 Found 44 hits with Last Name = 'yang' and Initial = 'yj'
Found 44 hits with Last Name = 'yang' and Initial = 'yj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458125
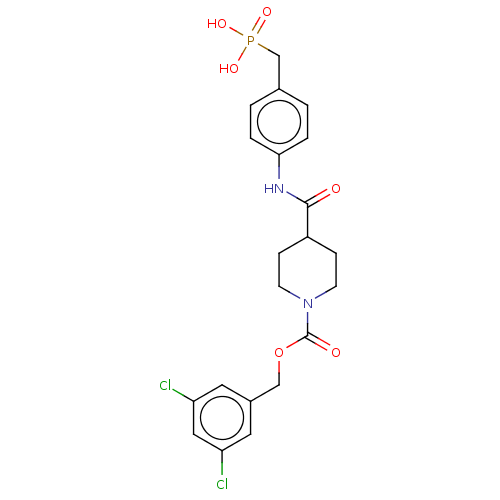
(CHEMBL4217352)Show SMILES OP(O)(=O)Cc1ccc(NC(=O)C2CCN(CC2)C(=O)OCc2cc(Cl)cc(Cl)c2)cc1 Show InChI InChI=1S/C21H23Cl2N2O6P/c22-17-9-15(10-18(23)11-17)12-31-21(27)25-7-5-16(6-8-25)20(26)24-19-3-1-14(2-4-19)13-32(28,29)30/h1-4,9-11,16H,5-8,12-13H2,(H,24,26)(H2,28,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of human ENPP2 using FS3 as substrate measured every 5 mins for 30 mins by fluorescence assay |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458125

(CHEMBL4217352)Show SMILES OP(O)(=O)Cc1ccc(NC(=O)C2CCN(CC2)C(=O)OCc2cc(Cl)cc(Cl)c2)cc1 Show InChI InChI=1S/C21H23Cl2N2O6P/c22-17-9-15(10-18(23)11-17)12-31-21(27)25-7-5-16(6-8-25)20(26)24-19-3-1-14(2-4-19)13-32(28,29)30/h1-4,9-11,16H,5-8,12-13H2,(H,24,26)(H2,28,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of ENPP2 in human A2058 cells using LPC as substrate preincubated for 15 mins followed by substrate addition measured after 3 hrs by LC-MS... |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458132

(CHEMBL4205127)Show SMILES OP(O)(=O)Cc1ccc(cc1)C(=O)NC1CCN(CC1)C(=O)OCc1cc(Cl)cc(Cl)c1 Show InChI InChI=1S/C21H23Cl2N2O6P/c22-17-9-15(10-18(23)11-17)12-31-21(27)25-7-5-19(6-8-25)24-20(26)16-3-1-14(2-4-16)13-32(28,29)30/h1-4,9-11,19H,5-8,12-13H2,(H,24,26)(H2,28,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of human ENPP2 using FS3 as substrate measured every 5 mins for 30 mins by fluorescence assay |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458135
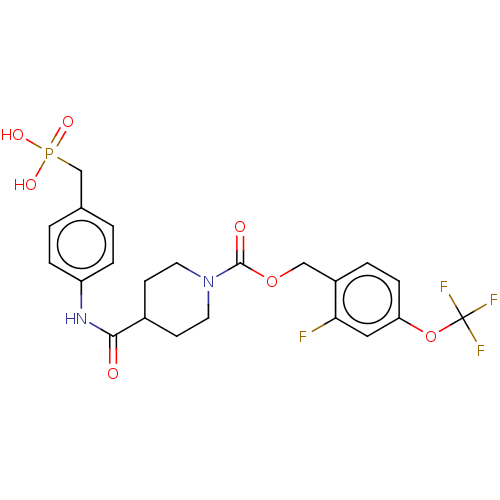
(CHEMBL4214731)Show SMILES OP(O)(=O)Cc1ccc(NC(=O)C2CCN(CC2)C(=O)OCc2ccc(OC(F)(F)F)cc2F)cc1 Show InChI InChI=1S/C22H23F4N2O7P/c23-19-11-18(35-22(24,25)26)6-3-16(19)12-34-21(30)28-9-7-15(8-10-28)20(29)27-17-4-1-14(2-5-17)13-36(31,32)33/h1-6,11,15H,7-10,12-13H2,(H,27,29)(H2,31,32,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of human ENPP2 using FS3 as substrate measured every 5 mins for 30 mins by fluorescence assay |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458127
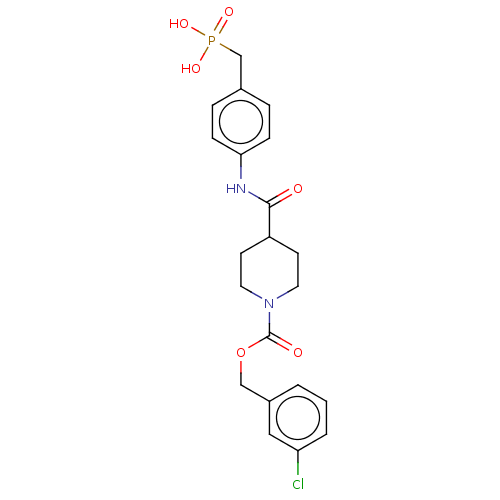
(CHEMBL4211269)Show SMILES OP(O)(=O)Cc1ccc(NC(=O)C2CCN(CC2)C(=O)OCc2cccc(Cl)c2)cc1 Show InChI InChI=1S/C21H24ClN2O6P/c22-18-3-1-2-16(12-18)13-30-21(26)24-10-8-17(9-11-24)20(25)23-19-6-4-15(5-7-19)14-31(27,28)29/h1-7,12,17H,8-11,13-14H2,(H,23,25)(H2,27,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of human ENPP2 using FS3 as substrate measured every 5 mins for 30 mins by fluorescence assay |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458131

