

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Succinate dehydrogenase cytochrome b560 subunit, mitochondrial (Sus scrofa) | BDBM50079610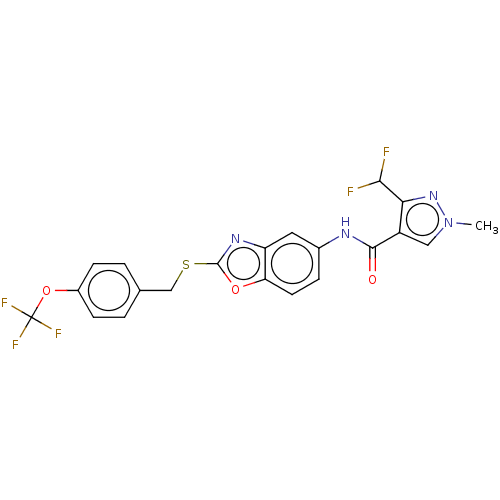 (CHEMBL3417782) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University Curated by ChEMBL | Assay Description Non-competitive inhibition of porcine heart SQR in SCR complex with respect to DCIP substrate at 23 degC by double-reciprocal plot | Eur J Med Chem 95: 424-34 (2015) Article DOI: 10.1016/j.ejmech.2015.03.060 BindingDB Entry DOI: 10.7270/Q27946D8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Succinate dehydrogenase cytochrome b560 subunit, mitochondrial (Sus scrofa) | BDBM50079600 (CHEBI:83138 | PENTHIOPYRAD) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 327 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University Curated by ChEMBL | Assay Description Non-competitive inhibition of porcine heart SQR in SCR complex with respect to DCIP substrate at 23 degC by double-reciprocal plot | Eur J Med Chem 95: 424-34 (2015) Article DOI: 10.1016/j.ejmech.2015.03.060 BindingDB Entry DOI: 10.7270/Q27946D8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50449386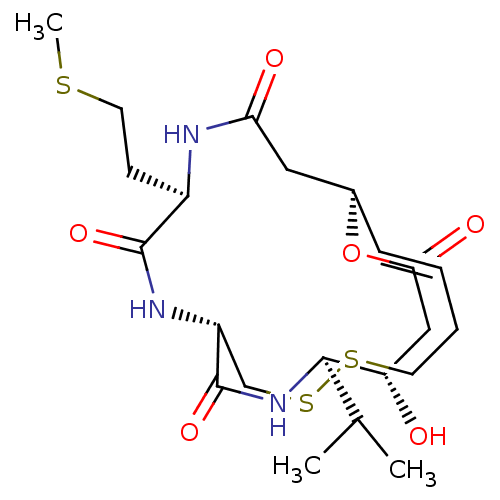 (CHEMBL3126833) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 after 1 hr by luminescence assay | Medchemcomm 3: 976-981 (2012) Article DOI: 10.1039/c2md20024d BindingDB Entry DOI: 10.7270/Q21G0Q7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50436850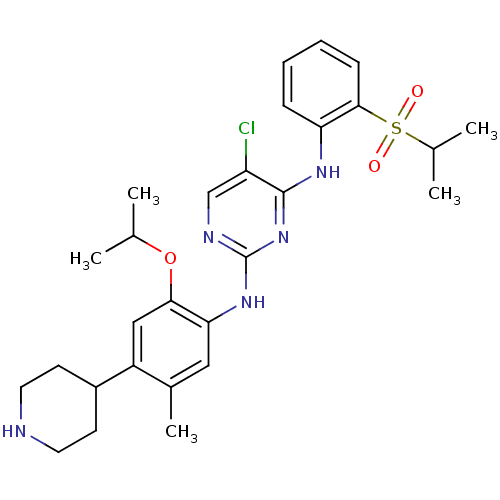 (CERITINIB | CHEMBL2403108 | LDK378 | US10053458, C...