 Found 935 hits with Last Name = 'ali' and Initial = 'y'
Found 935 hits with Last Name = 'ali' and Initial = 'y' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cholinesterase
(Homo sapiens (Human)) | BDBM50092532
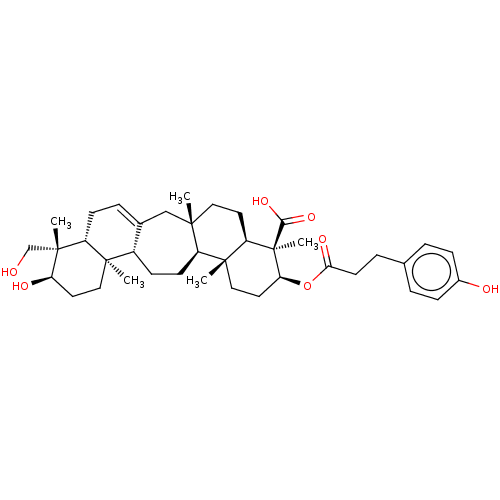
(CHEMBL3586200)Show SMILES [H][C@@]12CC=C3C[C@]4(C)CC[C@]5([H])[C@](C)(CC[C@H](OC(=O)CCc6ccc(O)cc6)[C@]5(C)C(O)=O)[C@@]4([H])CC[C@]3([H])[C@@]1(C)CC[C@@H](O)[C@]2(C)CO |r,t:3| Show InChI InChI=1S/C39H56O7/c1-35-19-16-30-37(3,21-18-32(39(30,5)34(44)45)46-33(43)15-8-24-6-10-26(41)11-7-24)28(35)14-12-27-25(22-35)9-13-29-36(27,2)20-17-31(42)38(29,4)23-40/h6-7,9-11,27-32,40-42H,8,12-23H2,1-5H3,(H,44,45)/t27-,28-,29+,30+,31+,32-,35-,36+,37+,38+,39+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu
Curated by ChEMBL
| Assay Description
Inhibition of BChE (unknown origin) by Dixon plot analysis |
Bioorg Med Chem 23: 3126-34 (2015)
Article DOI: 10.1016/j.bmc.2015.04.080
BindingDB Entry DOI: 10.7270/Q24X59JZ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50092540

(CHEMBL3586207)Show InChI InChI=1S/C10H16O3/c1-2-3-6-9(11)7-4-5-8-10(12)13/h4-5,7-9,11H,2-3,6H2,1H3,(H,12,13)/b7-4+,8-5+/t9-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) by Dixon plot analysis |
Bioorg Med Chem 23: 3126-34 (2015)
Article DOI: 10.1016/j.bmc.2015.04.080
BindingDB Entry DOI: 10.7270/Q24X59JZ |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50267969

(Sargahydroquinoic Acid)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-c1cc(-[#8])cc(-[#6])c1-[#8])-[#6](-[#8])=O Show InChI InChI=1S/C27H38O4/c1-19(2)9-6-13-23(27(30)31)14-8-12-20(3)10-7-11-21(4)15-16-24-18-25(28)17-22(5)26(24)29/h9-10,14-15,17-18,28-29H,6-8,11-13,16H2,1-5H3,(H,30,31)/b20-10+,21-15+,23-14- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Food and Life Science, Pukyong National University, Busan 608-737, Republic of Korea.
Curated by ChEMBL
| Assay Description
Mixed type inhibition of recombinant human BACE1 using Rh-EVNLDAEFK-Quencher as substrate after 60 mins by Dixon plot analysis |
Bioorg Med Chem 25: 3964-3970 (2017)
Article DOI: 10.1016/j.bmc.2017.05.033
BindingDB Entry DOI: 10.7270/Q2K076RW |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50092531

(CHEMBL3586199)Show SMILES [H][C@@]12CC[C@@]3(C)C[C@@]4(O)CC[C@@]5([H])C(C)(C)[C@H](CC[C@]5(C)[C@@]4([H])CC[C@]3([H])[C@@]1(C)CC[C@H](OC(C)=O)C2(C)C)OC(C)=O |r| Show InChI InChI=1S/C34H56O5/c1-21(35)38-27-14-17-32(8)23(29(27,3)4)12-16-31(7)20-34(37)19-13-24-30(5,6)28(39-22(2)36)15-18-33(24,9)26(34)11-10-25(31)32/h23-28,37H,10-20H2,1-9H3/t23-,24-,25-,26+,27-,28-,31-,32-,33-,34-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) by Dixon plot analysis |
Bioorg Med Chem 23: 3126-34 (2015)
Article DOI: 10.1016/j.bmc.2015.04.080
BindingDB Entry DOI: 10.7270/Q24X59JZ |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50267968
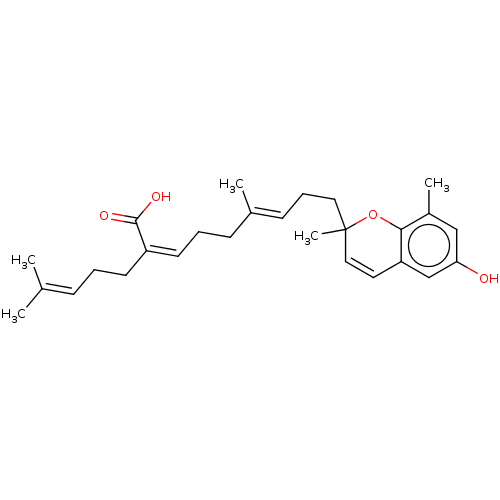
(CHEMBL4085945)Show SMILES [#6]\[#6](-[#6])=[#6]/[#6]-[#6]\[#6](=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#6]C1([#6])[#8]-c2c(-[#6])cc(-[#8])cc2-[#6]=[#6]1)-[#6](-[#8])=O |c:27| Show InChI InChI=1S/C27H36O4/c1-19(2)9-6-12-22(26(29)30)13-7-10-20(3)11-8-15-27(5)16-14-23-18-24(28)17-21(4)25(23)31-27/h9,11,13-14,16-18,28H,6-8,10,12,15H2,1-5H3,(H,29,30)/b20-11+,22-13- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Food and Life Science, Pukyong National University, Busan 608-737, Republic of Korea.
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of recombinant human BACE1 using Rh-EVNLDAEFK-Quencher as substrate after 60 mins by Dixon plot analysis |
Bioorg Med Chem 25: 3964-3970 (2017)
Article DOI: 10.1016/j.bmc.2017.05.033
BindingDB Entry DOI: 10.7270/Q2K076RW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50060922

