 Found 69 hits with Last Name = 'murai' and Initial = 'y'
Found 69 hits with Last Name = 'murai' and Initial = 'y' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
AMP deaminase 2
(Homo sapiens) | BDBM50610224

(CHEMBL5269095) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
AMP deaminase 2
(Mus musculus) | BDBM50610231
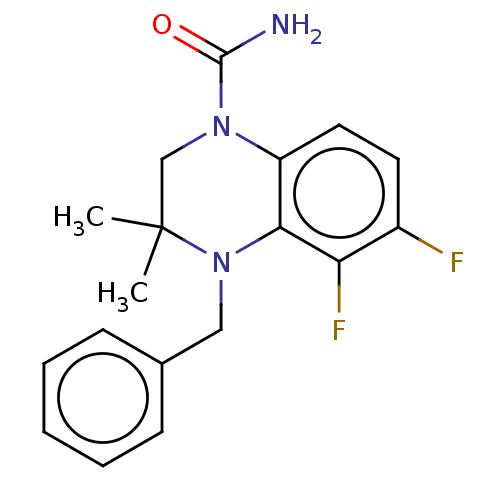
(CHEMBL5271711) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
AMP deaminase 2
(Homo sapiens) | BDBM50610231

(CHEMBL5271711) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
AMP deaminase 2
(Homo sapiens) | BDBM50610244
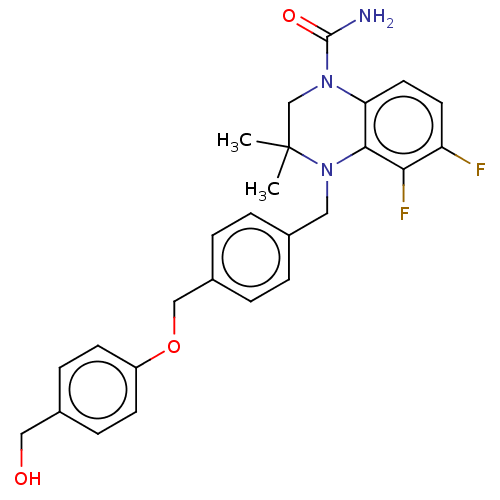
(CHEMBL5272816)Show SMILES CC1(C)CN(C(N)=O)c2ccc(F)c(F)c2N1Cc1ccc(COc2ccc(CO)cc2)cc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
AMP deaminase 2
(Mus musculus) | BDBM50610244

(CHEMBL5272816)Show SMILES CC1(C)CN(C(N)=O)c2ccc(F)c(F)c2N1Cc1ccc(COc2ccc(CO)cc2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
AMP deaminase 2
(Homo sapiens) | BDBM50610242
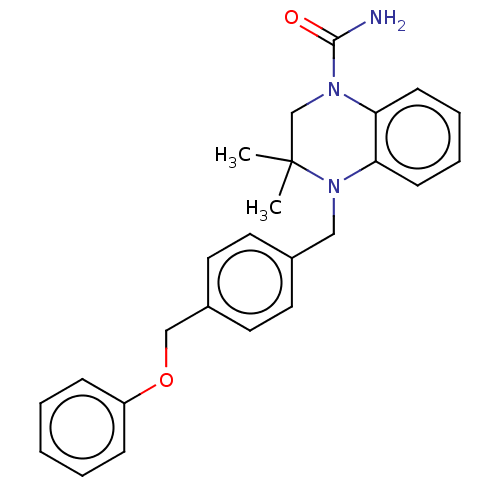
(CHEMBL5270167)Show SMILES CC1(C)CN(C(N)=O)c2ccccc2N1Cc1ccc(COc2ccccc2)cc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
AMP deaminase 2
(Homo sapiens) | BDBM50610243
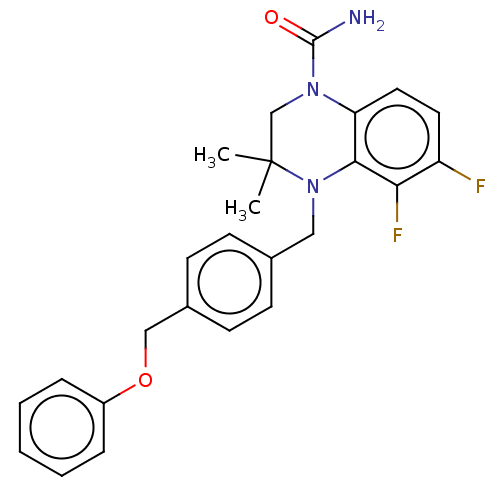
(CHEMBL5287644)Show SMILES CC1(C)CN(C(N)=O)c2ccc(F)c(F)c2N1Cc1ccc(COc2ccccc2)cc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
AMP deaminase 2
(Homo sapiens) | BDBM50610237