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of ALK (unknown origin) after 60 mins | J Med Chem 56: 5675-90 (2014) Article DOI: 10.1021/jm400402q BindingDB Entry DOI: 10.7270/Q2G1627J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50354088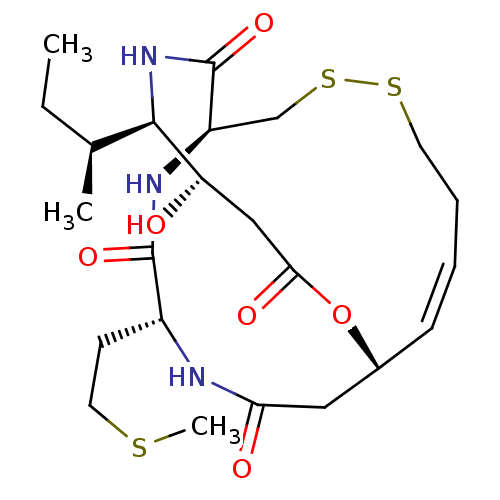 (CHEMBL1836142) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 after 1 hr by luminescence assay | Medchemcomm 3: 976-981 (2012) Article DOI: 10.1039/c2md20024d BindingDB Entry DOI: 10.7270/Q21G0Q7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM19151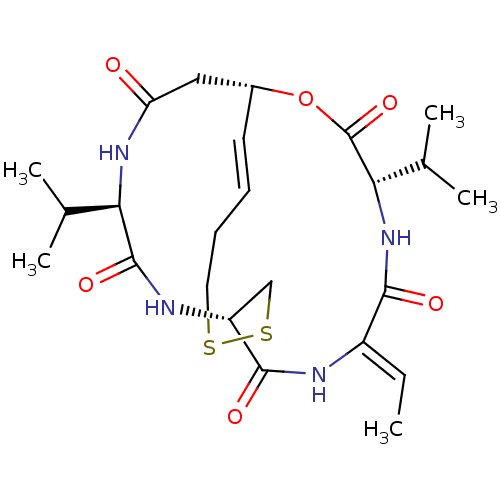 ((1S,4S,7Z,10S,16E,21R)-7-ethylidene-4,21-bis(propa...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 after 1 hr by luminescence assay | Medchemcomm 3: 976-981 (2012) Article DOI: 10.1039/c2md20024d BindingDB Entry DOI: 10.7270/Q21G0Q7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50496770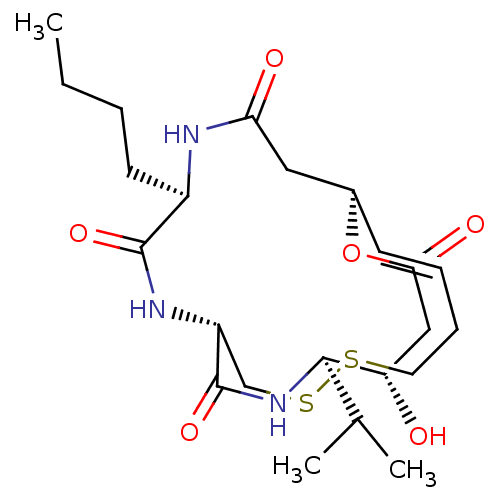 (CHEMBL3218562) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 after 1 hr by luminescence assay | Medchemcomm 3: 976-981 (2012) Article DOI: 10.1039/c2md20024d BindingDB Entry DOI: 10.7270/Q21G0Q7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50496768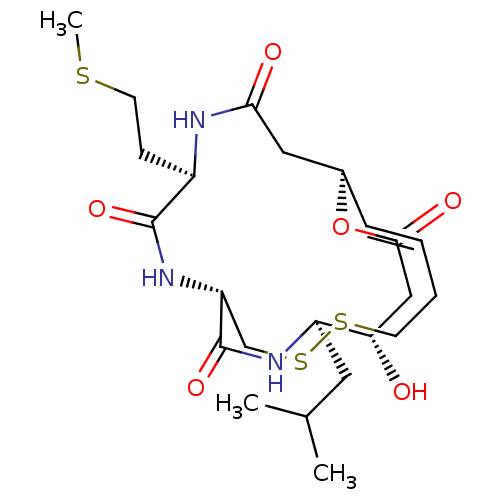 (CHEMBL3218563) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 after 1 hr by luminescence assay | Medchemcomm 3: 976-981 (2012) Article DOI: 10.1039/c2md20024d BindingDB Entry DOI: 10.7270/Q21G0Q7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50354084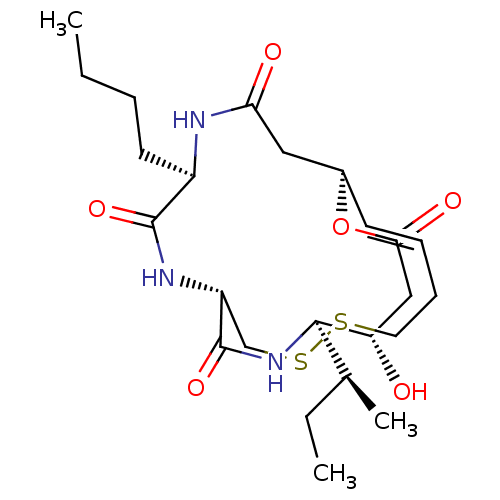 (CHEMBL1836145) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 after 1 hr by luminescence assay | Medchemcomm 3: 976-981 (2012) Article DOI: 10.1039/c2md20024d BindingDB Entry DOI: 10.7270/Q21G0Q7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50436850 (CERITINIB | CHEMBL2403108 | LDK378 | US10053458, C...