(CHEMBL4212398)Show SMILES OP(O)(=O)Cc1csc(NC(=O)C2CCN(CC2)C(=O)OCc2cc(Cl)cc(Cl)c2)n1 Show InChI InChI=1S/C18H20Cl2N3O6PS/c19-13-5-11(6-14(20)7-13)8-29-18(25)23-3-1-12(2-4-23)16(24)22-17-21-15(10-31-17)9-30(26,27)28/h5-7,10,12H,1-4,8-9H2,(H,21,22,24)(H2,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of human ENPP2 using FS3 as substrate measured every 5 mins for 30 mins by fluorescence assay |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458126

(CHEMBL4203076)Show SMILES OP(O)(=O)Cc1ccc(NC(=O)C2CCN(CC2)C(=O)OCc2ccc(OC(F)(F)F)cc2)cc1 Show InChI InChI=1S/C22H24F3N2O7P/c23-22(24,25)34-19-7-3-15(4-8-19)13-33-21(29)27-11-9-17(10-12-27)20(28)26-18-5-1-16(2-6-18)14-35(30,31)32/h1-8,17H,9-14H2,(H,26,28)(H2,30,31,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of human ENPP2 using FS3 as substrate measured every 5 mins for 30 mins by fluorescence assay |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458125

(CHEMBL4217352)Show SMILES OP(O)(=O)Cc1ccc(NC(=O)C2CCN(CC2)C(=O)OCc2cc(Cl)cc(Cl)c2)cc1 Show InChI InChI=1S/C21H23Cl2N2O6P/c22-17-9-15(10-18(23)11-17)12-31-21(27)25-7-5-16(6-8-25)20(26)24-19-3-1-14(2-4-19)13-32(28,29)30/h1-4,9-11,16H,5-8,12-13H2,(H,24,26)(H2,28,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of human ENPP2 using bis-pNPP as substrate measured every 10 secs for 10 mins by spectrophotometric assay |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458135

(CHEMBL4214731)Show SMILES OP(O)(=O)Cc1ccc(NC(=O)C2CCN(CC2)C(=O)OCc2ccc(OC(F)(F)F)cc2F)cc1 Show InChI InChI=1S/C22H23F4N2O7P/c23-19-11-18(35-22(24,25)26)6-3-16(19)12-34-21(30)28-9-7-15(8-10-28)20(29)27-17-4-1-14(2-5-17)13-36(31,32)33/h1-6,11,15H,7-10,12-13H2,(H,27,29)(H2,31,32,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of human ENPP2 using bis-pNPP as substrate measured every 10 secs for 10 mins by spectrophotometric assay |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458126

(CHEMBL4203076)Show SMILES OP(O)(=O)Cc1ccc(NC(=O)C2CCN(CC2)C(=O)OCc2ccc(OC(F)(F)F)cc2)cc1 Show InChI InChI=1S/C22H24F3N2O7P/c23-22(24,25)34-19-7-3-15(4-8-19)13-33-21(29)27-11-9-17(10-12-27)20(28)26-18-5-1-16(2-6-18)14-35(30,31)32/h1-8,17H,9-14H2,(H,26,28)(H2,30,31,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of human ENPP2 using bis-pNPP as substrate measured every 10 secs for 10 mins by spectrophotometric assay |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458136
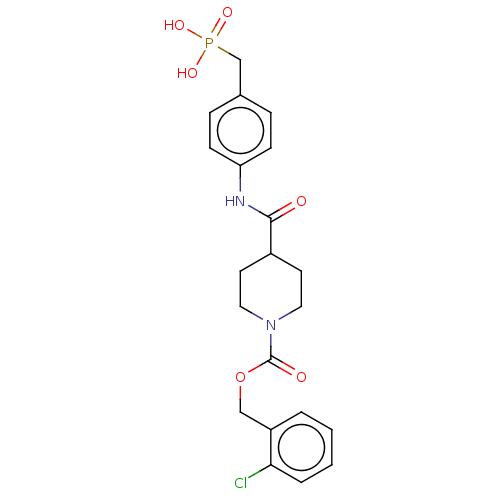
(CHEMBL4216236)Show SMILES OP(O)(=O)Cc1ccc(NC(=O)C2CCN(CC2)C(=O)OCc2ccccc2Cl)cc1 Show InChI InChI=1S/C21H24ClN2O6P/c22-19-4-2-1-3-17(19)13-30-21(26)24-11-9-16(10-12-24)20(25)23-18-7-5-15(6-8-18)14-31(27,28)29/h1-8,16H,9-14H2,(H,23,25)(H2,27,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of human ENPP2 using FS3 as substrate measured every 5 mins for 30 mins by fluorescence assay |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458133