(CHEMBL3394769)Show SMILES [#8]-[#6]-c1ccc(-c2ccc(-[#8])cc2)c(\[#6](=[#6]-2/[#6]=[#6]-[#6](=O)-[#6]=[#6]-2)-c2ccc(-[#8])cc2)c1C#Cc1ccc(-[#8])cc1 |c:17,21| Show InChI InChI=1S/C34H24O5/c35-21-26-10-20-31(23-4-13-28(37)14-5-23)34(32(26)19-3-22-1-11-27(36)12-2-22)33(24-6-15-29(38)16-7-24)25-8-17-30(39)18-9-25/h1-2,4-18,20,35-38H,21H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu
Curated by ChEMBL
| Assay Description
Mixed-competitive inhibition of human recombinant PTP1B using p-nitrophenylphosphate as substrate by Lineweaver-Burk plot analysis |
J Nat Prod 78: 34-42 (2015)
Article DOI: 10.1021/np5005856
BindingDB Entry DOI: 10.7270/Q2TX3H15 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50267967
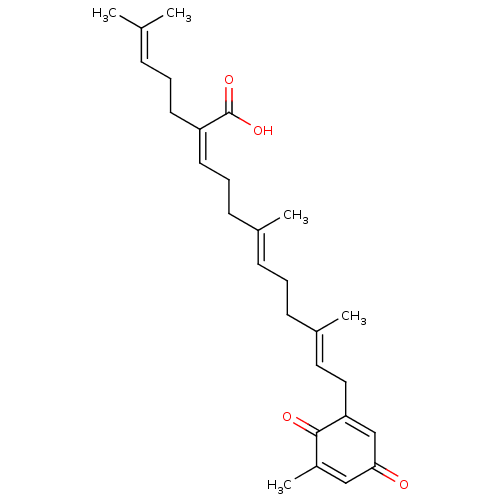
(CHEMBL4064412)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#6]-1=[#6]-[#6](=O)-[#6]=[#6](-[#6])-[#6]-1=O)-[#6](-[#8])=O |t:19,23| Show InChI InChI=1S/C27H36O4/c1-19(2)9-6-13-23(27(30)31)14-8-12-20(3)10-7-11-21(4)15-16-24-18-25(28)17-22(5)26(24)29/h9-10,14-15,17-18H,6-8,11-13,16H2,1-5H3,(H,30,31)/b20-10+,21-15+,23-14- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Food and Life Science, Pukyong National University, Busan 608-737, Republic of Korea.
Curated by ChEMBL
| Assay Description
Mixed type inhibition of recombinant human BACE1 using Rh-EVNLDAEFK-Quencher as substrate after 60 mins by Dixon plot analysis |
Bioorg Med Chem 25: 3964-3970 (2017)
Article DOI: 10.1016/j.bmc.2017.05.033
BindingDB Entry DOI: 10.7270/Q2K076RW |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50092529

(CHEMBL3586197)Show SMILES [H][C@]12CC[C@@]3([H])[C@@](C)(CC[C@@]4([H])C(C)(C)C(=O)CC[C@]34C)CC1=CC(=O)[C@@]1([H])C(C)(C)[C@H](O)CC[C@]21C |r,c:25| Show InChI InChI=1S/C30H46O3/c1-26(2)21-10-13-28(5)17-18-16-20(31)25-27(3,4)24(33)11-14-29(25,6)19(18)8-9-22(28)30(21,7)15-12-23(26)32/h16,19,21-22,24-25,33H,8-15,17H2,1-7H3/t19-,21-,22-,24+,25-,28-,29+,30-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu
Curated by ChEMBL
| Assay Description
Inhibition of BChE (unknown origin) by Dixon plot analysis |
Bioorg Med Chem 23: 3126-34 (2015)
Article DOI: 10.1016/j.bmc.2015.04.080
BindingDB Entry DOI: 10.7270/Q24X59JZ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50092533

(CHEMBL3586201)Show SMILES [H][C@]12CC[C@@]3([H])[C@@](C)(CC[C@@]4([H])C(C)(C)[C@H](O)CC[C@]34C)CC1=CC[C@@]1([H])C(C)(C)[C@@H](O)CC[C@]21C |r,c:25| Show InChI InChI=1S/C30H50O2/c1-26(2)21-10-8-19-18-28(5)15-12-22-27(3,4)25(32)14-17-30(22,7)23(28)11-9-20(19)29(21,6)16-13-24(26)31/h8,20-25,31-32H,9-18H2,1-7H3/t20-,21-,22-,23-,24-,25+,28-,29+,30-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu
Curated by ChEMBL
| Assay Description
Inhibition of BChE (unknown origin) by Dixon plot analysis |
Bioorg Med Chem 23: 3126-34 (2015)
Article DOI: 10.1016/j.bmc.2015.04.080
BindingDB Entry DOI: 10.7270/Q24X59JZ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50092532

(CHEMBL3586200)Show SMILES [H][C@@]12CC=C3C[C@]4(C)CC[C@]5([H])[C@](C)(CC[C@H](OC(=O)CCc6ccc(O)cc6)[C@]5(C)C(O)=O)[C@@]4([H])CC[C@]3([H])[C@@]1(C)CC[C@@H](O)[C@]2(C)CO |r,t:3| Show InChI InChI=1S/C39H56O7/c1-35-19-16-30-37(3,21-18-32(39(30,5)34(44)45)46-33(43)15-8-24-6-10-26(41)11-7-24)28(35)14-12-27-25(22-35)9-13-29-36(27,2)20-17-31(42)38(29,4)23-40/h6-7,9-11,27-32,40-42H,8,12-23H2,1-5H3,(H,44,45)/t27-,28-,29+,30+,31+,32-,35-,36+,37+,38+,39+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.97E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) by Dixon plot analysis |
Bioorg Med Chem 23: 3126-34 (2015)
Article DOI: 10.1016/j.bmc.2015.04.080
BindingDB Entry DOI: 10.7270/Q24X59JZ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50060921

(CHEMBL3394770)Show SMILES [#6]-[#8]-c1ccc(cc1)-[#6](=[#6]-1\[#6]=[#6]-[#6](=O)-[#6]=[#6]-1)\c1c(ccc(-[#6]-[#8])c1C#Cc1ccc(-[#8])cc1)-c1ccc(-[#8])cc1 |c:11,15| Show InChI InChI=1S/C35H26O5/c1-40-31-18-9-26(10-19-31)34(25-7-16-30(39)17-8-25)35-32(24-5-14-29(38)15-6-24)21-11-27(22-36)33(35)20-4-23-2-12-28(37)13-3-23/h2-3,5-19,21,36-38H,22H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu
Curated by ChEMBL
| Assay Description
Mixed-competitive inhibition of human recombinant PTP1B using p-nitrophenylphosphate as substrate by Lineweaver-Burk plot analysis |
J Nat Prod 78: 34-42 (2015)
Article DOI: 10.1021/np5005856
BindingDB Entry DOI: 10.7270/Q2TX3H15 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50060920

(CHEMBL3394771)Show SMILES OCc1ccc(-c2ccc(O)cc2O)c(c1C#Cc1ccc(O)cc1)C(O)(c1ccc(O)cc1)c1ccc(O)cc1 Show InChI InChI=1S/C34H26O7/c35-20-22-4-17-31(30-18-15-28(39)19-32(30)40)33(29(22)16-3-21-1-9-25(36)10-2-21)34(41,23-5-11-26(37)12-6-23)24-7-13-27(38)14-8-24/h1-2,4-15,17-19,35-41H,20H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of human recombinant PTP1B using p-nitrophenylphosphate as substrate by Lineweaver-Burk plot analysis |
J Nat Prod 78: 34-42 (2015)
Article DOI: 10.1021/np5005856
BindingDB Entry DOI: 10.7270/Q2TX3H15 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50060918
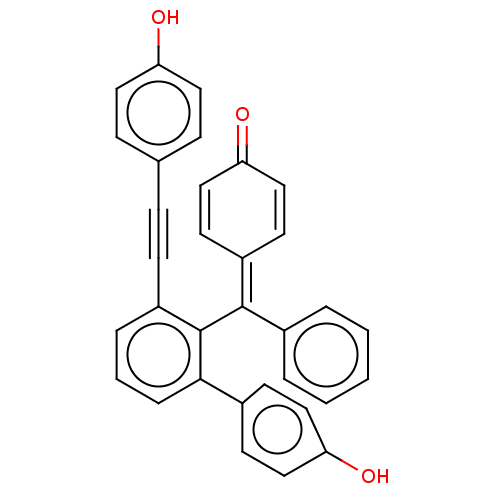
(Selaginellin)Show SMILES [#8]-c1ccc(cc1)C#Cc1cccc(-c2ccc(-[#8])cc2)c1\[#6](=[#6]-1/[#6]=[#6]-[#6](=O)-[#6]=[#6]-1)-c1ccccc1 |c:27,31| Show InChI InChI=1S/C33H22O3/c34-28-17-10-23(11-18-28)9-12-26-7-4-8-31(24-13-19-29(35)20-14-24)33(26)32(25-5-2-1-3-6-25)27-15-21-30(36)22-16-27/h1-8,10-11,13-22,34-35H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.39E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu
Curated by ChEMBL
| Assay Description
Mixed-competitive inhibition of human recombinant PTP1B using p-nitrophenylphosphate as substrate by Lineweaver-Burk plot analysis |
J Nat Prod 78: 34-42 (2015)
Article DOI: 10.1021/np5005856
BindingDB Entry DOI: 10.7270/Q2TX3H15 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50060919