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of EML4-fused ALK (unknown origin) by cell-based assay | J Med Chem 56: 5675-90 (2014) Article DOI: 10.1021/jm400402q BindingDB Entry DOI: 10.7270/Q2G1627J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50496769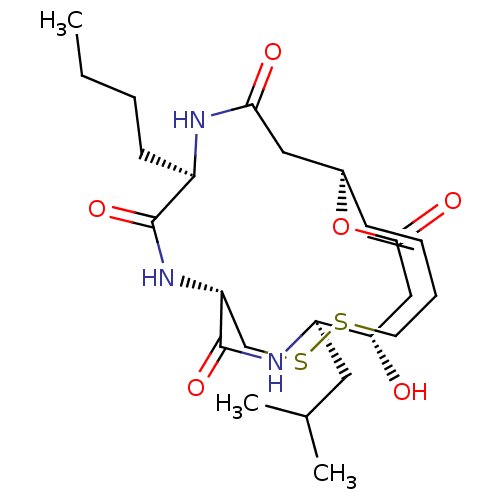 (CHEMBL3218564) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 after 1 hr by luminescence assay | Medchemcomm 3: 976-981 (2012) Article DOI: 10.1039/c2md20024d BindingDB Entry DOI: 10.7270/Q21G0Q7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM482158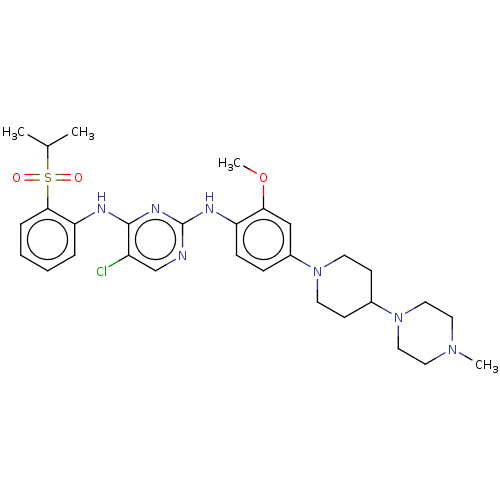 (BDBM50242742 | TAE684) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of NPM-fused ALK (unknown origin) expressed in mouse BAF3 cells after 2 to 3 days by luciferase reporter gene assay | J Med Chem 56: 5675-90 (2014) Article DOI: 10.1021/jm400402q BindingDB Entry DOI: 10.7270/Q2G1627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3/Nuclear receptor corepressor 2 (HDAC3/NCoR2) (Homo sapiens (Human)) | BDBM50354089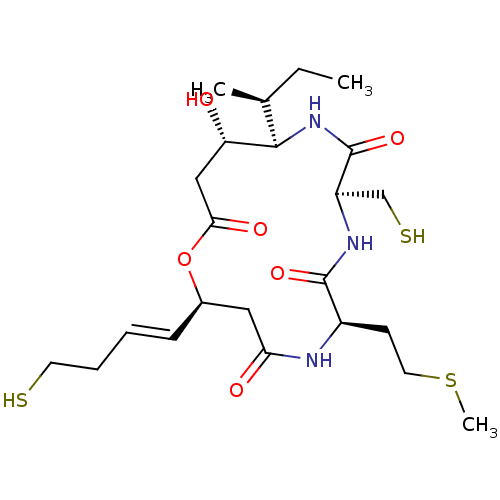 (CHEMBL1836144) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC3/NCOR2 using Boc-L-Lys(epsilon-trifluoroacetyl)-AMC as substrate by two-step fluorogenic assay | J Nat Prod 74: 2031-8 (2011) Article DOI: 10.1021/np200324x BindingDB Entry DOI: 10.7270/Q2MW2HJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3/Nuclear receptor corepressor 2 (HDAC3/NCoR2) (Homo sapiens (Human)) | BDBM50354087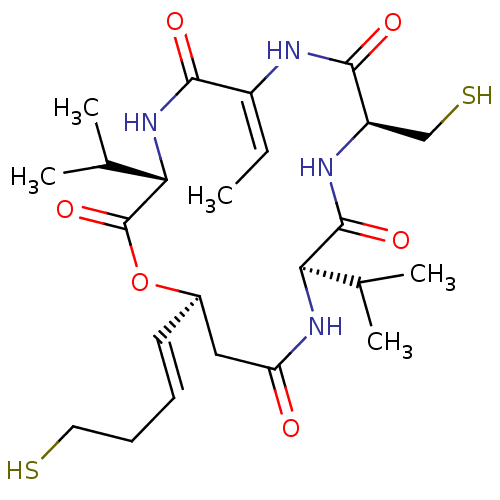 (CHEMBL1836143) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC3/NCOR2 using Boc-L-Lys(epsilon-trifluoroacetyl)-AMC as substrate by two-step fluorogenic assay | J Nat Prod 74: 2031-8 (2011) Article DOI: 10.1021/np200324x BindingDB Entry DOI: 10.7270/Q2MW2HJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50436850 (CERITINIB | CHEMBL2403108 | LDK378 | US10053458, C...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of insulin receptor (unknown origin) after 60 mins | J Med Chem 56: 5675-90 (2014) Article DOI: 10.1021/jm400402q BindingDB Entry DOI: 10.7270/Q2G1627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM50436850 (CERITINIB | CHEMBL2403108 | LDK378 | US10053458, C...