(CHEMBL4216825)Show SMILES OP(O)(=O)Cc1csc(n1)C(=O)NC1CCN(C1)C(=O)OCc1cc(Cl)cc(Cl)c1 Show InChI InChI=1S/C17H18Cl2N3O6PS/c18-11-3-10(4-12(19)5-11)7-28-17(24)22-2-1-13(6-22)20-15(23)16-21-14(9-30-16)8-29(25,26)27/h3-5,9,13H,1-2,6-8H2,(H,20,23)(H2,25,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of human ENPP2 using FS3 as substrate measured every 5 mins for 30 mins by fluorescence assay |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458124
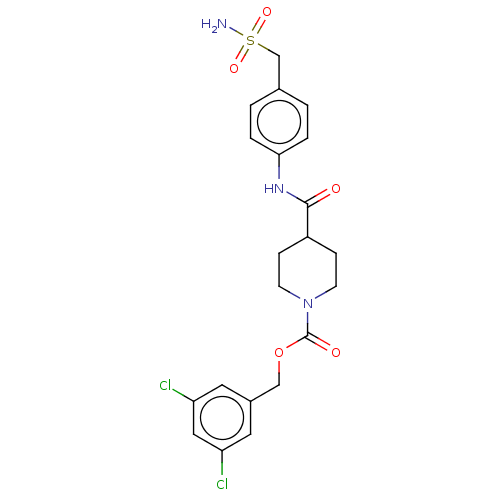
(CHEMBL4208368)Show SMILES NS(=O)(=O)Cc1ccc(NC(=O)C2CCN(CC2)C(=O)OCc2cc(Cl)cc(Cl)c2)cc1 Show InChI InChI=1S/C21H23Cl2N3O5S/c22-17-9-15(10-18(23)11-17)12-31-21(28)26-7-5-16(6-8-26)20(27)25-19-3-1-14(2-4-19)13-32(24,29)30/h1-4,9-11,16H,5-8,12-13H2,(H,25,27)(H2,24,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of human ENPP2 using FS3 as substrate measured every 5 mins for 30 mins by fluorescence assay |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458132

(CHEMBL4205127)Show SMILES OP(O)(=O)Cc1ccc(cc1)C(=O)NC1CCN(CC1)C(=O)OCc1cc(Cl)cc(Cl)c1 Show InChI InChI=1S/C21H23Cl2N2O6P/c22-17-9-15(10-18(23)11-17)12-31-21(27)25-7-5-19(6-8-25)24-20(26)16-3-1-14(2-4-16)13-32(28,29)30/h1-4,9-11,19H,5-8,12-13H2,(H,24,26)(H2,28,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of human ENPP2 using bis-pNPP as substrate measured every 10 secs for 10 mins by spectrophotometric assay |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458128

(CHEMBL4206899)Show SMILES OP(O)(=O)Cc1ccc(NC(=O)C2CCN(CC2)C(=O)OCc2ccccc2F)cc1 Show InChI InChI=1S/C21H24FN2O6P/c22-19-4-2-1-3-17(19)13-30-21(26)24-11-9-16(10-12-24)20(25)23-18-7-5-15(6-8-18)14-31(27,28)29/h1-8,16H,9-14H2,(H,23,25)(H2,27,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of human ENPP2 using FS3 as substrate measured every 5 mins for 30 mins by fluorescence assay |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458129
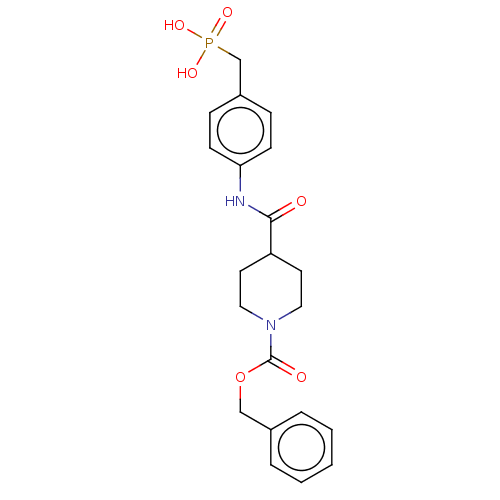
(CHEMBL4209155)Show SMILES OP(O)(=O)Cc1ccc(NC(=O)C2CCN(CC2)C(=O)OCc2ccccc2)cc1 Show InChI InChI=1S/C21H25N2O6P/c24-20(22-19-8-6-17(7-9-19)15-30(26,27)28)18-10-12-23(13-11-18)21(25)29-14-16-4-2-1-3-5-16/h1-9,18H,10-15H2,(H,22,24)(H2,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of human ENPP2 using FS3 as substrate measured every 5 mins for 30 mins by fluorescence assay |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458125

