

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glutamate receptor 2 (Homo sapiens (Human)) | BDBM50427677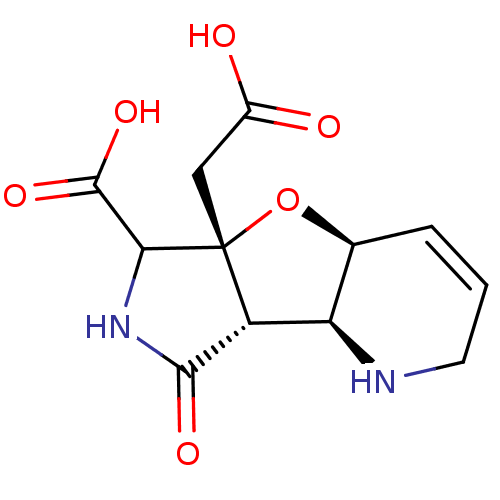 (CHEMBL2323527) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 5.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yokohama City University Curated by ChEMBL | Assay Description Binding affinity to GluA2 ligand binding domain (unknown origin) by competitive binding assay | J Med Chem 56: 2283-93 (2013) Article DOI: 10.1021/jm301590z BindingDB Entry DOI: 10.7270/Q2SJ1MZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Mus musculus (Mouse)) | BDBM50462596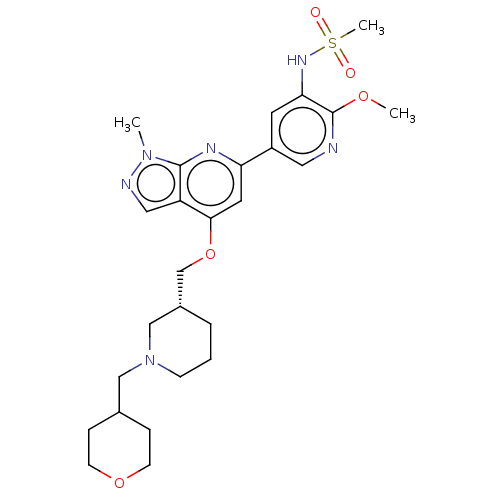 (CHEMBL4242659) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Inhibition of PI3Kdelta in Balb/c mouse splenocytes-derived B cells assessed as reduction in anti-IgM antibody-stimulated B cell proliferation after ... | Bioorg Med Chem 26: 3917-3924 (2018) Article DOI: 10.1016/j.bmc.2018.06.012 BindingDB Entry DOI: 10.7270/Q2ZP48RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Mus musculus (Mouse)) | BDBM50462587 (CHEMBL4250271) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Inhibition of PI3Kdelta in Balb/c mouse splenocytes-derived B cells assessed as reduction in anti-IgM antibody-stimulated B cell proliferation after ... | Bioorg Med Chem 26: 3917-3924 (2018) Article DOI: 10.1016/j.bmc.2018.06.012 BindingDB Entry DOI: 10.7270/Q2ZP48RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50461380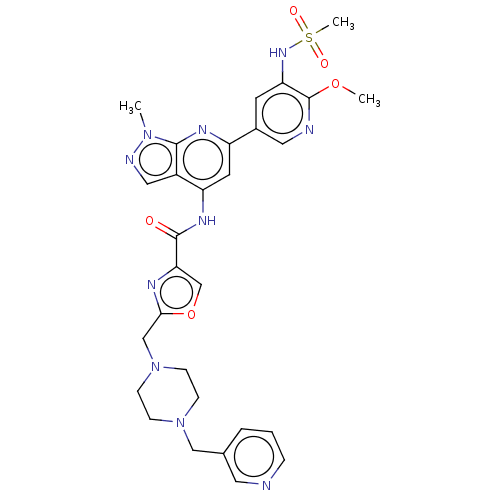 (CHEMBL4225418) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human full length N-terminal His6-tagged PI3K p110delta/untagged recombinant full length human p85alpha expressed in baculo... | Bioorg Med Chem 26: 2410-2419 (2018) Article DOI: 10.1016/j.bmc.2018.03.042 BindingDB Entry DOI: 10.7270/Q2PN988P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50462596 (CHEMBL4242659) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human full length N-terminal His6-tagged PI3K p110delta/p85alpha expressed in baculovirus infected Sf21 cells using PIP2 as... | Bioorg Med Chem 26: 3917-3924 (2018) Article DOI: 10.1016/j.bmc.2018.06.012 BindingDB Entry DOI: 10.7270/Q2ZP48RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50462587 (CHEMBL4250271) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human full length N-terminal His6-tagged PI3K p110delta/p85alpha expressed in baculovirus infected Sf21 cells using PIP2 as... | Bioorg Med Chem 26: 3917-3924 (2018) Article DOI: 10.1016/j.bmc.2018.06.012 BindingDB Entry DOI: 10.7270/Q2ZP48RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50462588 (CHEMBL4248505) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human