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of IGF1 receptor (unknown origin) after 60 mins | J Med Chem 56: 5675-90 (2014) Article DOI: 10.1021/jm400402q BindingDB Entry DOI: 10.7270/Q2G1627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50436863 (CHEMBL2403836) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of NPM-fused ALK (unknown origin) expressed in mouse BAF3 cells after 2 to 3 days by luciferase reporter gene assay | J Med Chem 56: 5675-90 (2014) Article DOI: 10.1021/jm400402q BindingDB Entry DOI: 10.7270/Q2G1627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3/Nuclear receptor corepressor 2 (HDAC3/NCoR2) (Homo sapiens (Human)) | BDBM50354085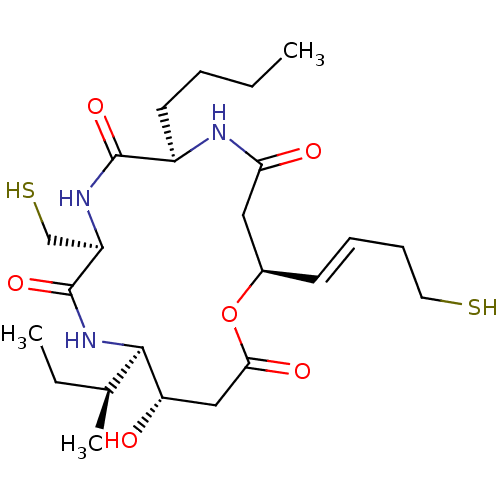 (CHEMBL1836042) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC3/NCOR2 using Boc-L-Lys(epsilon-trifluoroacetyl)-AMC as substrate by two-step fluorogenic assay | J Nat Prod 74: 2031-8 (2011) Article DOI: 10.1021/np200324x BindingDB Entry DOI: 10.7270/Q2MW2HJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50436862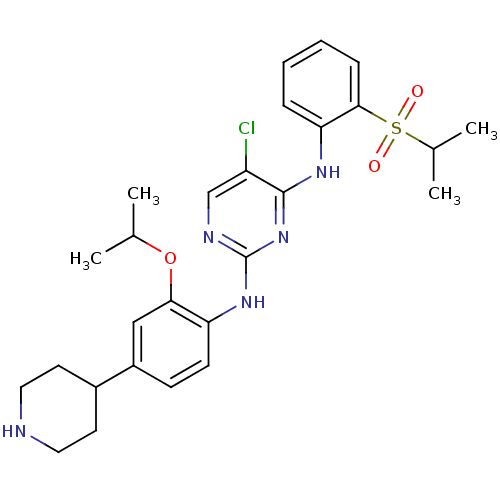 (CHEMBL2403845) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of NPM-fused ALK (unknown origin) expressed in mouse BAF3 cells after 2 to 3 days by luciferase reporter gene assay | J Med Chem 56: 5675-90 (2014) Article DOI: 10.1021/jm400402q BindingDB Entry DOI: 10.7270/Q2G1627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Succinate dehydrogenase cytochrome b560 subunit, mitochondrial (Sus scrofa) | BDBM50079610 (CHEMBL3417782) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University Curated by ChEMBL | Assay Description Inhibition of porcine heart SQR in SCR complex assessed as reduction in ubiquinol-cytochrome c reductase (complex 3) activity by measuring