(CHEMBL5287535)Show SMILES CC1(C)CN(C(N)=O)c2ccccc2N1Cc1cccc(OC(F)(F)F)c1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
AMP deaminase 2
(Homo sapiens) | BDBM50610246
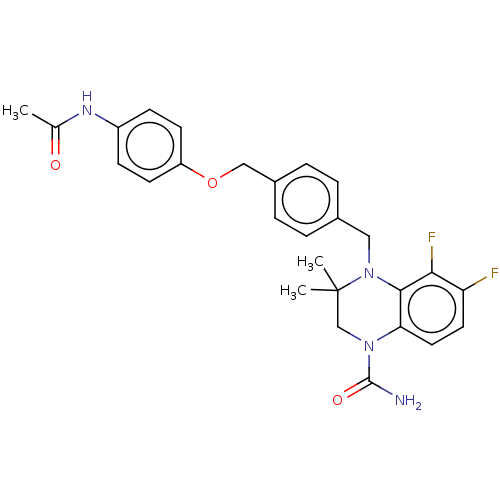
(CHEMBL5281489)Show SMILES CC(=O)Nc1ccc(OCc2ccc(CN3c4c(F)c(F)ccc4N(CC3(C)C)C(N)=O)cc2)cc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
AMP deaminase 2
(Homo sapiens) | BDBM50610236
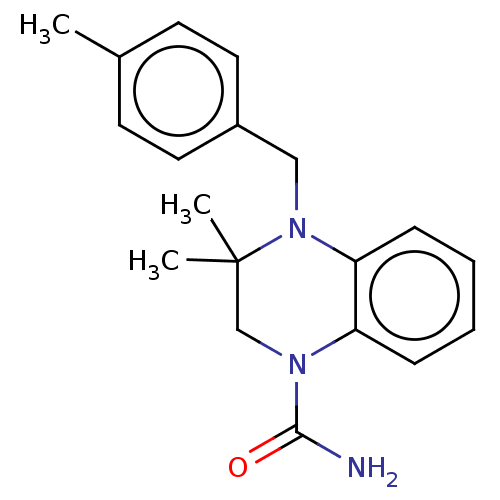
(CHEMBL5276735) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
AMP deaminase 2
(Homo sapiens) | BDBM50610228