full length N-terminal His6-tagged PI3K p110delta/p85alpha expressed in baculovirus infected Sf21 cells using PIP2 as... | Bioorg Med Chem 26: 3917-3924 (2018) Article DOI: 10.1016/j.bmc.2018.06.012 BindingDB Entry DOI: 10.7270/Q2ZP48RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Mus musculus (Mouse)) | BDBM50462594 (CHEMBL4249087) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Inhibition of PI3Kdelta in Balb/c mouse splenocytes-derived B cells assessed as reduction in anti-IgM antibody-stimulated B cell proliferation after ... | Bioorg Med Chem 26: 3917-3924 (2018) Article DOI: 10.1016/j.bmc.2018.06.012 BindingDB Entry DOI: 10.7270/Q2ZP48RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Mus musculus (Mouse)) | BDBM50462589 (CHEMBL4247328) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Inhibition of PI3Kdelta in Balb/c mouse splenocytes-derived B cells assessed as reduction in anti-IgM antibody-stimulated B cell proliferation after ... | Bioorg Med Chem 26: 3917-3924 (2018) Article DOI: 10.1016/j.bmc.2018.06.012 BindingDB Entry DOI: 10.7270/Q2ZP48RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Mus musculus (Mouse)) | BDBM50462588 (CHEMBL4248505) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Inhibition of PI3Kdelta in Balb/c mouse splenocytes-derived B cells assessed as reduction in anti-IgM antibody-stimulated B cell proliferation after ... | Bioorg Med Chem 26: 3917-3924 (2018) Article DOI: 10.1016/j.bmc.2018.06.012 BindingDB Entry DOI: 10.7270/Q2ZP48RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM166423 (US9073821, 550) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | 8.0 | 25 |
TAISHO PHARMACEUTICAL CO., LTD; TOYAMA CHEMICAL CO., LTD. US Patent | Assay Description To assay the activity of Pseudomonas aeruginosa LpxC enzyme, LpxC was reacted with its substrate UDP-3-O-(R-3-hydroxydecanoyl)-N-acetylglucosamine an... | US Patent US9073821 (2015) BindingDB Entry DOI: 10.7270/Q2ZG6R0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50462589 (CHEMBL4247328) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human full length N-terminal His6-tagged PI3K p110delta/p85alpha expressed in baculovirus infected Sf21 cells using PIP2 as... | Bioorg Med Chem 26: 3917-3924 (2018) Article DOI: 10.1016/j.bmc.2018.06.012 BindingDB Entry DOI: 10.7270/Q2ZP48RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM166411 (US9073821, 376) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 8.0 | 25 |