cytochrome... | Eur J Med Chem 95: 424-34 (2015) Article DOI: 10.1016/j.ejmech.2015.03.060 BindingDB Entry DOI: 10.7270/Q27946D8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50436861 (CHEMBL2403849) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of NPM-fused ALK (unknown origin) expressed in mouse BAF3 cells after 2 to 3 days by luciferase reporter gene assay | J Med Chem 56: 5675-90 (2014) Article DOI: 10.1021/jm400402q BindingDB Entry DOI: 10.7270/Q2G1627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50436860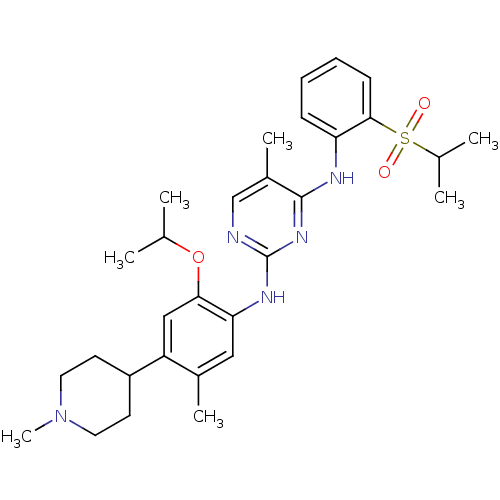 (CHEMBL2403843) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of NPM-fused ALK (unknown origin) expressed in mouse BAF3 cells after 2 to 3 days by luciferase reporter gene assay | J Med Chem 56: 5675-90 (2014) Article DOI: 10.1021/jm400402q BindingDB Entry DOI: 10.7270/Q2G1627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50436858 (CHEMBL2403848) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of NPM-fused ALK (unknown origin) expressed in mouse BAF3 cells after 2 to 3 days by luciferase reporter gene assay | J Med Chem 56: 5675-90 (2014) Article DOI: 10.1021/jm400402q BindingDB Entry DOI: 10.7270/Q2G1627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50436859 (CHEMBL2403841) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of NPM-fused ALK (unknown origin) expressed in mouse BAF3 cells after 2 to 3 days by luciferase reporter gene assay | J Med Chem 56: 5675-90 (2014) Article DOI: 10.1021/jm400402q BindingDB Entry DOI: 10.7270/Q2G1627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3/Nuclear receptor corepressor 2 (HDAC3/NCoR2) (Homo sapiens (Human)) | BDBM50354086 (FK-228 | Istodax | ROMIDEPSIN) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC3/NCOR2 using Boc-L-Lys(epsilon-trifluoroacetyl)-AMC as substrate by two-step fluorogenic