(CHEMBL3394772)Show SMILES COCc1ccc(-c2ccc(O)cc2O)c(c1C#Cc1ccc(O)cc1)C(O)(c1ccc(O)cc1)c1ccc(O)cc1 Show InChI InChI=1S/C35H28O7/c1-42-21-23-5-18-32(31-19-16-29(39)20-33(31)40)34(30(23)17-4-22-2-10-26(36)11-3-22)35(41,24-6-12-27(37)13-7-24)25-8-14-28(38)15-9-25/h2-3,5-16,18-20,36-41H,21H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu
Curated by ChEMBL
| Assay Description
Uncompetitive inhibition of human recombinant PTP1B using p-nitrophenylphosphate as substrate by Lineweaver-Burk plot analysis |
J Nat Prod 78: 34-42 (2015)
Article DOI: 10.1021/np5005856
BindingDB Entry DOI: 10.7270/Q2TX3H15 |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM18207

((1R,2S,10S,11S,13R,14R,15S,17S)-1-fluoro-14,17-dih...)Show SMILES [H][C@@]12C[C@@H](C)[C@](O)(C(=O)CO)[C@@]1(C)C[C@H](O)[C@@]1(F)[C@@]2([H])CCC2=CC(=O)C=C[C@]12C |c:28,t:24| Show InChI InChI=1S/C22H29FO5/c1-12-8-16-15-5-4-13-9-14(25)6-7-19(13,2)21(15,23)17(26)10-20(16,3)22(12,28)18(27)11-24/h6-7,9,12,15-17,24,26,28H,4-5,8,10-11H2,1-3H3/t12-,15+,16+,17+,19+,20+,21+,22+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at glucocorticoid receptor in HFF assessed as inhibition of IL1-induced IL-6 production after 18 to 24 hrs |
Bioorg Med Chem Lett 21: 6842-51 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.018
BindingDB Entry DOI: 10.7270/Q2CV4J51 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50356050
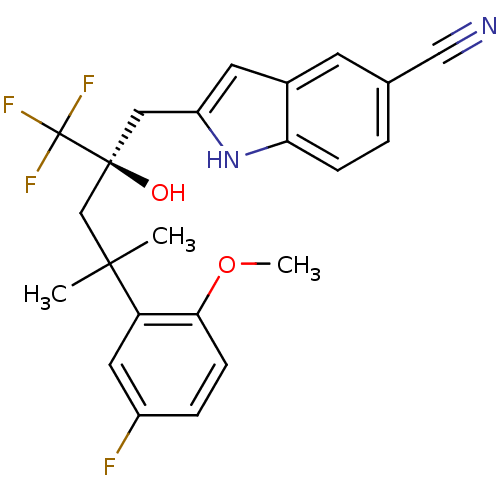
(CHEMBL1911650)Show SMILES COc1ccc(F)cc1C(C)(C)C[C@@](O)(Cc1cc2cc(ccc2[nH]1)C#N)C(F)(F)F |r| Show InChI InChI=1S/C23H22F4N2O2/c1-21(2,18-10-16(24)5-7-20(18)31-3)13-22(30,23(25,26)27)11-17-9-15-8-14(12-28)4-6-19(15)29-17/h4-10,29-30H,11,13H2,1-3H3/t22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of tetramethylrhodamine-labeled Dexamethasone from human recombinant glucocorticoid receptor expressed in baculovirus infected insect ce... |
Bioorg Med Chem Lett 21: 6842-51 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.018
BindingDB Entry DOI: 10.7270/Q2CV4J51 |
More data for this
Ligand-Target Pair | |
Glutaminyl-peptide cyclotransferase
(Homo sapiens (Human)) | BDBM50519290
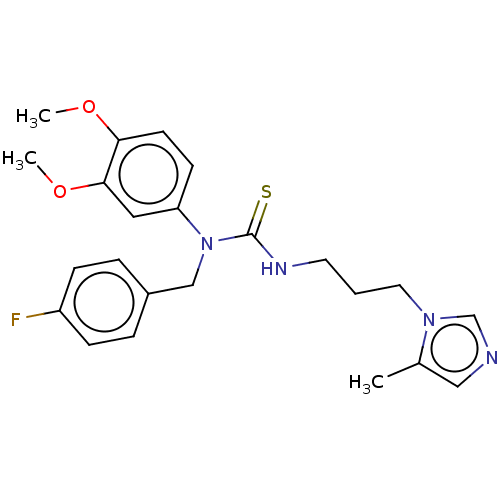
(CHEMBL4525926)Show SMILES COc1ccc(cc1OC)N(Cc1ccc(F)cc1)C(=S)NCCCn1cncc1C Show InChI InChI=1S/C23H27FN4O2S/c1-17-14-25-16-27(17)12-4-11-26-23(31)28(15-18-5-7-19(24)8-6-18)20-9-10-21(29-2)22(13-20)30-3/h5-10,13-14,16H,4,11-12,15H2,1-3H3,(H,26,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal His6-tagged glutaminyl cyclase (Ala33 to Leu361 residues) expressed in baculovirus infected Sf21 insect ce... |
J Med Chem 62: 8011-8027 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00751
BindingDB Entry DOI: 10.7270/Q2W0999V |
More data for this
Ligand-Target Pair | |
Glutaminyl-peptide cyclotransferase
(Homo sapiens (Human)) | BDBM50519285
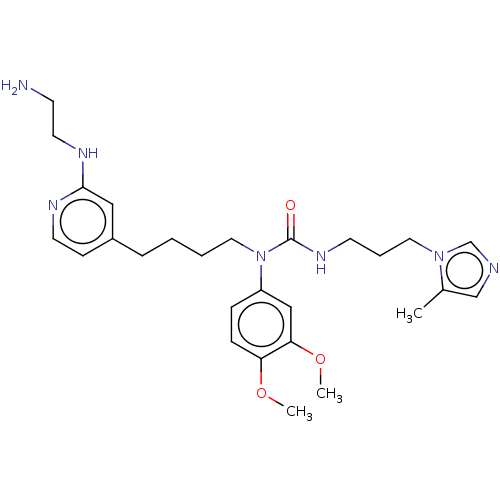
(CHEMBL4445407)Show SMILES COc1ccc(cc1OC)N(CCCCc1ccnc(NCCN)c1)C(=O)NCCCn1cncc1C Show InChI InChI=1S/C27H39N7O3/c1-21-19-29-20-33(21)15-6-12-32-27(35)34(23-8-9-24(36-2)25(18-23)37-3)16-5-4-7-22-10-13-30-26(17-22)31-14-11-28/h8-10,13,17-20H,4-7,11-12,14-16,28H2,1-3H3,(H,30,31)(H,32,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal His6-tagged glutaminyl cyclase (Ala33 to Leu361 residues) expressed in baculovirus infected Sf21 insect ce... |