TAISHO PHARMACEUTICAL CO., LTD; TOYAMA CHEMICAL CO., LTD. US Patent | Assay Description To assay the activity of Pseudomonas aeruginosa LpxC enzyme, LpxC was reacted with its substrate UDP-3-O-(R-3-hydroxydecanoyl)-N-acetylglucosamine an... | US Patent US9073821 (2015) BindingDB Entry DOI: 10.7270/Q2ZG6R0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NADH-ubiquinone oxidoreductase chain 1 (Bos taurus (Bovine)) | BDBM50427137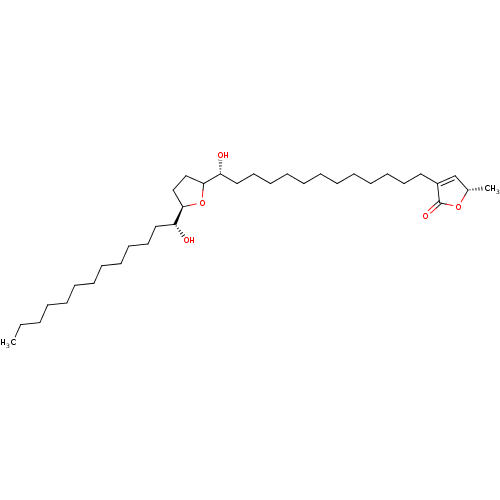 (SOLAMIN) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of bovine heart mitochondrial complex 1 | Bioorg Med Chem Lett 23: 1217-9 (2013) Article DOI: 10.1016/j.bmcl.2013.01.018 BindingDB Entry DOI: 10.7270/Q28P61VW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Mus musculus (Mouse)) | BDBM50461379 (CHEMBL4227156) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Inhibition of PI3Kdelta in Balb/c mouse splenocytes-derived B cells assessed as reduction in anti-IgM antibody-stimulated B cell proliferation after ... | Bioorg Med Chem 26: 3917-3924 (2018) Article DOI: 10.1016/j.bmc.2018.06.012 BindingDB Entry DOI: 10.7270/Q2ZP48RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM166426 (US9073821, 557) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | 8.0 | 25 |
TAISHO PHARMACEUTICAL CO., LTD; TOYAMA CHEMICAL CO., LTD. US Patent | Assay Description To assay the activity of Pseudomonas aeruginosa LpxC enzyme, LpxC was reacted with its substrate UDP-3-O-(R-3-hydroxydecanoyl)-N-acetylglucosamine an... | US Patent US9073821 (2015) BindingDB Entry DOI: 10.7270/Q2ZG6R0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Mus musculus (Mouse)) | BDBM50461373 (CHEMBL4228899) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Inhibition of PI3Kdelta in Balb/c mouse splenocytes-derived B cells assessed as reduction in anti-IgM antibody-stimulated B cell proliferation after ... | Bioorg Med Chem 26: 3917-3924 (2018) Article DOI: 10.1016/j.bmc.2018.06.012 BindingDB Entry DOI: 10.7270/Q2ZP48RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM166418 (US9073821, 434) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | 8.0 | 25 |
TAISHO PHARMACEUTICAL CO., LTD; TOYAMA CHEMICAL CO., LTD. US Patent | Assay Description To assay the activity of Pseudomonas aeruginosa LpxC enzyme, LpxC was reacted with its substrate UDP-3-O-(R-3-hydroxydecanoyl)-N-acetylglucosamine an... | US Patent US9073821 (2015) BindingDB Entry DOI: 10.7270/Q2ZG6R0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM166415 (US9073821, 405) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | 8.0 | 25 |