(CHEMBL5284620) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
AMP deaminase 2
(Homo sapiens) | BDBM50610234
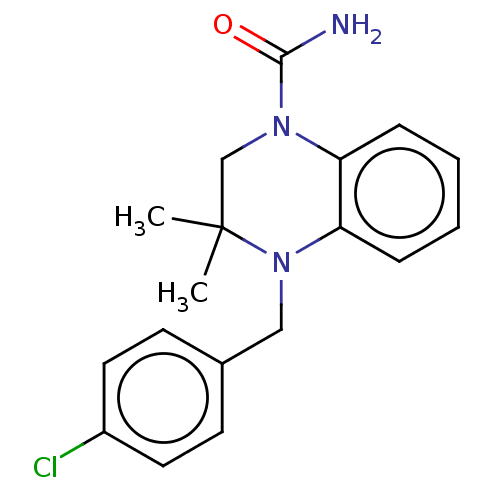
(CHEMBL5289502) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
AMP deaminase 2
(Homo sapiens) | BDBM50610233
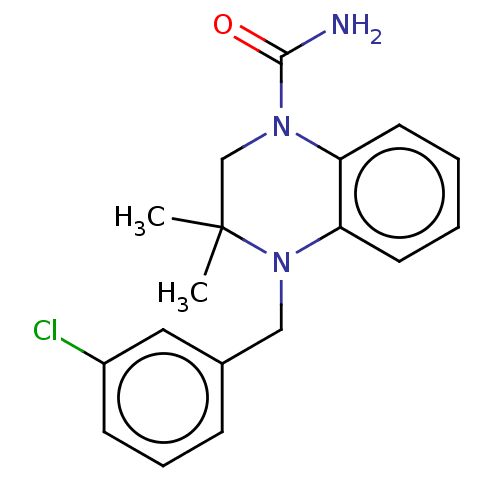
(CHEMBL5271550) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
AMP deaminase 2
(Homo sapiens) | BDBM50610245
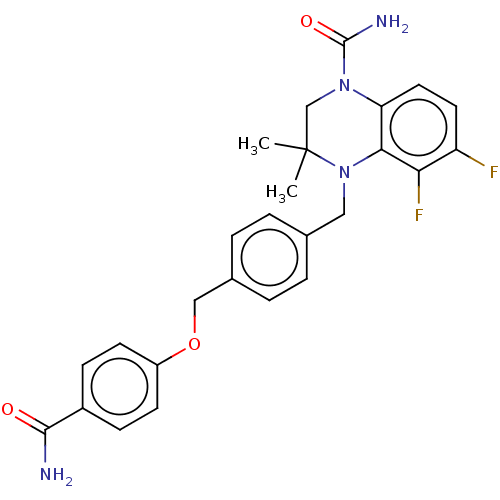
(CHEMBL5281034)Show SMILES CC1(C)CN(C(N)=O)c2ccc(F)c(F)c2N1Cc1ccc(COc2ccc(cc2)C(N)=O)cc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
AMP deaminase 2
(Homo sapiens) | BDBM50610226

(CHEMBL5266374) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
AMP deaminase 2
(Homo sapiens) | BDBM50610230

(CHEMBL5283087) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
AMP deaminase 2
(Homo sapiens) | BDBM50610223

(CHEMBL5280447) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
AMP deaminase 2
(Homo sapiens) | BDBM50610238

(CHEMBL5284666)Show SMILES CC1(C)CN(C(N)=O)c2ccccc2N1Cc1ccc(OC(F)(F)F)cc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
AMP deaminase 2
(Homo sapiens) | BDBM50610235
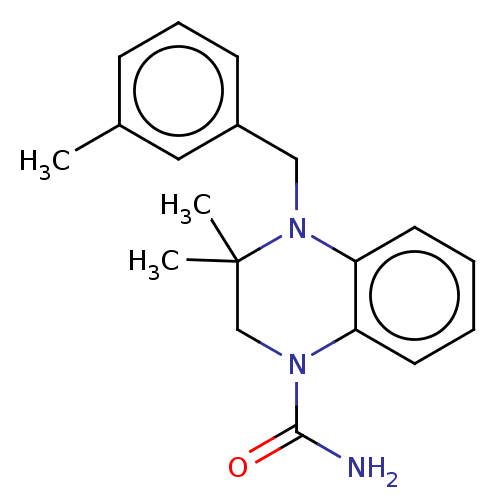
(CHEMBL5277889) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
AMP deaminase 2
(Homo sapiens) | BDBM50610240

(CHEMBL5280490)Show SMILES CC1(C)CN(C(N)=O)c2ccccc2N1Cc1ccc(CCc2ccccc2)cc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
AMP deaminase 2
(Homo sapiens) | BDBM50610227