J Med Chem 62: 8011-8027 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00751
BindingDB Entry DOI: 10.7270/Q2W0999V |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50208836
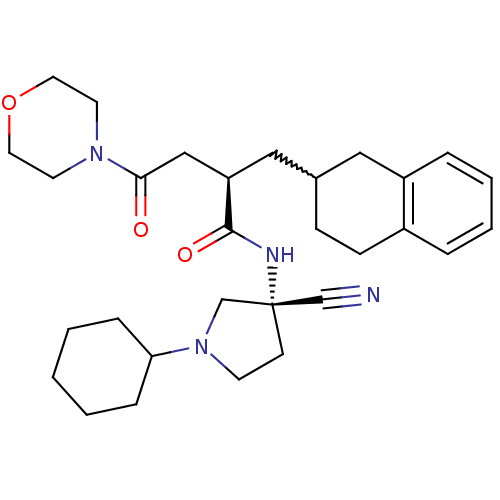
((R)-N-((R)-3-cyano-1-cyclohexylpyrrolidin-3-yl)-4-...)Show SMILES O=C(C[C@@H](CC1CCc2ccccc2C1)C(=O)N[C@@]1(CCN(C1)C1CCCCC1)C#N)N1CCOCC1 |w:5.4| Show InChI InChI=1S/C30H42N4O3/c31-21-30(12-13-34(22-30)27-8-2-1-3-9-27)32-29(36)26(20-28(35)33-14-16-37-17-15-33)19-23-10-11-24-6-4-5-7-25(24)18-23/h4-7,23,26-27H,1-3,8-20,22H2,(H,32,36)/t23?,26-,30+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin S |
Bioorg Med Chem Lett 17: 2465-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.046
BindingDB Entry DOI: 10.7270/Q24X57G7 |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50356060
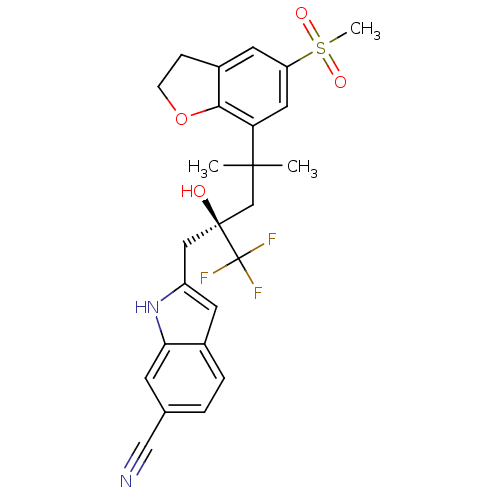
(CHEMBL1911664)Show SMILES CC(C)(C[C@@](O)(Cc1cc2ccc(cc2[nH]1)C#N)C(F)(F)F)c1cc(cc2CCOc12)S(C)(=O)=O |r| Show InChI InChI=1S/C25H25F3N2O4S/c1-23(2,20-11-19(35(3,32)33)10-17-6-7-34-22(17)20)14-24(31,25(26,27)28)12-18-9-16-5-4-15(13-29)8-21(16)30-18/h4-5,8-11,30-31H,6-7,12,14H2,1-3H3/t24-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of tetramethylrhodamine-labeled Dexamethasone from human recombinant glucocorticoid receptor expressed in baculovirus infected insect ce... |
Bioorg Med Chem Lett 21: 6842-51 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.018
BindingDB Entry DOI: 10.7270/Q2CV4J51 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50593227
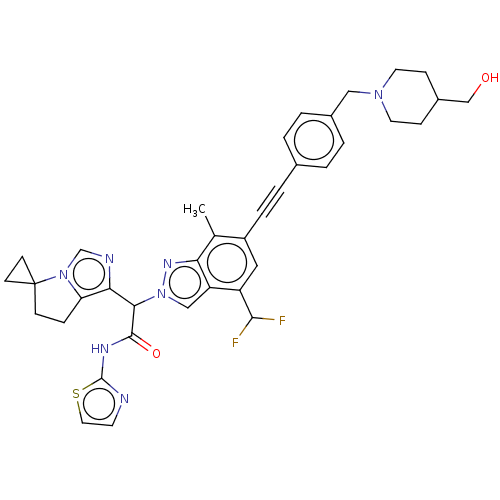
(CHEMBL5177023)Show SMILES Cc1c(cc(C(F)F)c2cn(nc12)C(C(=O)Nc1nccs1)c1ncn2c1CCC21CC1)C#Cc1ccc(CN2CCC(CO)CC2)cc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00893
BindingDB Entry DOI: 10.7270/Q2RN3CVW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutaminyl-peptide cyclotransferase
(Homo sapiens (Human)) | BDBM50519280
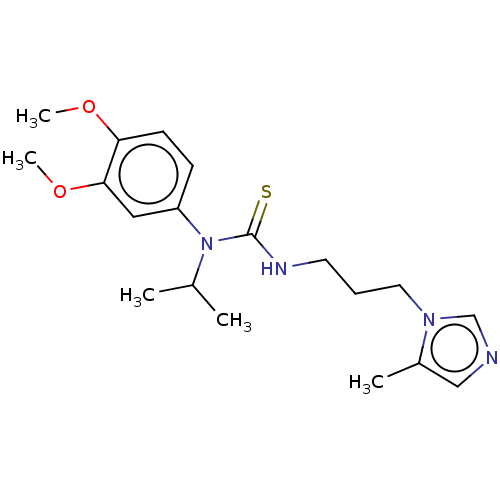
(CHEMBL4464130)Show InChI InChI=1S/C19H28N4O2S/c1-14(2)23(16-7-8-17(24-4)18(11-16)25-5)19(26)21-9-6-10-22-13-20-12-15(22)3/h7-8,11-14H,6,9-10H2,1-5H3,(H,21,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal His6-tagged glutaminyl cyclase (Ala33 to Leu361 residues) expressed in baculovirus infected Sf21 insect ce... |
J Med Chem 62: 8011-8027 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00751
BindingDB Entry DOI: 10.7270/Q2W0999V |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM18207

((1R,2S,10S,11S,13R,14R,15S,17S)-1-fluoro-14,17-dih...)Show SMILES [H][C@@]12C[C@@H](C)[C@](O)(C(=O)CO)[C@@]1(C)C[C@H](O)[C@@]1(F)[C@@]2([H])CCC2=CC(=O)C=C[C@]12C |c:28,t:24| Show InChI InChI=1S/C22H29FO5/c1-12-8-16-15-5-4-13-9-14(25)6-7-19(13,2)21(15,23)17(26)10-20(16,3)22(12,28)18(27)11-24/h6-7,9,12,15-17,24,26,28H,4-5,8,10-11H2,1-3H3/t12-,15+,16+,17+,19+,20+,21+,22+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of tetramethylrhodamine-labeled Dexamethasone from human recombinant glucocorticoid receptor expressed in baculovirus infected insect ce... |
Bioorg Med Chem Lett 21: 6842-51 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.018
BindingDB Entry DOI: 10.7270/Q2CV4J51 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM18207