TAISHO PHARMACEUTICAL CO., LTD; TOYAMA CHEMICAL CO., LTD. US Patent | Assay Description To assay the activity of Pseudomonas aeruginosa LpxC enzyme, LpxC was reacted with its substrate UDP-3-O-(R-3-hydroxydecanoyl)-N-acetylglucosamine an... | US Patent US9073821 (2015) BindingDB Entry DOI: 10.7270/Q2ZG6R0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Mus musculus (Mouse)) | BDBM50462595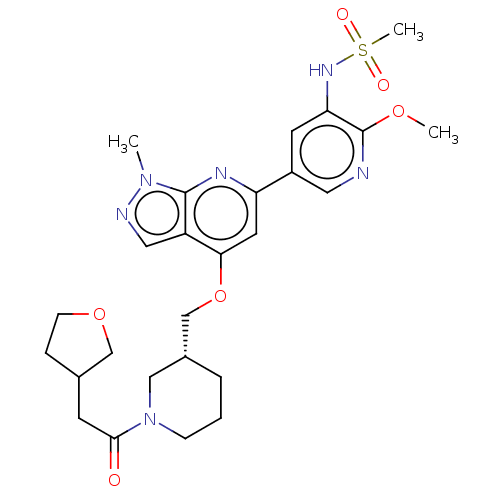 (CHEMBL4250966) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Inhibition of PI3Kdelta in Balb/c mouse splenocytes-derived B cells assessed as reduction in anti-IgM antibody-stimulated B cell proliferation after ... | Bioorg Med Chem 26: 3917-3924 (2018) Article DOI: 10.1016/j.bmc.2018.06.012 BindingDB Entry DOI: 10.7270/Q2ZP48RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NADH-ubiquinone oxidoreductase chain 1 (Bos taurus (Bovine)) | BDBM50427136 (CHEMBL1834283) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of bovine heart mitochondrial complex 1 | Bioorg Med Chem Lett 23: 1217-9 (2013) Article DOI: 10.1016/j.bmcl.2013.01.018 BindingDB Entry DOI: 10.7270/Q28P61VW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM166409 (US9073821, 211) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | 8.0 | 25 |
TAISHO PHARMACEUTICAL CO., LTD; TOYAMA CHEMICAL CO., LTD. US Patent | Assay Description To assay the activity of Pseudomonas aeruginosa LpxC enzyme, LpxC was reacted with its substrate UDP-3-O-(R-3-hydroxydecanoyl)-N-acetylglucosamine an... | US Patent US9073821 (2015) BindingDB Entry DOI: 10.7270/Q2ZG6R0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM166412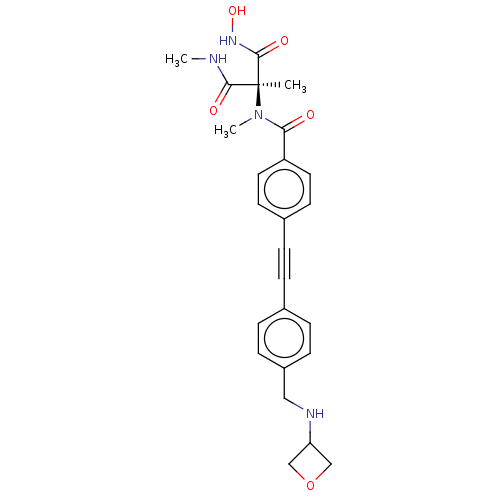 (US9073821, 396) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | 8.0 | 25 |
TAISHO PHARMACEUTICAL CO., LTD; TOYAMA CHEMICAL CO., LTD. US Patent | Assay Description To assay the activity of Pseudomonas aeruginosa LpxC enzyme, LpxC was reacted with its substrate UDP-3-O-(R-3-hydroxydecanoyl)-N-acetylglucosamine an... | US Patent US9073821 (2015) BindingDB Entry DOI: 10.7270/Q2ZG6R0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50462593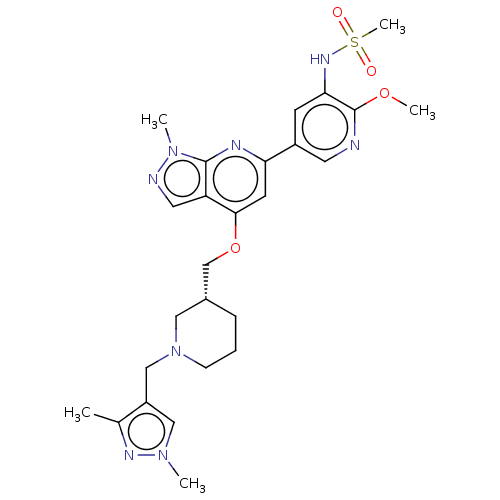 (CHEMBL4246031) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human full length N-terminal His6-tagged PI3K p110delta/p85alpha expressed in baculovirus infected Sf21 cells using PIP2 as... | Bioorg Med Chem 26: 3917-3924 (2018) Article DOI: 10.1016/j.bmc.2018.06.012 BindingDB Entry DOI: 10.7270/Q2ZP48RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM166388 (US9073821, 3) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | 8.0 | 25 |
TAISHO PHARMACEUTICAL CO., LTD; TOYAMA CHEMICAL CO., LTD. US Patent | Assay Description To assay the activity of Pseudomonas aeruginosa LpxC enzyme, LpxC was reacted with its substrate UDP-3-O-(R-3-hydroxydecanoyl)-N-acetylglucosamine an... | US Patent US9073821 (2015) BindingDB Entry DOI: 10.7270/Q2ZG6R0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50461383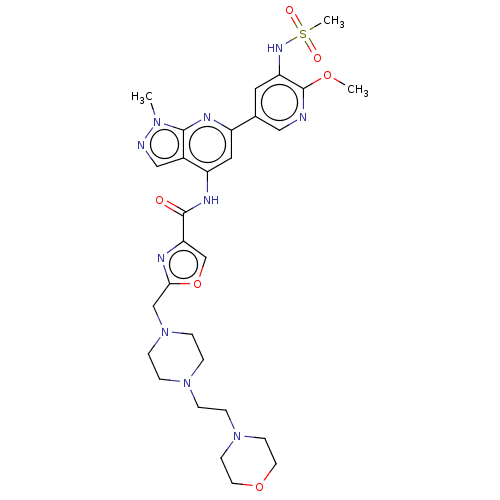 (CHEMBL4229164) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human full length N-terminal His6-tagged PI3K p110delta/untagged recombinant full length human p85alpha expressed in baculo... | Bioorg Med Chem 26: 2410-2419 (2018) Article DOI: 10.1016/j.bmc.2018.03.042 BindingDB Entry DOI: 10.7270/Q2PN988P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM166432 (US9073821, 585) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | 8.0 | 25 |