(CHEMBL5284646) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
AMP deaminase 2
(Homo sapiens) | BDBM50610239
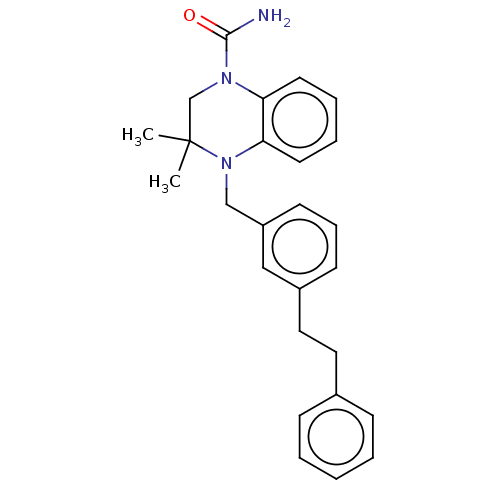
(CHEMBL5282638)Show SMILES CC1(C)CN(C(N)=O)c2ccccc2N1Cc1cccc(CCc2ccccc2)c1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
AMP deaminase 2
(Homo sapiens) | BDBM50610241
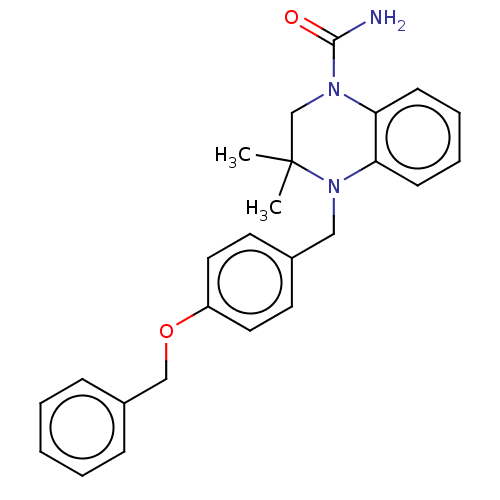
(CHEMBL5290923)Show SMILES CC1(C)CN(C(N)=O)c2ccccc2N1Cc1ccc(OCc2ccccc2)cc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylcholine:ceramide cholinephosphotransferase 2
(Homo sapiens (Human)) | BDBM50182486
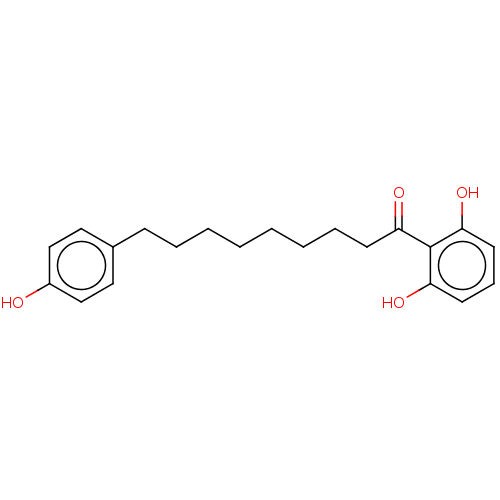
(MALABARICONE B)Show InChI InChI=1S/C21H26O4/c22-17-14-12-16(13-15-17)8-5-3-1-2-4-6-9-18(23)21-19(24)10-7-11-20(21)25/h7,10-15,22,24-25H,1-6,8-9H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of SMS2 (unknown origin) stably expressed in mouse ZS cells using 5 to 50 uM C6-NBD-ceramide as substrate preincubated for ... |
ACS Med Chem Lett 10: 1154-1158 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00171
BindingDB Entry DOI: 10.7270/Q2WS8XPS |
More data for this
Ligand-Target Pair | |
Phosphatidylcholine:ceramide cholinephosphotransferase 2
(Homo sapiens (Human)) | BDBM50182487

(Malabaricone A)Show InChI InChI=1S/C21H26O3/c22-18(21-19(23)15-10-16-20(21)24)14-9-4-2-1-3-6-11-17-12-7-5-8-13-17/h5,7-8,10,12-13,15-16,23-24H,1-4,6,9,11,14H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of SMS2 (unknown origin) stably expressed in mouse ZS cells using 5 to 50 uM C6-NBD-ceramide as substrate preincubated for ... |
ACS Med Chem Lett 10: 1154-1158 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00171
BindingDB Entry DOI: 10.7270/Q2WS8XPS |
More data for this
Ligand-Target Pair | |
Phosphatidylcholine:ceramide cholinephosphotransferase 2
(Homo sapiens (Human)) | BDBM50182491

(CHEBI:69015 | Malabaricone C)Show InChI InChI=1S/C21H26O5/c22-16-13-12-15(14-20(16)26)8-5-3-1-2-4-6-9-17(23)21-18(24)10-7-11-19(21)25/h7,10-14,22,24-26H,1-6,8-9H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of SMS2 (unknown origin) stably expressed in mouse ZS cells using 5 to 50 uM C6-NBD-ceramide as substrate preincubated for ... |
ACS Med Chem Lett 10: 1154-1158 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00171
BindingDB Entry DOI: 10.7270/Q2WS8XPS |
More data for this
Ligand-Target Pair | |
AMP deaminase 2
(Homo sapiens) | BDBM50610224