assay | J Nat Prod 74: 2031-8 (2011) Article DOI: 10.1021/np200324x BindingDB Entry DOI: 10.7270/Q2MW2HJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3/Nuclear receptor corepressor 2 (HDAC3/NCoR2) (Homo sapiens (Human)) | BDBM50354086 (FK-228 | Istodax | ROMIDEPSIN) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC3/N-CoR2 using Boc-L-Lys(epsilon-trifluoroacetyl)-AMC as substrate by two-step fluorogenic assay in presence of 0... | J Nat Prod 74: 2031-8 (2011) Article DOI: 10.1021/np200324x BindingDB Entry DOI: 10.7270/Q2MW2HJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3/Nuclear receptor corepressor 2 (HDAC3/NCoR2) (Homo sapiens (Human)) | BDBM50354084 (CHEMBL1836145) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC3/NCOR2 using Boc-L-Lys(epsilon-trifluoroacetyl)-AMC as substrate by two-step fluorogenic assay | J Nat Prod 74: 2031-8 (2011) Article DOI: 10.1021/np200324x BindingDB Entry DOI: 10.7270/Q2MW2HJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Testis-specific serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50436850 (CERITINIB | CHEMBL2403108 | LDK378 | US10053458, C...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of STK22D (unknown origin) after 60 mins | J Med Chem 56: 5675-90 (2014) Article DOI: 10.1021/jm400402q BindingDB Entry DOI: 10.7270/Q2G1627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50436857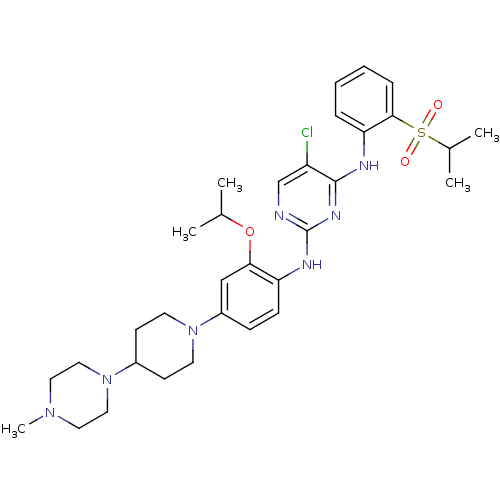 (CHEMBL2403844) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of NPM-fused ALK (unknown origin) expressed in mouse BAF3 cells after 2 to 3 days by luciferase reporter gene assay | J Med Chem 56: 5675-90 (2014) Article DOI: 10.1021/jm400402q BindingDB Entry DOI: 10.7270/Q2G1627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50436856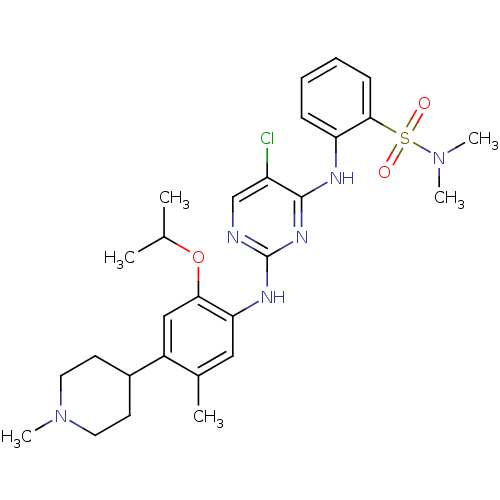 (CHEMBL2403833) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of