TAISHO PHARMACEUTICAL CO., LTD; TOYAMA CHEMICAL CO., LTD. US Patent | Assay Description To assay the activity of Pseudomonas aeruginosa LpxC enzyme, LpxC was reacted with its substrate UDP-3-O-(R-3-hydroxydecanoyl)-N-acetylglucosamine an... | US Patent US9073821 (2015) BindingDB Entry DOI: 10.7270/Q2ZG6R0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM166390 (US9073821, 5) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | 8.0 | 25 |
TAISHO PHARMACEUTICAL CO., LTD; TOYAMA CHEMICAL CO., LTD. US Patent | Assay Description To assay the activity of Pseudomonas aeruginosa LpxC enzyme, LpxC was reacted with its substrate UDP-3-O-(R-3-hydroxydecanoyl)-N-acetylglucosamine an... | US Patent US9073821 (2015) BindingDB Entry DOI: 10.7270/Q2ZG6R0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM166430 (US9073821, 563) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | 8.0 | 25 |
TAISHO PHARMACEUTICAL CO., LTD; TOYAMA CHEMICAL CO., LTD. US Patent | Assay Description To assay the activity of Pseudomonas aeruginosa LpxC enzyme, LpxC was reacted with its substrate UDP-3-O-(R-3-hydroxydecanoyl)-N-acetylglucosamine an... | US Patent US9073821 (2015) BindingDB Entry DOI: 10.7270/Q2ZG6R0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50462594 (CHEMBL4249087) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human full length N-terminal His6-tagged PI3K p110delta/p85alpha expressed in baculovirus infected Sf21 cells using PIP2 as... | Bioorg Med Chem 26: 3917-3924 (2018) Article DOI: 10.1016/j.bmc.2018.06.012 BindingDB Entry DOI: 10.7270/Q2ZP48RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM166408 (US9073821, 168) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | 8.0 | 25 |
TAISHO PHARMACEUTICAL CO., LTD; TOYAMA CHEMICAL CO., LTD. US Patent | Assay Description To assay the activity of Pseudomonas aeruginosa LpxC enzyme, LpxC was reacted with its substrate UDP-3-O-(R-3-hydroxydecanoyl)-N-acetylglucosamine an... | US Patent US9073821 (2015) BindingDB Entry DOI: 10.7270/Q2ZG6R0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Mus musculus (Mouse)) | BDBM50462590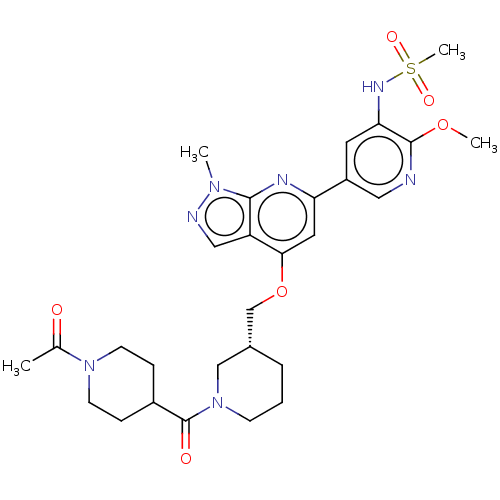 (CHEMBL4244862) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Inhibition of PI3Kdelta in Balb/c mouse splenocytes-derived B cells assessed as reduction in anti-IgM antibody-stimulated B cell proliferation after ... | Bioorg Med Chem 26: 3917-3924 (2018) Article DOI: 10.1016/j.bmc.2018.06.012 BindingDB Entry DOI: 10.7270/Q2ZP48RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM166419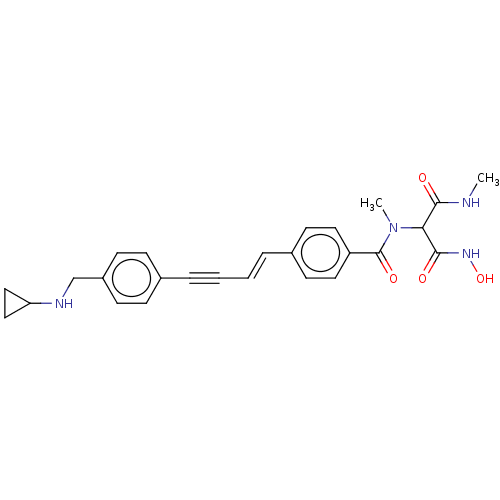 (US9073821, 477) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | 8.0 | 25 |