((1R,2S,10S,11S,13R,14R,15S,17S)-1-fluoro-14,17-dih...)Show SMILES [H][C@@]12C[C@@H](C)[C@](O)(C(=O)CO)[C@@]1(C)C[C@H](O)[C@@]1(F)[C@@]2([H])CCC2=CC(=O)C=C[C@]12C |c:28,t:24| Show InChI InChI=1S/C22H29FO5/c1-12-8-16-15-5-4-13-9-14(25)6-7-19(13,2)21(15,23)17(26)10-20(16,3)22(12,28)18(27)11-24/h6-7,9,12,15-17,24,26,28H,4-5,8,10-11H2,1-3H3/t12-,15+,16+,17+,19+,20+,21+,22+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of tetramethylrhodamine labeled dexamethosone binding to GR by FP assay |
J Med Chem 49: 7887-96 (2006)
Article DOI: 10.1021/jm061273t
BindingDB Entry DOI: 10.7270/Q2BG2PTB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50201093
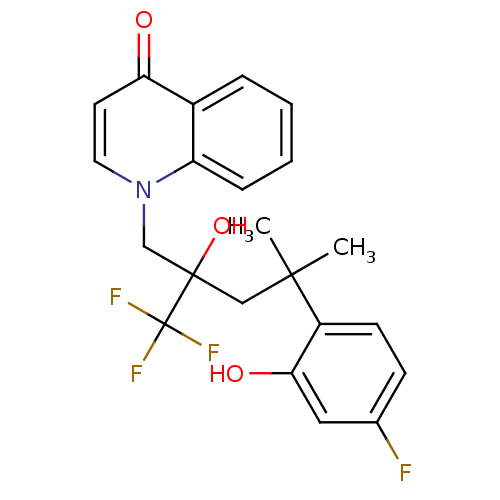
(1-[4-(4-fluoro-2-hydroxyphenyl)-2-hydroxy-4-methyl...)Show SMILES CC(C)(CC(O)(Cn1ccc(=O)c2ccccc12)C(F)(F)F)c1ccc(F)cc1O Show InChI InChI=1S/C22H21F4NO3/c1-20(2,16-8-7-14(23)11-19(16)29)12-21(30,22(24,25)26)13-27-10-9-18(28)15-5-3-4-6-17(15)27/h3-11,29-30H,12-13H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of tetramethylrhodamine labeled dexamethosone binding to GR by FP assay |
J Med Chem 49: 7887-96 (2006)
Article DOI: 10.1021/jm061273t
BindingDB Entry DOI: 10.7270/Q2BG2PTB |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50593214
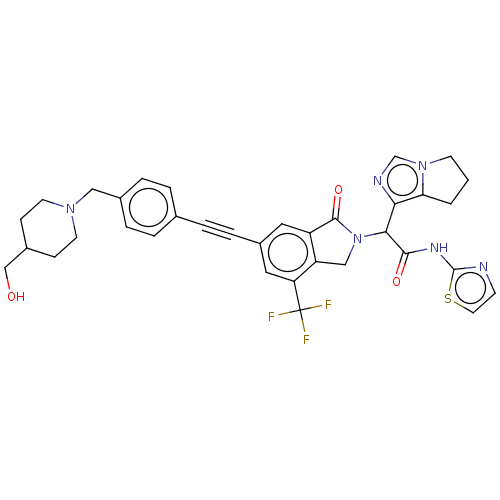
(CHEMBL5189574)Show SMILES OCC1CCN(Cc2ccc(cc2)C#Cc2cc3C(=O)N(Cc3c(c2)C(F)(F)F)C(C(=O)Nc2nccs2)c2ncn3CCCc23)CC1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00893
BindingDB Entry DOI: 10.7270/Q2RN3CVW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM18207

((1R,2S,10S,11S,13R,14R,15S,17S)-1-fluoro-14,17-dih...)Show SMILES [H][C@@]12C[C@@H](C)[C@](O)(C(=O)CO)[C@@]1(C)C[C@H](O)[C@@]1(F)[C@@]2([H])CCC2=CC(=O)C=C[C@]12C |c:28,t:24| Show InChI InChI=1S/C22H29FO5/c1-12-8-16-15-5-4-13-9-14(25)6-7-19(13,2)21(15,23)17(26)10-20(16,3)22(12,28)18(27)11-24/h6-7,9,12,15-17,24,26,28H,4-5,8,10-11H2,1-3H3/t12-,15+,16+,17+,19+,20+,21+,22+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Displacement of TAMRA labeled Dexamethasone at human glucocorticoid receptor in insect cell |
Bioorg Med Chem Lett 17: 5025-31 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.031
BindingDB Entry DOI: 10.7270/Q2G73FJ2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50356050

(CHEMBL1911650)Show SMILES COc1ccc(F)cc1C(C)(C)C[C@@](O)(Cc1cc2cc(ccc2[nH]1)C#N)C(F)(F)F |r| Show InChI InChI=1S/C23H22F4N2O2/c1-21(2,18-10-16(24)5-7-20(18)31-3)13-22(30,23(25,26)27)11-17-9-15-8-14(12-28)4-6-19(15)29-17/h4-10,29-30H,11,13H2,1-3H3/t22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at glucocorticoid receptor in HFF assessed as inhibition of IL1-induced IL-6 production after 18 to 24 hrs |
Bioorg Med Chem Lett 21: 6842-51 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.018
BindingDB Entry DOI: 10.7270/Q2CV4J51 |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50201099
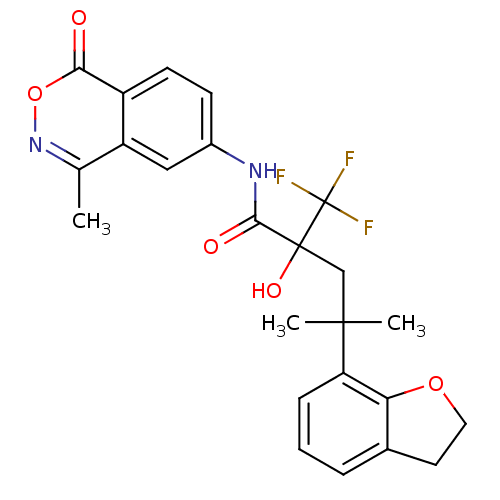
(4-(2,3-dihydrobenzofuran-7-yl)-2-hydroxy-4-methyl-...)Show SMILES Cc1noc(=O)c2ccc(NC(=O)C(O)(CC(C)(C)c3cccc4CCOc34)C(F)(F)F)cc12 Show InChI InChI=1S/C24H23F3N2O5/c1-13-17-11-15(7-8-16(17)20(30)34-29-13)28-21(31)23(32,24(25,26)27)12-22(2,3)18-6-4-5-14-9-10-33-19(14)18/h4-8,11,32H,9-10,12H2,1-3H3,(H,28,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of tetramethylrhodamine labeled dexamethosone binding to GR by FP assay |
J Med Chem 49: 7887-96 (2006)
Article DOI: 10.1021/jm061273t
BindingDB Entry DOI: 10.7270/Q2BG2PTB |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50593226
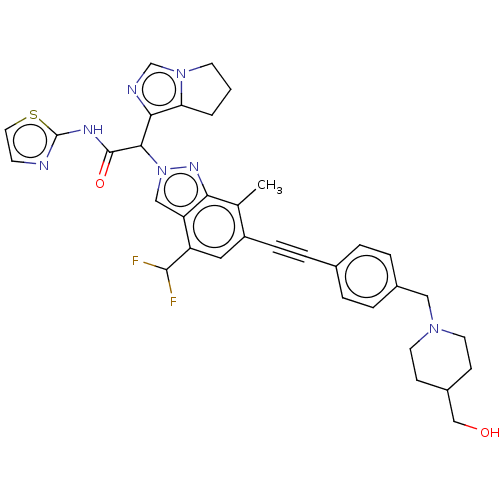
(CHEMBL5184760)Show SMILES Cc1c(cc(C(F)F)c2cn(nc12)C(C(=O)Nc1nccs1)c1ncn2CCCc12)C#Cc1ccc(CN2CCC(CO)CC2)cc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00893
BindingDB Entry DOI: 10.7270/Q2RN3CVW |
More data for this
Ligand-Target Pair | |
Glutaminyl-peptide cyclotransferase
(Homo sapiens (Human)) | BDBM50519269
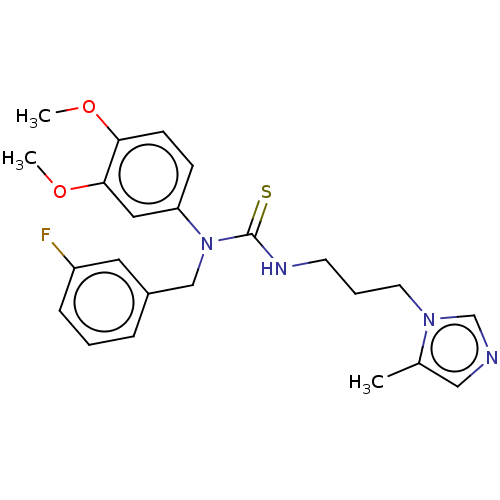
(CHEMBL4539841)Show SMILES COc1ccc(cc1OC)N(Cc1cccc(F)c1)C(=S)NCCCn1cncc1C Show InChI InChI=1S/C23H27FN4O2S/c1-17-14-25-16-27(17)11-5-10-26-23(31)28(15-18-6-4-7-19(24)12-18)20-8-9-21(29-2)22(13-20)30-3/h4,6-9,12-14,16H,5,10-11,15H2,1-3H3,(H,26,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal His6-tagged glutaminyl cyclase (Ala33 to Leu361 residues) expressed in baculovirus infected Sf21 insect ce... |
J Med Chem 62: 8011-8027 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00751
BindingDB Entry DOI: 10.7270/Q2W0999V |
More data for this
Ligand-Target Pair | |
Glutaminyl-peptide cyclotransferase
(Homo sapiens (Human)) | BDBM50519268
(CHEMBL4586501)Show SMILES COc1ccc(cc1OC)N(Cc1cccc(Cl)c1)C(=S)NCCCn1cncc1C Show InChI InChI=1S/C23H27ClN4O2S/c1-17-14-25-16-27(17)11-5-10-26-23(31)28(15-18-6-4-7-19(24)12-18)20-8-9-21(29-2)22(13-20)30-3/h4,6-9,12-14,16H,5,10-11,15H2,1-3H3,(H,26,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal His6-tagged glutaminyl cyclase (Ala33 to Leu361 residues) expressed in baculovirus infected Sf21 insect ce... |
J Med Chem 62: 8011-8027 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00751
BindingDB Entry DOI: 10.7270/Q2W0999V |
More data for this
Ligand-Target Pair | |
Glutaminyl-peptide cyclotransferase
(Homo sapiens (Human)) | BDBM50519240

