24)12-13-31(26,2)28(25)37-30(33)36/h5-7,9,11,15-17,23-24,26-28,34H,3-4,8,10,12-14H2,1-2H3,(H2,32,35)/t23-,24-,26+,27+,28+,31+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Laval
Curated by ChEMBL
| Assay Description
Inhibition of 17 beta-HSD1 in human T47D cells assessed as inhibition of transformation of [14C]E1 to [14C]E2 after overnight incubation |
Eur J Med Chem 93: 470-80 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.059
BindingDB Entry DOI: 10.7270/Q2KP83WQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50373700

(CHEMBL410242)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(O)ccc34)[C@@H]1C[C@H](Cc1cccc(c1)C(N)=O)[C@@H]2O |r| Show InChI InChI=1S/C26H31NO3/c1-26-10-9-21-20-8-6-19(28)13-16(20)5-7-22(21)23(26)14-18(24(26)29)12-15-3-2-4-17(11-15)25(27)30/h2-4,6,8,11,13,18,21-24,28-29H,5,7,9-10,12,14H2,1H3,(H2,27,30)/t18-,21+,22+,23-,24-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laval University
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) by fluorescence assay |
J Med Chem 57: 204-22 (2014)
Article DOI: 10.1021/jm401639v
BindingDB Entry DOI: 10.7270/Q2V98C2Z |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50400509

(CHEMBL2203397)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(CCBr)ccc34)[C@@H]1C[C@H](Cc1cccc(c1)C(N)=O)[C@@H]2O |r| Show InChI InChI=1S/C28H34BrNO2/c1-28-11-9-23-22-7-5-17(10-12-29)13-19(22)6-8-24(23)25(28)16-21(26(28)31)15-18-3-2-4-20(14-18)27(30)32/h2-5,7,13-14,21,23-26,31H,6,8-12,15-16H2,1H3,(H2,30,32)/t21-,23+,24+,25-,26-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laval University
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) by fluorescence assay |
J Med Chem 57: 204-22 (2014)
Article DOI: 10.1021/jm401639v
BindingDB Entry DOI: 10.7270/Q2V98C2Z |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50078674

(CHEMBL3415431)Show SMILES [H][C@@]12C[C@]3(O)[C@@H](N(CCCCCC)C(=O)O[C@]3([H])[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H])c1cccc(c1)C(N)=O |r| Show InChI InChI=1S/C33H42N2O5/c1-3-4-5-6-16-35-28(21-8-7-9-22(17-21)29(34)37)33(39)19-27-26-12-10-20-18-23(36)11-13-24(20)25(26)14-15-32(27,2)30(33)40-31(35)38/h7-9,11,13,17-18,25-28,30,36,39H,3-6,10,12,14-16,19H2,1-2H3,(H2,34,37)/t25-,26-,27+,28+,30-,32+,33+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Laval
Curated by ChEMBL
| Assay Description
Inhibition of 17 beta-HSD1 in human T47D cells assessed as inhibition of transformation of [14C]E1 to [14C]E2 after overnight incubation |
Eur J Med Chem 93: 470-80 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.059
BindingDB Entry DOI: 10.7270/Q2KP83WQ |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50134329

(CHEMBL122708 | Sulfamic acid (11R,12S,15S,16S)-13-...)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(OS(N)(=O)=O)ccc34)[C@@H]1CCC2=O Show InChI InChI=1S/C18H23NO4S/c1-18-9-8-14-13-5-3-12(23-24(19,21)22)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-16H,2,4,6-9H2,1H3,(H2,19,21,22)/t14-,15-,16+,18+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CHU de Qu£bec - Research Center
Curated by ChEMBL
| Assay Description
Reversible inhibition of steroid sulfatase in human JEG3 cells using [3H] E1S as substrate by scintillation counting method |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115368
BindingDB Entry DOI: 10.7270/Q2V98CNK |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data