TAISHO PHARMACEUTICAL CO., LTD; TOYAMA CHEMICAL CO., LTD. US Patent | Assay Description To assay the activity of Pseudomonas aeruginosa LpxC enzyme, LpxC was reacted with its substrate UDP-3-O-(R-3-hydroxydecanoyl)-N-acetylglucosamine an... | US Patent US9073821 (2015) BindingDB Entry DOI: 10.7270/Q2ZG6R0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM166406 (US9073821, 237) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | 8.0 | 25 |
TAISHO PHARMACEUTICAL CO., LTD; TOYAMA CHEMICAL CO., LTD. US Patent | Assay Description To assay the activity of Pseudomonas aeruginosa LpxC enzyme, LpxC was reacted with its substrate UDP-3-O-(R-3-hydroxydecanoyl)-N-acetylglucosamine an... | US Patent US9073821 (2015) BindingDB Entry DOI: 10.7270/Q2ZG6R0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50461379 (CHEMBL4227156) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human full length N-terminal His6-tagged PI3K p110delta/untagged recombinant full length human p85alpha expressed in baculo... | Bioorg Med Chem 26: 2410-2419 (2018) Article DOI: 10.1016/j.bmc.2018.03.042 BindingDB Entry DOI: 10.7270/Q2PN988P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50461378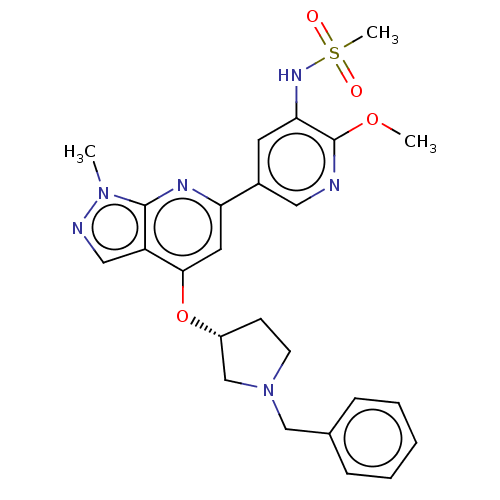 (CHEMBL4229226) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human full length N-terminal His6-tagged PI3K p110delta/untagged recombinant full length human p85alpha expressed in baculo... | Bioorg Med Chem 26: 2410-2419 (2018) Article DOI: 10.1016/j.bmc.2018.03.042 BindingDB Entry DOI: 10.7270/Q2PN988P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM166396 (US9073821, 52) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | 8.0 | 25 |
TAISHO PHARMACEUTICAL CO., LTD; TOYAMA CHEMICAL CO., LTD. US Patent | Assay Description To assay the activity of Pseudomonas aeruginosa LpxC enzyme, LpxC was reacted with its substrate UDP-3-O-(R-3-hydroxydecanoyl)-N-acetylglucosamine an... | US Patent US9073821 (2015) BindingDB Entry DOI: 10.7270/Q2ZG6R0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50462590 (CHEMBL4244862) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human full length N-terminal His6-tagged PI3K p110delta/p85alpha expressed in baculovirus infected Sf21 cells using PIP2 as... | Bioorg Med Chem 26: 3917-3924 (2018) Article DOI: 10.1016/j.bmc.2018.06.012 BindingDB Entry DOI: 10.7270/Q2ZP48RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM166394 (US9073821, 40) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | 8.0 | 25 |