(CHEMBL4547037)Show SMILES COc1ccc(cc1OC)N(CCCCc1ccnc(N)c1)C(=O)NCCCn1cncc1C Show InChI InChI=1S/C25H34N6O3/c1-19-17-27-18-30(19)13-6-11-29-25(32)31(21-8-9-22(33-2)23(16-21)34-3)14-5-4-7-20-10-12-28-24(26)15-20/h8-10,12,15-18H,4-7,11,13-14H2,1-3H3,(H2,26,28)(H,29,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal His6-tagged glutaminyl cyclase (Ala33 to Leu361 residues) expressed in baculovirus infected Sf21 insect ce... |
J Med Chem 62: 8011-8027 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00751
BindingDB Entry DOI: 10.7270/Q2W0999V |
More data for this
Ligand-Target Pair | |
Glutaminyl-peptide cyclotransferase
(Homo sapiens (Human)) | BDBM50519259
(CHEMBL4448303)Show SMILES COc1ccc(cc1OC)N(Cc1ccc(F)cc1)C(=O)NCCCn1cncc1C Show InChI InChI=1S/C23H27FN4O3/c1-17-14-25-16-27(17)12-4-11-26-23(29)28(15-18-5-7-19(24)8-6-18)20-9-10-21(30-2)22(13-20)31-3/h5-10,13-14,16H,4,11-12,15H2,1-3H3,(H,26,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal His6-tagged glutaminyl cyclase (Ala33 to Leu361 residues) expressed in baculovirus infected Sf21 insect ce... |
J Med Chem 62: 8011-8027 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00751
BindingDB Entry DOI: 10.7270/Q2W0999V |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50593216

(CHEMBL5183324)Show SMILES CC1(C)CCc2c(ncn12)C(N1Cc2c(cc(cc2C(F)F)C#Cc2ccc(CN3CCC(CO)CC3)cc2)C1=O)C(=O)Nc1nccs1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00893
BindingDB Entry DOI: 10.7270/Q2RN3CVW |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50201100
(1-[4-(5-fluoro-2-methoxyphenyl)-2-hydroxy-4-methyl...)Show SMILES COc1ccc(F)cc1C(C)(C)CC(O)(CN1CCC(=O)c2ccccc12)C(F)(F)F Show InChI InChI=1S/C23H25F4NO3/c1-21(2,17-12-15(24)8-9-20(17)31-3)13-22(30,23(25,26)27)14-28-11-10-19(29)16-6-4-5-7-18(16)28/h4-9,12,30H,10-11,13-14H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of tetramethylrhodamine labeled dexamethosone binding to GR by FP assay |
J Med Chem 49: 7887-96 (2006)
Article DOI: 10.1021/jm061273t
BindingDB Entry DOI: 10.7270/Q2BG2PTB |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50201081
(1-[4-(2,3-dihydrobenzofuran-7-yl)-2-hydroxy-4-meth...)Show SMILES CC(C)(CC(O)(Cn1ccc(=O)c2ccccc12)C(F)(F)F)c1cccc2CCOc12 Show InChI InChI=1S/C24H24F3NO3/c1-22(2,18-8-5-6-16-11-13-31-21(16)18)14-23(30,24(25,26)27)15-28-12-10-20(29)17-7-3-4-9-19(17)28/h3-10,12,30H,11,13-15H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of tetramethylrhodamine labeled dexamethosone binding to GR by FP assay |
J Med Chem 49: 7887-96 (2006)
Article DOI: 10.1021/jm061273t
BindingDB Entry DOI: 10.7270/Q2BG2PTB |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50593204