(CHEMBL4217352)Show SMILES OP(O)(=O)Cc1ccc(NC(=O)C2CCN(CC2)C(=O)OCc2cc(Cl)cc(Cl)c2)cc1 Show InChI InChI=1S/C21H23Cl2N2O6P/c22-17-9-15(10-18(23)11-17)12-31-21(27)25-7-5-16(6-8-25)20(26)24-19-3-1-14(2-4-19)13-32(28,29)30/h1-4,9-11,16H,5-8,12-13H2,(H,24,26)(H2,28,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of ENPP2 in human SKHEP1 cells using LPC as substrate preincubated for 15 mins followed by substrate addition measured after 3 hrs by LC-M... |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458130
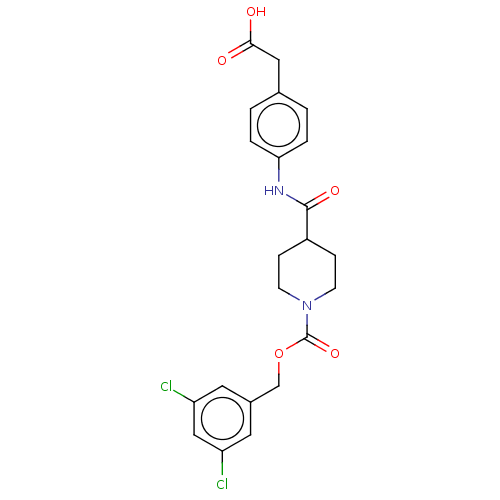
(CHEMBL4204027)Show SMILES OC(=O)Cc1ccc(NC(=O)C2CCN(CC2)C(=O)OCc2cc(Cl)cc(Cl)c2)cc1 Show InChI InChI=1S/C22H22Cl2N2O5/c23-17-9-15(10-18(24)12-17)13-31-22(30)26-7-5-16(6-8-26)21(29)25-19-3-1-14(2-4-19)11-20(27)28/h1-4,9-10,12,16H,5-8,11,13H2,(H,25,29)(H,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of human ENPP2 using FS3 as substrate measured every 5 mins for 30 mins by fluorescence assay |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458134

(CHEMBL4203499)Show SMILES NS(=O)(=O)Nc1ccc(NC(=O)C2CCN(CC2)C(=O)OCc2cc(Cl)cc(Cl)c2)cc1 Show InChI InChI=1S/C20H22Cl2N4O5S/c21-15-9-13(10-16(22)11-15)12-31-20(28)26-7-5-14(6-8-26)19(27)24-17-1-3-18(4-2-17)25-32(23,29)30/h1-4,9-11,14,25H,5-8,12H2,(H,24,27)(H2,23,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of human ENPP2 using FS3 as substrate measured every 5 mins for 30 mins by fluorescence assay |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50269714

(CHEMBL4092264)Show SMILES OP(O)(=O)Cc1ccc(cc1)-c1csc(n1)C(=O)NC1CCN(CC1)C(=O)OCc1cc(Cl)cc(Cl)c1 Show InChI InChI=1S/C24H24Cl2N3O6PS/c25-18-9-16(10-19(26)11-18)12-35-24(31)29-7-5-20(6-8-29)27-22(30)23-28-21(14-37-23)17-3-1-15(2-4-17)13-36(32,33)34/h1-4,9-11,14,20H,5-8,12-13H2,(H,27,30)(H2,32,33,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of ENPP2 in human plasma using LPC as substrate preincubated for 15 mins followed by substrate addition measured after 3 hrs by LC-MS/MS a... |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458123

(CHEMBL4215710)Show SMILES ONC(=O)c1ccc(NC(=O)C2CCN(CC2)C(=O)OCc2cc(Cl)cc(Cl)c2)cc1 Show InChI InChI=1S/C21H21Cl2N3O5/c22-16-9-13(10-17(23)11-16)12-31-21(29)26-7-5-15(6-8-26)19(27)24-18-3-1-14(2-4-18)20(28)25-30/h1-4,9-11,15,30H,5-8,12H2,(H,24,27)(H,25,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of human ENPP2 using FS3 as substrate measured every 5 mins for 30 mins by fluorescence assay |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458131

(CHEMBL4212398)Show SMILES OP(O)(=O)Cc1csc(NC(=O)C2CCN(CC2)C(=O)OCc2cc(Cl)cc(Cl)c2)n1 Show InChI InChI=1S/C18H20Cl2N3O6PS/c19-13-5-11(6-14(20)7-13)8-29-18(25)23-3-1-12(2-4-23)16(24)22-17-21-15(10-31-17)9-30(26,27)28/h5-7,10,12H,1-4,8-9H2,(H,21,22,24)(H2,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of human ENPP2 using bis-pNPP as substrate measured every 10 secs for 10 mins by spectrophotometric assay |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458127

(CHEMBL4211269)Show SMILES OP(O)(=O)Cc1ccc(NC(=O)C2CCN(CC2)C(=O)OCc2cccc(Cl)c2)cc1 Show InChI InChI=1S/C21H24ClN2O6P/c22-18-3-1-2-16(12-18)13-30-21(26)24-10-8-17(9-11-24)20(25)23-19-6-4-15(5-7-19)14-31(27,28)29/h1-7,12,17H,8-11,13-14H2,(H,23,25)(H2,27,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of human ENPP2 using bis-pNPP as substrate measured every 10 secs for 10 mins by spectrophotometric assay |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458133

(CHEMBL4216825)Show SMILES OP(O)(=O)Cc1csc(n1)C(=O)NC1CCN(C1)C(=O)OCc1cc(Cl)cc(Cl)c1 Show InChI InChI=1S/C17H18Cl2N3O6PS/c18-11-3-10(4-12(19)5-11)7-28-17(24)22-2-1-13(6-22)20-15(23)16-21-14(9-30-16)8-29(25,26)27/h3-5,9,13H,1-2,6-8H2,(H,20,23)(H2,25,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of human ENPP2 using bis-pNPP as substrate measured every 10 secs for 10 mins by spectrophotometric assay |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458124