(CHEMBL5269095) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
AMP deaminase 2
(Homo sapiens) | BDBM50610225

(CHEMBL5284837) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
AMP deaminase 2
(Homo sapiens) | BDBM50610237

(CHEMBL5287535)Show SMILES CC1(C)CN(C(N)=O)c2ccccc2N1Cc1cccc(OC(F)(F)F)c1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
AMP deaminase 2
(Homo sapiens) | BDBM50610236

(CHEMBL5276735) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylcholine:ceramide cholinephosphotransferase 2
(Homo sapiens (Human)) | BDBM50242188

(6-(8'Z-pentadecenyl)-salicylicacid | 6-[8'(Z)-pent...)Show InChI InChI=1S/C22H34O3/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-16-19-17-15-18-20(23)21(19)22(24)25/h7-8,15,17-18,23H,2-6,9-14,16H2,1H3,(H,24,25)/b8-7- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of SMS2 (unknown origin) |
ACS Med Chem Lett 10: 1154-1158 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00171
BindingDB Entry DOI: 10.7270/Q2WS8XPS |
More data for this
Ligand-Target Pair | |
Phosphatidylcholine:ceramide cholinephosphotransferase 2
(Homo sapiens (Human)) | BDBM50182491

(CHEBI:69015 | Malabaricone C)Show InChI InChI=1S/C21H26O5/c22-16-13-12-15(14-20(16)26)8-5-3-1-2-4-6-9-17(23)21-18(24)10-7-11-19(21)25/h7,10-14,22,24-26H,1-6,8-9H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of SMS2 (unknown origin) stably expressed in mouse ZS cells using C6-NBD-ceramide as substrate preincubated for 30 mins followed by substr... |
ACS Med Chem Lett 10: 1154-1158 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00171
BindingDB Entry DOI: 10.7270/Q2WS8XPS |
More data for this
Ligand-Target Pair | |
Phosphatidylcholine:ceramide cholinephosphotransferase 1
(Homo sapiens (Human)) | BDBM50242188

(6-(8'Z-pentadecenyl)-salicylicacid | 6-[8'(Z)-pent...)Show InChI InChI=1S/C22H34O3/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-16-19-17-15-18-20(23)21(19)22(24)25/h7-8,15,17-18,23H,2-6,9-14,16H2,1H3,(H,24,25)/b8-7- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of SMS1 (unknown origin) |
ACS Med Chem Lett 10: 1154-1158 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00171
BindingDB Entry DOI: 10.7270/Q2WS8XPS |
More data for this
Ligand-Target Pair | |
AMP deaminase 2
(Homo sapiens) | BDBM50610228

(CHEMBL5284620) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
AMP deaminase 2
(Homo sapiens) | BDBM50610234

(CHEMBL5289502) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
AMP deaminase 2
(Homo sapiens) | BDBM50610231

(CHEMBL5271711) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
AMP deaminase 2
(Homo sapiens) | BDBM50610233

(CHEMBL5271550) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
AMP deaminase 2
(Homo sapiens) | BDBM50610226

(CHEMBL5266374) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylcholine:ceramide cholinephosphotransferase 1
(Homo sapiens (Human)) | BDBM50182486

(MALABARICONE B)Show InChI InChI=1S/C21H26O4/c22-17-14-12-16(13-15-17)8-5-3-1-2-4-6-9-18(23)21-19(24)10-7-11-20(21)25/h7,10-15,22,24-25H,1-6,8-9H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of SMS1 (unknown origin) stably expressed in mouse ZS cells using 5 to 50 uM C6-NBD-ceramide as substrate preincubated for ... |
ACS Med Chem Lett 10: 1154-1158 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00171
BindingDB Entry DOI: 10.7270/Q2WS8XPS |
More data for this
Ligand-Target Pair | |
Phosphatidylcholine:ceramide cholinephosphotransferase 1
(Homo sapiens (Human)) | BDBM50182487