(CHEMBL5173979)Show SMILES Clc1cccc2C(=O)N(Cc12)C(C(=O)Nc1nccs1)c1ncn2CCCc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00893
BindingDB Entry DOI: 10.7270/Q2RN3CVW |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50593219
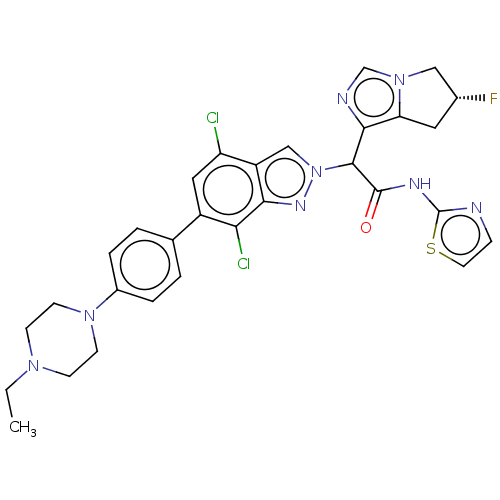
(CHEMBL5202945)Show SMILES CCN1CCN(CC1)c1ccc(cc1)-c1cc(Cl)c2cn(nc2c1Cl)C(C(=O)Nc1nccs1)c1ncn2C[C@H](F)Cc12 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00893
BindingDB Entry DOI: 10.7270/Q2RN3CVW |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50593215
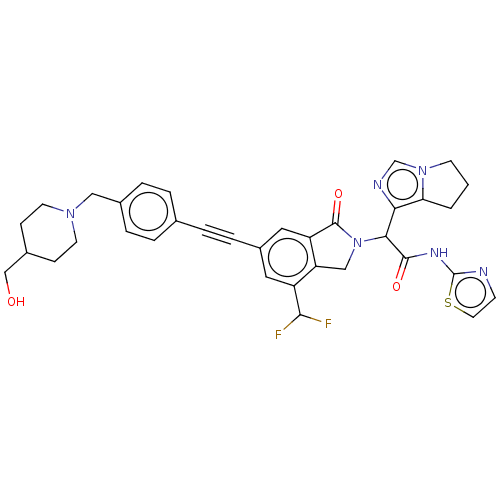
(CHEMBL5184539)Show SMILES OCC1CCN(Cc2ccc(cc2)C#Cc2cc3C(=O)N(Cc3c(c2)C(F)F)C(C(=O)Nc2nccs2)c2ncn3CCCc23)CC1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00893
BindingDB Entry DOI: 10.7270/Q2RN3CVW |
More data for this
Ligand-Target Pair | |
Glutaminyl-peptide cyclotransferase
(Homo sapiens (Human)) | BDBM50519272
(CHEMBL4445143)Show InChI InChI=1S/C22H32N4O2S/c1-17-14-23-16-25(17)12-6-11-24-22(29)26(15-18-7-4-5-8-18)19-9-10-20(27-2)21(13-19)28-3/h9-10,13-14,16,18H,4-8,11-12,15H2,1-3H3,(H,24,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal His6-tagged glutaminyl cyclase (Ala33 to Leu361 residues) expressed in baculovirus infected Sf21 insect ce... |
J Med Chem 62: 8011-8027 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00751
BindingDB Entry DOI: 10.7270/Q2W0999V |
More data for this
Ligand-Target Pair | |
Glutaminyl-peptide cyclotransferase
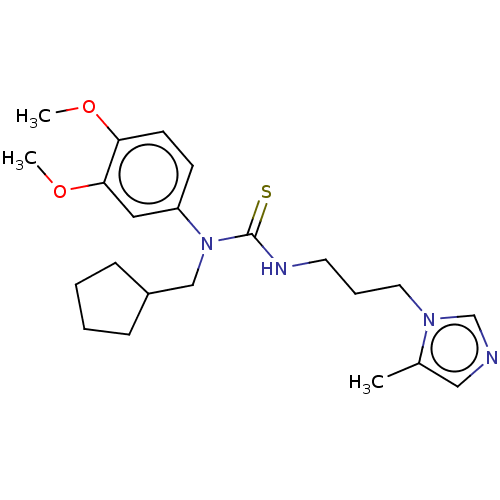
(Homo sapiens (Human)) | BDBM50237457
(CHEMBL4097599)Show SMILES COc1cc(NC(=S)NCCCn2cncc2C)ccc1OCCCCc1ccnc(N)c1 Show InChI InChI=1S/C24H32N6O2S/c1-18-16-26-17-30(18)12-5-10-28-24(33)29-20-7-8-21(22(15-20)31-2)32-13-4-3-6-19-9-11-27-23(25)14-19/h7-9,11,14-17H,3-6,10,12-13H2,1-2H3,(H2,25,27)(H2,28,29,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal His6-tagged glutaminyl cyclase (Ala33 to Leu361 residues) expressed in baculovirus infected Sf21 insect ce... |
J Med Chem 62: 8011-8027 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00751
BindingDB Entry DOI: 10.7270/Q2W0999V |
More data for this
Ligand-Target Pair | |
Glutaminyl-peptide cyclotransferase
(Homo sapiens (Human)) | BDBM50519261
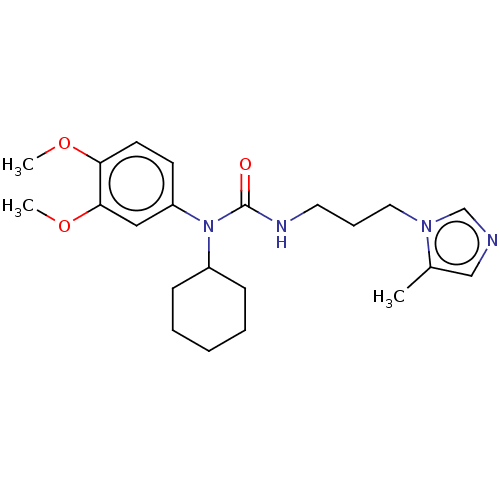
(CHEMBL4549443)Show InChI InChI=1S/C22H32N4O3/c1-17-15-23-16-25(17)13-7-12-24-22(27)26(18-8-5-4-6-9-18)19-10-11-20(28-2)21(14-19)29-3/h10-11,14-16,18H,4-9,12-13H2,1-3H3,(H,24,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal His6-tagged glutaminyl cyclase (Ala33 to Leu361 residues) expressed in baculovirus infected Sf21 insect ce... |
J Med Chem 62: 8011-8027 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00751
BindingDB Entry DOI: 10.7270/Q2W0999V |
More data for this
Ligand-Target Pair | |
Glutaminyl-peptide cyclotransferase
(Homo sapiens (Human)) | BDBM50519298
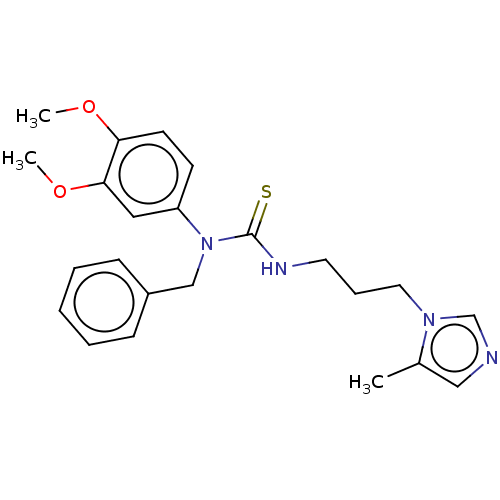
(CHEMBL4459792)Show SMILES COc1ccc(cc1OC)N(Cc1ccccc1)C(=S)NCCCn1cncc1C Show InChI InChI=1S/C23H28N4O2S/c1-18-15-24-17-26(18)13-7-12-25-23(30)27(16-19-8-5-4-6-9-19)20-10-11-21(28-2)22(14-20)29-3/h4-6,8-11,14-15,17H,7,12-13,16H2,1-3H3,(H,25,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal His6-tagged glutaminyl cyclase (Ala33 to Leu361 residues) expressed in baculovirus infected Sf21 insect ce... |
J Med Chem 62: 8011-8027 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00751
BindingDB Entry DOI: 10.7270/Q2W0999V |
More data for this
Ligand-Target Pair | |
Glutaminyl-peptide cyclotransferase