(CHEMBL4208368)Show SMILES NS(=O)(=O)Cc1ccc(NC(=O)C2CCN(CC2)C(=O)OCc2cc(Cl)cc(Cl)c2)cc1 Show InChI InChI=1S/C21H23Cl2N3O5S/c22-17-9-15(10-18(23)11-17)12-31-21(28)26-7-5-16(6-8-26)20(27)25-19-3-1-14(2-4-19)13-32(24,29)30/h1-4,9-11,16H,5-8,12-13H2,(H,25,27)(H2,24,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of human ENPP2 using bis-pNPP as substrate measured every 10 secs for 10 mins by spectrophotometric assay |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458125

(CHEMBL4217352)Show SMILES OP(O)(=O)Cc1ccc(NC(=O)C2CCN(CC2)C(=O)OCc2cc(Cl)cc(Cl)c2)cc1 Show InChI InChI=1S/C21H23Cl2N2O6P/c22-17-9-15(10-18(23)11-17)12-31-21(27)25-7-5-16(6-8-25)20(26)24-19-3-1-14(2-4-19)13-32(28,29)30/h1-4,9-11,16H,5-8,12-13H2,(H,24,26)(H2,28,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 136 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of ENPP2 in human plasma using LPC as substrate preincubated for 15 mins followed by substrate addition measured after 3 hrs by LC-MS/MS a... |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458136

(CHEMBL4216236)Show SMILES OP(O)(=O)Cc1ccc(NC(=O)C2CCN(CC2)C(=O)OCc2ccccc2Cl)cc1 Show InChI InChI=1S/C21H24ClN2O6P/c22-19-4-2-1-3-17(19)13-30-21(26)24-11-9-16(10-12-24)20(25)23-18-7-5-15(6-8-18)14-31(27,28)29/h1-8,16H,9-14H2,(H,23,25)(H2,27,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 213 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of human ENPP2 using bis-pNPP as substrate measured every 10 secs for 10 mins by spectrophotometric assay |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458134

(CHEMBL4203499)Show SMILES NS(=O)(=O)Nc1ccc(NC(=O)C2CCN(CC2)C(=O)OCc2cc(Cl)cc(Cl)c2)cc1 Show InChI InChI=1S/C20H22Cl2N4O5S/c21-15-9-13(10-16(22)11-15)12-31-20(28)26-7-5-14(6-8-26)19(27)24-17-1-3-18(4-2-17)25-32(23,29)30/h1-4,9-11,14,25H,5-8,12H2,(H,24,27)(H2,23,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of human ENPP2 using bis-pNPP as substrate measured every 10 secs for 10 mins by spectrophotometric assay |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458129

(CHEMBL4209155)Show SMILES OP(O)(=O)Cc1ccc(NC(=O)C2CCN(CC2)C(=O)OCc2ccccc2)cc1 Show InChI InChI=1S/C21H25N2O6P/c24-20(22-19-8-6-17(7-9-19)15-30(26,27)28)18-10-12-23(13-11-18)21(25)29-14-16-4-2-1-3-5-16/h1-9,18H,10-15H2,(H,22,24)(H2,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 255 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of human ENPP2 using bis-pNPP as substrate measured every 10 secs for 10 mins by spectrophotometric assay |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458130

(CHEMBL4204027)Show SMILES OC(=O)Cc1ccc(NC(=O)C2CCN(CC2)C(=O)OCc2cc(Cl)cc(Cl)c2)cc1 Show InChI InChI=1S/C22H22Cl2N2O5/c23-17-9-15(10-18(24)12-17)13-31-22(30)26-7-5-16(6-8-26)21(29)25-19-3-1-14(2-4-19)11-20(27)28/h1-4,9-10,12,16H,5-8,11,13H2,(H,25,29)(H,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 439 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of human ENPP2 using bis-pNPP as substrate measured every 10 secs for 10 mins by spectrophotometric assay |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458128

(CHEMBL4206899)Show SMILES OP(O)(=O)Cc1ccc(NC(=O)C2CCN(CC2)C(=O)OCc2ccccc2F)cc1 Show InChI InChI=1S/C21H24FN2O6P/c22-19-4-2-1-3-17(19)13-30-21(26)24-11-9-16(10-12-24)20(25)23-18-7-5-15(6-8-18)14-31(27,28)29/h1-8,16H,9-14H2,(H,23,25)(H2,27,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 453 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of human ENPP2 using bis-pNPP as substrate measured every 10 secs for 10 mins by spectrophotometric assay |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458123

(CHEMBL4215710)Show SMILES ONC(=O)c1ccc(NC(=O)C2CCN(CC2)C(=O)OCc2cc(Cl)cc(Cl)c2)cc1 Show InChI InChI=1S/C21H21Cl2N3O5/c22-16-9-13(10-17(23)11-16)12-31-21(29)26-7-5-15(6-8-26)19(27)24-18-3-1-14(2-4-18)20(28)25-30/h1-4,9-11,15,30H,5-8,12H2,(H,24,27)(H,25,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 496 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of human ENPP2 using bis-pNPP as substrate measured every 10 secs for 10 mins by spectrophotometric assay |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50353025