(Malabaricone A)Show InChI InChI=1S/C21H26O3/c22-18(21-19(23)15-10-16-20(21)24)14-9-4-2-1-3-6-11-17-12-7-5-8-13-17/h5,7-8,10,12-13,15-16,23-24H,1-4,6,9,11,14H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of SMS1 (unknown origin) stably expressed in mouse ZS cells using 5 to 50 uM C6-NBD-ceramide as substrate preincubated for ... |
ACS Med Chem Lett 10: 1154-1158 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00171
BindingDB Entry DOI: 10.7270/Q2WS8XPS |
More data for this
Ligand-Target Pair | |
Phosphatidylcholine:ceramide cholinephosphotransferase 1
(Homo sapiens (Human)) | BDBM50182491

(CHEBI:69015 | Malabaricone C)Show InChI InChI=1S/C21H26O5/c22-16-13-12-15(14-20(16)26)8-5-3-1-2-4-6-9-17(23)21-18(24)10-7-11-19(21)25/h7,10-14,22,24-26H,1-6,8-9H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of SMS1 (unknown origin) stably expressed in mouse ZS cells using 5 to 50 uM C6-NBD-ceramide as substrate preincubated for ... |
ACS Med Chem Lett 10: 1154-1158 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00171
BindingDB Entry DOI: 10.7270/Q2WS8XPS |
More data for this
Ligand-Target Pair | |
AMP deaminase 2
(Homo sapiens) | BDBM50610229

(CHEMBL5282210) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
AMP deaminase 2
(Homo sapiens) | BDBM50610235

(CHEMBL5277889) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylcholine:ceramide cholinephosphotransferase 2
(Homo sapiens (Human)) | BDBM50182486

(MALABARICONE B)Show InChI InChI=1S/C21H26O4/c22-17-14-12-16(13-15-17)8-5-3-1-2-4-6-9-18(23)21-19(24)10-7-11-20(21)25/h7,10-15,22,24-25H,1-6,8-9H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of SMS2 (unknown origin) stably expressed in mouse ZS cells using C6-NBD-ceramide as substrate preincubated for 30 mins followed by substr... |
ACS Med Chem Lett 10: 1154-1158 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00171
BindingDB Entry DOI: 10.7270/Q2WS8XPS |
More data for this
Ligand-Target Pair | |
AMP deaminase 2
(Homo sapiens) | BDBM50610242

(CHEMBL5270167)Show SMILES CC1(C)CN(C(N)=O)c2ccccc2N1Cc1ccc(COc2ccccc2)cc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
AMP deaminase 2
(Homo sapiens) | BDBM50610239

(CHEMBL5282638)Show SMILES CC1(C)CN(C(N)=O)c2ccccc2N1Cc1cccc(CCc2ccccc2)c1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylcholine:ceramide cholinephosphotransferase 1
(Homo sapiens (Human)) | BDBM50182491

(CHEBI:69015 | Malabaricone C)Show InChI InChI=1S/C21H26O5/c22-16-13-12-15(14-20(16)26)8-5-3-1-2-4-6-9-17(23)21-18(24)10-7-11-19(21)25/h7,10-14,22,24-26H,1-6,8-9H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of SMS1 (unknown origin) stably expressed in mouse ZS cells using C6-NBD-ceramide as substrate preincubated for 30 mins followed by substr... |
ACS Med Chem Lett 10: 1154-1158 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00171
BindingDB Entry DOI: 10.7270/Q2WS8XPS |
More data for this
Ligand-Target Pair | |
AMP deaminase 2
(Homo sapiens) | BDBM50610223

(CHEMBL5280447) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
AMP deaminase 2
(Homo sapiens) | BDBM50610241

(CHEMBL5290923)Show SMILES CC1(C)CN(C(N)=O)c2ccccc2N1Cc1ccc(OCc2ccccc2)cc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylcholine:ceramide cholinephosphotransferase 1
(Homo sapiens (Human)) | BDBM50182486

(MALABARICONE B)Show InChI InChI=1S/C21H26O4/c22-17-14-12-16(13-15-17)8-5-3-1-2-4-6-9-18(23)21-19(24)10-7-11-20(21)25/h7,10-15,22,24-25H,1-6,8-9H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of SMS1 (unknown origin) stably expressed in mouse ZS cells using C6-NBD-ceramide as substrate preincubated for 30 mins followed by substr... |
ACS Med Chem Lett 10: 1154-1158 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00171
BindingDB Entry DOI: 10.7270/Q2WS8XPS |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data