(Homo sapiens (Human)) | BDBM50519274
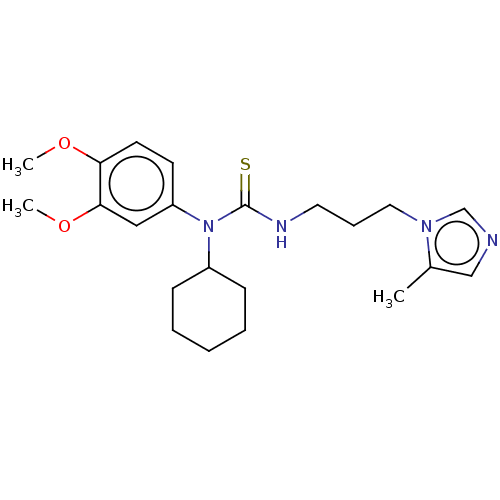
(CHEMBL4462968)Show InChI InChI=1S/C22H32N4O2S/c1-17-15-23-16-25(17)13-7-12-24-22(29)26(18-8-5-4-6-9-18)19-10-11-20(27-2)21(14-19)28-3/h10-11,14-16,18H,4-9,12-13H2,1-3H3,(H,24,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal His6-tagged glutaminyl cyclase (Ala33 to Leu361 residues) expressed in baculovirus infected Sf21 insect ce... |
J Med Chem 62: 8011-8027 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00751
BindingDB Entry DOI: 10.7270/Q2W0999V |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50356044
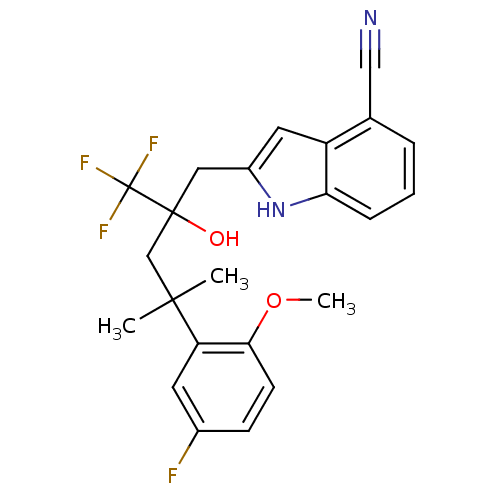
(CHEMBL1911644)Show SMILES COc1ccc(F)cc1C(C)(C)CC(O)(Cc1cc2c(cccc2[nH]1)C#N)C(F)(F)F Show InChI InChI=1S/C23H22F4N2O2/c1-21(2,18-9-15(24)7-8-20(18)31-3)13-22(30,23(25,26)27)11-16-10-17-14(12-28)5-4-6-19(17)29-16/h4-10,29-30H,11,13H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of tetramethylrhodamine-labeled Dexamethasone from human recombinant glucocorticoid receptor expressed in baculovirus infected insect ce... |
Bioorg Med Chem Lett 21: 6842-51 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.018
BindingDB Entry DOI: 10.7270/Q2CV4J51 |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50356063
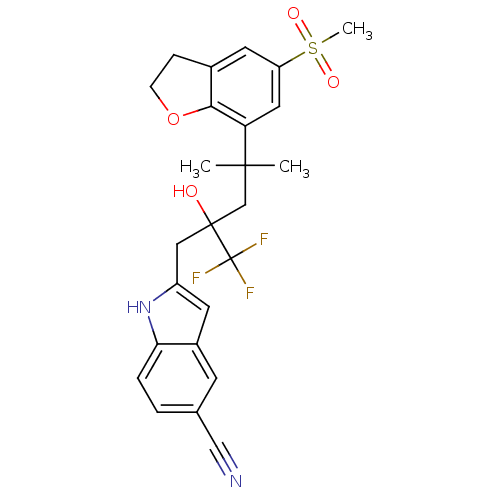
(CHEMBL1911661)Show SMILES CC(C)(CC(O)(Cc1cc2cc(ccc2[nH]1)C#N)C(F)(F)F)c1cc(cc2CCOc12)S(C)(=O)=O Show InChI InChI=1S/C25H25F3N2O4S/c1-23(2,20-11-19(35(3,32)33)10-16-6-7-34-22(16)20)14-24(31,25(26,27)28)12-18-9-17-8-15(13-29)4-5-21(17)30-18/h4-5,8-11,30-31H,6-7,12,14H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of tetramethylrhodamine-labeled Dexamethasone from human recombinant glucocorticoid receptor expressed in baculovirus infected insect ce... |
Bioorg Med Chem Lett 21: 6842-51 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.018
BindingDB Entry DOI: 10.7270/Q2CV4J51 |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50356059
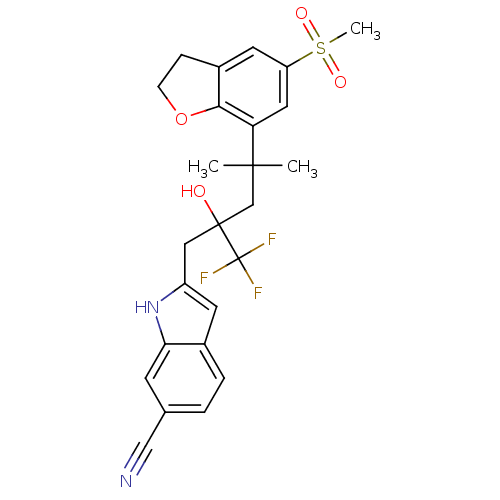
(CHEMBL1911662)Show SMILES CC(C)(CC(O)(Cc1cc2ccc(cc2[nH]1)C#N)C(F)(F)F)c1cc(cc2CCOc12)S(C)(=O)=O Show InChI InChI=1S/C25H25F3N2O4S/c1-23(2,20-11-19(35(3,32)33)10-17-6-7-34-22(17)20)14-24(31,25(26,27)28)12-18-9-16-5-4-15(13-29)8-21(16)30-18/h4-5,8-11,30-31H,6-7,12,14H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of tetramethylrhodamine-labeled Dexamethasone from human recombinant glucocorticoid receptor expressed in baculovirus infected insect ce... |
Bioorg Med Chem Lett 21: 6842-51 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.018
BindingDB Entry DOI: 10.7270/Q2CV4J51 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50121542
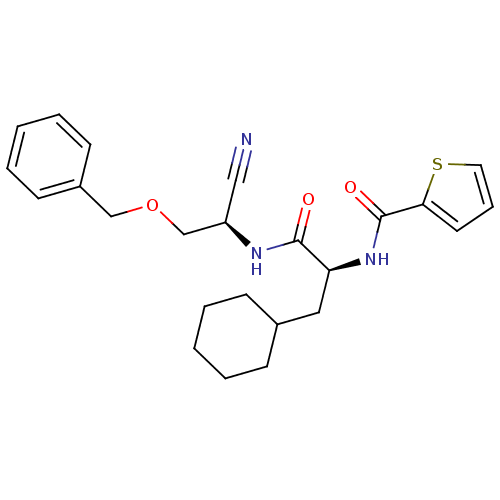
(CHEMBL155560 | Thiophene-2-carboxylic acid {1-[(be...)Show SMILES O=C(N[C@@H](COCc1ccccc1)C#N)[C@H](CC1CCCCC1)NC(=O)c1cccs1 Show InChI InChI=1S/C24H29N3O3S/c25-15-20(17-30-16-19-10-5-2-6-11-19)26-23(28)21(14-18-8-3-1-4-9-18)27-24(29)22-12-7-13-31-22/h2,5-7,10-13,18,20-21H,1,3-4,8-9,14,16-17H2,(H,26,28)(H,27,29)/t20-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus |
J Med Chem 45: 5471-82 (2002)
BindingDB Entry DOI: 10.7270/Q2P26XG7 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50593212

(CHEMBL5207479)Show SMILES OC1CCN(Cc2ccc(cc2)C#Cc2cc3C(=O)N(Cc3c(F)c2)C(C(=O)Nc2nccs2)c2ncn3CCCc23)CC1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00893
BindingDB Entry DOI: 10.7270/Q2RN3CVW |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data