(CHEMBL1821987)Show SMILES CC(C)CNC(=O)[C@@H]1C=C[C@@H]2C[C@H]3[C@H](Cc4ccc5OCOc5c4)[C@H]4C=C[C@@H]1[C@@H]2[C@@H]34 |r,c:8,27| Show InChI InChI=1S/C26H31NO3/c1-14(2)12-27-26(28)19-5-4-16-11-21-20(18-7-6-17(19)24(16)25(18)21)9-15-3-8-22-23(10-15)30-13-29-22/h3-8,10,14,16-21,24-25H,9,11-13H2,1-2H3,(H,27,28)/t16-,17+,18-,19-,20-,21+,24-,25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University
Curated by ChEMBL
| Assay Description
Inhibition of iNOS in mouse RAW264.7 cells assessed as anti-inflammatory activity by measuring maximum inhibition of LPS-induced nitric oxide product... |
J Nat Prod 74: 1875-80 (2011)
Article DOI: 10.1021/np200279r
BindingDB Entry DOI: 10.7270/Q2474B72 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50353027

(CHEMBL1821990)Show SMILES CC(C)CNC(=O)[C@@H]1[C@H]2C[C@H]3[C@H](CCCc4ccc5OCOc5c4)[C@@H]4[C@H]3[C@@H]2C=C[C@H]14 |r,c:31,TLB:11:24:8.7:27.28,9:8:25.24:27.28,10:25:8.7:27.28,THB:11:10:26:7.29.24,5:7:25.24:27.28| Show InChI InChI=1S/C26H33NO3/c1-14(2)12-27-26(28)25-18-8-7-17-20(25)11-19-16(23(18)24(17)19)5-3-4-15-6-9-21-22(10-15)30-13-29-21/h6-10,14,16-20,23-25H,3-5,11-13H2,1-2H3,(H,27,28)/t16-,17+,18-,19-,20-,23-,24-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University
Curated by ChEMBL
| Assay Description
Inhibition of iNOS in mouse RAW264.7 cells assessed as anti-inflammatory activity by measuring maximum inhibition of LPS-induced nitric oxide product... |
J Nat Prod 74: 1875-80 (2011)
Article DOI: 10.1021/np200279r
BindingDB Entry DOI: 10.7270/Q2474B72 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50207159
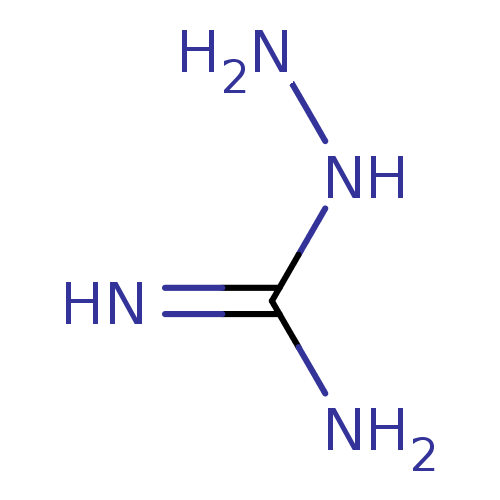
(2-aminoguanidine | 2-azanylguanidine | AMINOGUANID...)Show InChI InChI=1S/CH6N4/c2-1(3)5-4/h4H2,(H4,2,3,5) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 2.66E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University
Curated by ChEMBL
| Assay Description
Inhibition of iNOS in mouse RAW264.7 cells assessed as anti-inflammatory activity by measuring maximum inhibition of LPS-induced nitric oxide product... |
J Nat Prod 74: 1875-80 (2011)
Article DOI: 10.1021/np200279r
BindingDB Entry DOI: 10.7270/Q2474B72 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50353026