TAISHO PHARMACEUTICAL CO., LTD; TOYAMA CHEMICAL CO., LTD. US Patent | Assay Description To assay the activity of Pseudomonas aeruginosa LpxC enzyme, LpxC was reacted with its substrate UDP-3-O-(R-3-hydroxydecanoyl)-N-acetylglucosamine an... | US Patent US9073821 (2015) BindingDB Entry DOI: 10.7270/Q2ZG6R0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Mus musculus (Mouse)) | BDBM50462593 (CHEMBL4246031) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Inhibition of PI3Kdelta in Balb/c mouse splenocytes-derived B cells assessed as reduction in anti-IgM antibody-stimulated B cell proliferation after ... | Bioorg Med Chem 26: 3917-3924 (2018) Article DOI: 10.1016/j.bmc.2018.06.012 BindingDB Entry DOI: 10.7270/Q2ZP48RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50462595 (CHEMBL4250966) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human full length N-terminal His6-tagged PI3K p110delta/p85alpha expressed in baculovirus infected Sf21 cells using PIP2 as... | Bioorg Med Chem 26: 3917-3924 (2018) Article DOI: 10.1016/j.bmc.2018.06.012 BindingDB Entry DOI: 10.7270/Q2ZP48RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM166422 (US9073821, 528) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | 8.0 | 25 |
TAISHO PHARMACEUTICAL CO., LTD; TOYAMA CHEMICAL CO., LTD. US Patent | Assay Description To assay the activity of Pseudomonas aeruginosa LpxC enzyme, LpxC was reacted with its substrate UDP-3-O-(R-3-hydroxydecanoyl)-N-acetylglucosamine an... | US Patent US9073821 (2015) BindingDB Entry DOI: 10.7270/Q2ZG6R0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM166414 (US9073821, 402) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | 6.5 | 25 |
TAISHO PHARMACEUTICAL CO., LTD; TOYAMA CHEMICAL CO., LTD. US Patent | Assay Description To assay the activity of E. coli LpxC enzyme, LpxC was reacted with its substrate UDP-3-O-(R-3-hydroxytetradecanoyl)-N-acetylglucosamine and the amou... | US Patent US9073821 (2015) BindingDB Entry DOI: 10.7270/Q2ZG6R0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM166404 (US9073821, 188) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 8.0 | 25 |
TAISHO PHARMACEUTICAL CO., LTD; TOYAMA CHEMICAL CO., LTD. US Patent | Assay Description To assay the activity of Pseudomonas aeruginosa LpxC enzyme, LpxC was reacted with its substrate UDP-3-O-(R-3-hydroxydecanoyl)-N-acetylglucosamine an... | US Patent US9073821 (2015) BindingDB Entry DOI: 10.7270/Q2ZG6R0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM166407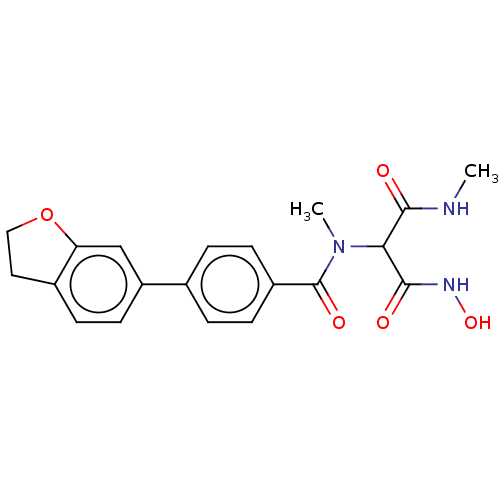 (US9073821, 271) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 8.0 | 25 |