(CHEMBL1821989)Show SMILES OC(=O)\C=C\[C@@H]1[C@@H]2C[C@H]3[C@H](CCCCCc4ccc5OCOc5c4)[C@H]4[C@H]3[C@@H]2C=C[C@@H]14 |r,c:31,TLB:7:6:25.24:27.28,8:25:6.5:27.28,9:24:6.5:27.28,THB:4:5:25.24:27.28| Show InChI InChI=1S/C26H30O4/c27-24(28)11-9-16-18-7-8-19-20(16)13-21-17(25(18)26(19)21)5-3-1-2-4-15-6-10-22-23(12-15)30-14-29-22/h6-12,16-21,25-26H,1-5,13-14H2,(H,27,28)/b11-9+/t16-,17-,18-,19+,20-,21-,25+,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.96E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University
Curated by ChEMBL
| Assay Description
Inhibition of iNOS in mouse RAW264.7 cells assessed as anti-inflammatory activity by measuring maximum inhibition of LPS-induced nitric oxide product... |
J Nat Prod 74: 1875-80 (2011)
Article DOI: 10.1021/np200279r
BindingDB Entry DOI: 10.7270/Q2474B72 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50353024
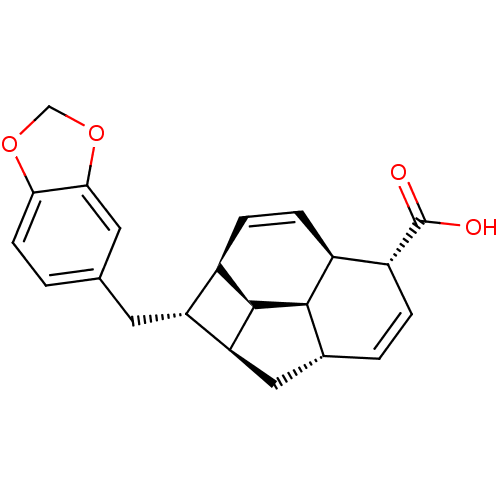
(CHEMBL1821986)Show SMILES OC(=O)[C@@H]1C=C[C@@H]2C[C@H]3[C@H](Cc4ccc5OCOc5c4)[C@H]4C=C[C@@H]1[C@@H]2[C@@H]34 |r,c:4,23| Show InChI InChI=1S/C22H22O4/c23-22(24)15-3-2-12-9-17-16(14-5-4-13(15)20(12)21(14)17)7-11-1-6-18-19(8-11)26-10-25-18/h1-6,8,12-17,20-21H,7,9-10H2,(H,23,24)/t12-,13+,14-,15-,16-,17+,20-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University
Curated by ChEMBL
| Assay Description
Inhibition of iNOS in mouse RAW264.7 cells assessed as anti-inflammatory activity by measuring maximum inhibition of LPS-induced nitric oxide product... |
J Nat Prod 74: 1875-80 (2011)
Article DOI: 10.1021/np200279r
BindingDB Entry DOI: 10.7270/Q2474B72 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50353030
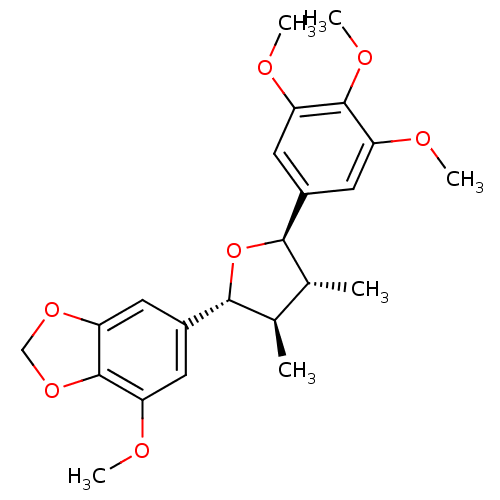
(CHEMBL1821993)Show SMILES COc1cc(cc2OCOc12)[C@@H]1O[C@H]([C@H](C)[C@H]1C)c1cc(OC)c(OC)c(OC)c1 |r| Show InChI InChI=1S/C23H28O7/c1-12-13(2)21(15-9-18(26-5)23-19(10-15)28-11-29-23)30-20(12)14-7-16(24-3)22(27-6)17(8-14)25-4/h7-10,12-13,20-21H,11H2,1-6H3/t12-,13-,20-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University
Curated by ChEMBL
| Assay Description
Inhibition of iNOS in mouse RAW264.7 cells assessed as anti-inflammatory activity by measuring maximum inhibition of LPS-induced nitric oxide product... |
J Nat Prod 74: 1875-80 (2011)
Article DOI: 10.1021/np200279r
BindingDB Entry DOI: 10.7270/Q2474B72 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50353023
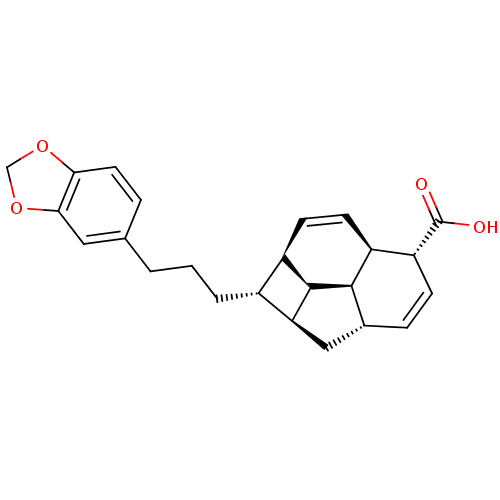
(CHEMBL1821985)Show SMILES OC(=O)[C@@H]1C=C[C@@H]2C[C@H]3[C@H](CCCc4ccc5OCOc5c4)[C@H]4C=C[C@@H]1[C@@H]2[C@@H]34 |r,c:4,25| Show InChI InChI=1S/C24H26O4/c25-24(26)18-6-5-14-11-19-15(16-7-8-17(18)22(14)23(16)19)3-1-2-13-4-9-20-21(10-13)28-12-27-20/h4-10,14-19,22-23H,1-3,11-12H2,(H,25,26)/t14-,15-,16-,17+,18-,19+,22-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.96E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University
Curated by ChEMBL
| Assay Description
Inhibition of iNOS in mouse RAW264.7 cells assessed as anti-inflammatory activity by measuring maximum inhibition of LPS-induced nitric oxide product... |
J Nat Prod 74: 1875-80 (2011)
Article DOI: 10.1021/np200279r
BindingDB Entry DOI: 10.7270/Q2474B72 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50353029
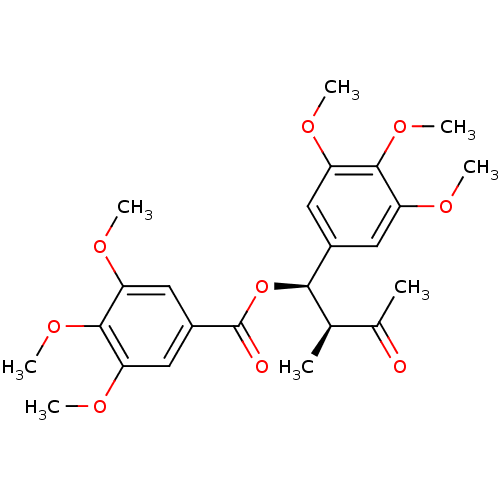
(CHEMBL1821992)Show SMILES COc1cc(cc(OC)c1OC)[C@H](OC(=O)c1cc(OC)c(OC)c(OC)c1)[C@H](C)C(C)=O |r| Show InChI InChI=1S/C24H30O9/c1-13(14(2)25)21(15-9-17(27-3)22(31-7)18(10-15)28-4)33-24(26)16-11-19(29-5)23(32-8)20(12-16)30-6/h9-13,21H,1-8H3/t13-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University
Curated by ChEMBL
| Assay Description
Inhibition of iNOS in mouse RAW264.7 cells assessed as anti-inflammatory activity by measuring maximum inhibition of LPS-induced nitric oxide product... |
J Nat Prod 74: 1875-80 (2011)
Article DOI: 10.1021/np200279r
BindingDB Entry DOI: 10.7270/Q2474B72 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50353028

(CHEMBL1821991)Show SMILES COc1cc(cc2OCOc12)[C@@H]1O[C@H]([C@H](C)[C@H]1C)c1cc(O)c(OC)c(OC)c1 |r| Show InChI InChI=1S/C22H26O7/c1-11-12(2)20(14-8-17(25-4)22-18(9-14)27-10-28-22)29-19(11)13-6-15(23)21(26-5)16(7-13)24-3/h6-9,11-12,19-20,23H,10H2,1-5H3/t11-,12-,19-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University
Curated by ChEMBL
| Assay Description
Inhibition of iNOS in mouse RAW264.7 cells assessed as anti-inflammatory activity by measuring maximum inhibition of LPS-induced nitric oxide product... |
J Nat Prod 74: 1875-80 (2011)
Article DOI: 10.1021/np200279r
BindingDB Entry DOI: 10.7270/Q2474B72 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50353031

(CHEMBL1821994)Show SMILES COc1cc(cc2OCOc12)[C@@H]1O[C@H]([C@H](C)[C@H]1C)c1cc2OCOc2c(OC)c1 |r| Show InChI InChI=1S/C22H24O7/c1-11-12(2)20(14-6-16(24-4)22-18(8-14)26-10-28-22)29-19(11)13-5-15(23-3)21-17(7-13)25-9-27-21/h5-8,11-12,19-20H,9-10H2,1-4H3/t11-,12-,19-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University
Curated by ChEMBL
| Assay Description
Inhibition of iNOS in mouse RAW264.7 cells assessed as anti-inflammatory activity by measuring maximum inhibition of LPS-induced nitric oxide product... |
J Nat Prod 74: 1875-80 (2011)
Article DOI: 10.1021/np200279r
BindingDB Entry DOI: 10.7270/Q2474B72 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50353032

(CHEMBL1821988)Show SMILES OC(=O)[C@@H]1[C@H]2C[C@H]3[C@H](CCCc4ccc5OCOc5c4)[C@@H]4[C@H]3[C@@H]2C=C[C@H]14 |r,c:27,TLB:7:20:4.3:23.24,5:4:21.20:23.24,6:21:4.3:23.24,THB:23:22:5.6:3.25.20,1:3:21.20:23.24| Show InChI InChI=1S/C22H24O4/c23-22(24)21-14-6-5-13-16(21)9-15-12(19(14)20(13)15)3-1-2-11-4-7-17-18(8-11)26-10-25-17/h4-8,12-16,19-21H,1-3,9-10H2,(H,23,24)/t12-,13+,14-,15-,16-,19-,20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.82E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University
Curated by ChEMBL
| Assay Description
Inhibition of iNOS in mouse RAW264.7 cells assessed as anti-inflammatory activity by measuring maximum inhibition of LPS-induced nitric oxide product... |
J Nat Prod 74: 1875-80 (2011)
Article DOI: 10.1021/np200279r
BindingDB Entry DOI: 10.7270/Q2474B72 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50225106
((2S)-2-amino-5-{[(E)-amino(nitroimino)methyl]amino...)Show SMILES N[C@@H](CCCNC(N)=N[N+]([O-])=O)C(O)=O |r,w:8.8| Show InChI InChI=1S/C6H13N5O4/c7-4(5(12)13)2-1-3-9-6(8)10-11(14)15/h4H,1-3,7H2,(H,12,13)(H3,8,9,10)/t4-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.52E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University
Curated by ChEMBL
| Assay Description
Inhibition of iNOS in mouse RAW264.7 cells assessed as anti-inflammatory activity by measuring maximum inhibition of LPS-induced nitric oxide product... |
J Nat Prod 74: 1875-80 (2011)
Article DOI: 10.1021/np200279r
BindingDB Entry DOI: 10.7270/Q2474B72 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data