TAISHO PHARMACEUTICAL CO., LTD; TOYAMA CHEMICAL CO., LTD. US Patent | Assay Description To assay the activity of Pseudomonas aeruginosa LpxC enzyme, LpxC was reacted with its substrate UDP-3-O-(R-3-hydroxydecanoyl)-N-acetylglucosamine an... | US Patent US9073821 (2015) BindingDB Entry DOI: 10.7270/Q2ZG6R0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM166393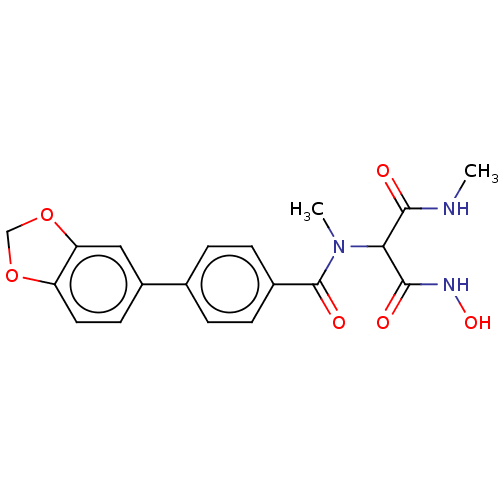 (US9073821, 8) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 8.0 | 25 |
TAISHO PHARMACEUTICAL CO., LTD; TOYAMA CHEMICAL CO., LTD. US Patent | Assay Description To assay the activity of Pseudomonas aeruginosa LpxC enzyme, LpxC was reacted with its substrate UDP-3-O-(R-3-hydroxydecanoyl)-N-acetylglucosamine an... | US Patent US9073821 (2015) BindingDB Entry DOI: 10.7270/Q2ZG6R0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM166417 (US9073821, 425) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | 8.0 | 25 |
TAISHO PHARMACEUTICAL CO., LTD; TOYAMA CHEMICAL CO., LTD. US Patent | Assay Description To assay the activity of Pseudomonas aeruginosa LpxC enzyme, LpxC was reacted with its substrate UDP-3-O-(R-3-hydroxydecanoyl)-N-acetylglucosamine an... | US Patent US9073821 (2015) BindingDB Entry DOI: 10.7270/Q2ZG6R0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50461373 (CHEMBL4228899) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human full length N-terminal His6-tagged PI3K p110delta/untagged recombinant full length human p85alpha expressed in baculo... | Bioorg Med Chem 26: 2410-2419 (2018) Article DOI: 10.1016/j.bmc.2018.03.042 BindingDB Entry DOI: 10.7270/Q2PN988P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM166401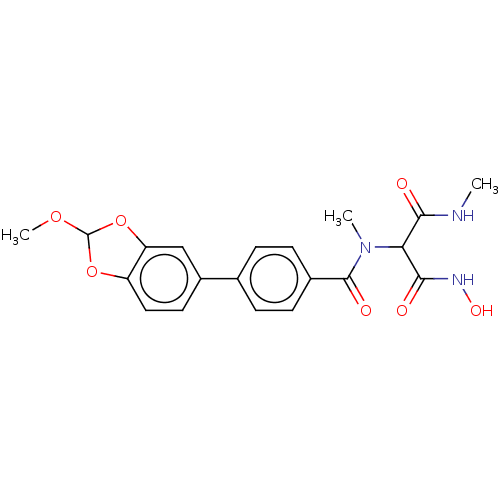 (US9073821, 94) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | 8.0 | 25 |
TAISHO PHARMACEUTICAL CO., LTD; TOYAMA CHEMICAL CO., LTD. US Patent | Assay Description To assay the activity of Pseudomonas aeruginosa LpxC enzyme, LpxC was reacted with its substrate UDP-3-O-(R-3-hydroxydecanoyl)-N-acetylglucosamine an... | US Patent US9073821 (2015) BindingDB Entry DOI: 10.7270/Q2ZG6R0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM166402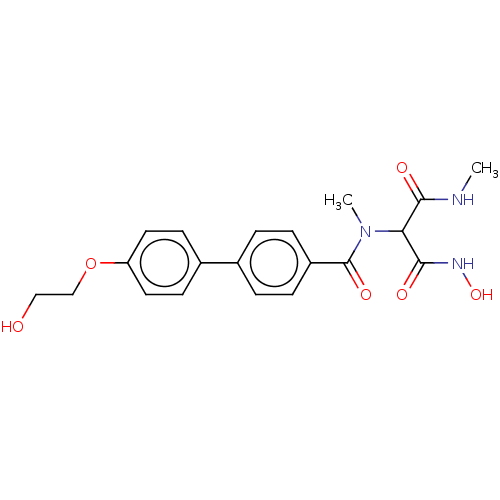 (US9073821, 153) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | 8.0 | 25 |
TAISHO PHARMACEUTICAL CO., LTD; TOYAMA CHEMICAL CO., LTD. US Patent | Assay Description To assay the activity of Pseudomonas aeruginosa LpxC enzyme, LpxC was reacted with its substrate UDP-3-O-(R-3-hydroxydecanoyl)-N-acetylglucosamine an... | US Patent US9073821 (2015) BindingDB Entry DOI: 10.7270/Q2ZG6R0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 351 total ) | Next | Last >> |