NPM-fused ALK (unknown origin) expressed in mouse BAF3 cells after 2 to 3 days by luciferase reporter gene assay | J Med Chem 56: 5675-90 (2014) Article DOI: 10.1021/jm400402q BindingDB Entry DOI: 10.7270/Q2G1627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50436855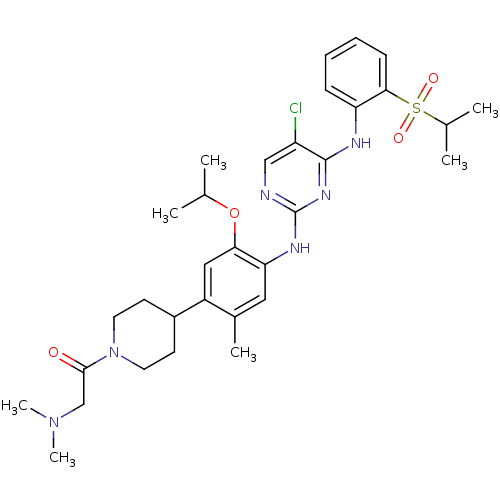 (CHEMBL2403842 | US8592432, 1) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of NPM-fused ALK (unknown origin) expressed in mouse BAF3 cells after 2 to 3 days by luciferase reporter gene assay | J Med Chem 56: 5675-90 (2014) Article DOI: 10.1021/jm400402q BindingDB Entry DOI: 10.7270/Q2G1627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50436850 (CERITINIB | CHEMBL2403108 | LDK378 | US10053458, C...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of NPM-fused ALK (unknown origin) expressed in mouse BAF3 cells after 2 to 3 days by luciferase reporter gene assay | J Med Chem 56: 5675-90 (2014) Article DOI: 10.1021/jm400402q BindingDB Entry DOI: 10.7270/Q2G1627J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50354087 (CHEMBL1836143) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC8 using Boc-L-Lys(epsilon-trifluoroacetyl)-AMC as substrate by two-step fluorogenic assay | J Nat Prod 74: 2031-8 (2011) Article DOI: 10.1021/np200324x BindingDB Entry DOI: 10.7270/Q2MW2HJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50436854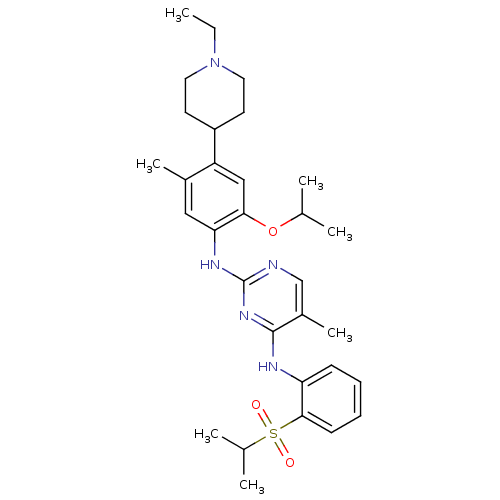 (CHEMBL2403827) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of NPM-fused ALK (unknown origin) expressed in mouse BAF3 cells after 2 to 3 days by luciferase reporter gene assay | J Med Chem 56: 5675-90 (2014) Article DOI: 10.1021/jm400402q BindingDB Entry DOI: 10.7270/Q2G1627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50436853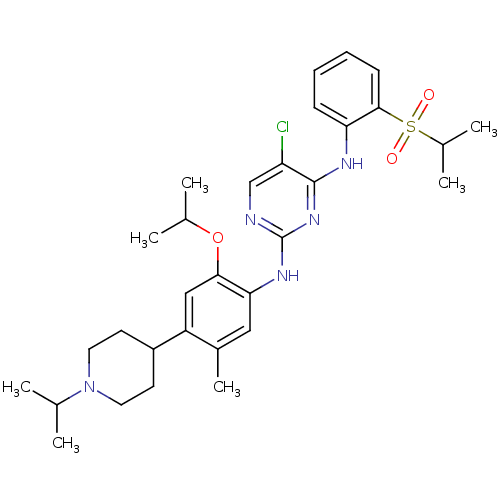 (CHEMBL2403835) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of NPM-fused ALK (unknown origin) expressed in mouse BAF3 cells after 2 to 3 days by luciferase reporter gene assay | J Med Chem 56: 5675-90 (2014) Article DOI: 10.1021/jm400402q BindingDB Entry DOI: 10.7270/Q2G1627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50436852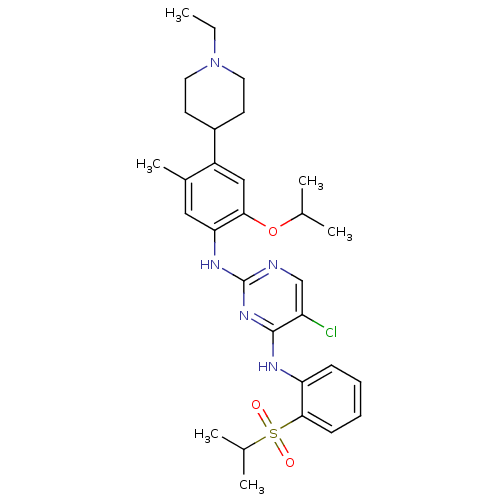 (CHEMBL2403834) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of NPM-fused ALK (unknown origin) expressed in mouse BAF3 cells after 2 to 3 days by luciferase reporter gene assay | J Med Chem 56: 5675-90 (2014) Article DOI: 10.1021/jm400402q BindingDB Entry DOI: 10.7270/Q2G1627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50436851 (CHEMBL2403831) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of NPM-fused ALK (unknown origin) expressed in mouse BAF3 cells after 2 to 3 days by luciferase reporter gene assay | J Med Chem 56: 5675-90 (2014) Article DOI: 10.1021/jm400402q BindingDB Entry DOI: 10.7270/Q2G1627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50436873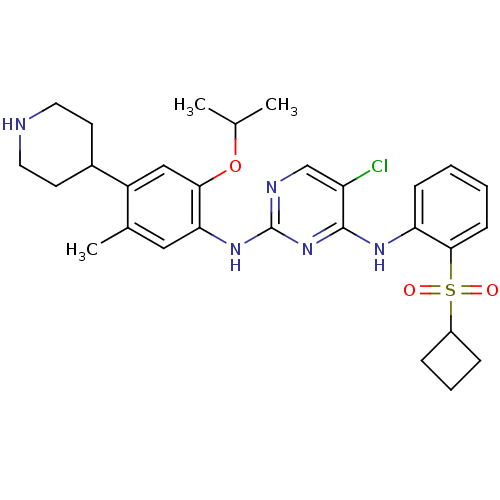 (CHEMBL2403847) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of NPM-fused ALK (unknown origin) expressed in mouse BAF3 cells after 2 to 3 days by luciferase reporter gene assay | J Med Chem 56: 5675-90 (2014) Article DOI: 10.1021/jm400402q BindingDB Entry DOI: 10.7270/Q2G1627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3/Nuclear receptor corepressor 2 (HDAC3/NCoR2) (Homo sapiens (Human)) | BDBM50354086 (FK-228 | Istodax | ROMIDEPSIN) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC3/N-CoR2 using Boc-L-Lys(epsilon-trifluoroacetyl)-AMC as substrate by two-step fluorogenic assay in presence of 0... | J Nat Prod 74: 2031-8 (2011) Article DOI: 10.1021/np200324x BindingDB Entry DOI: 10.7270/Q2MW2HJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50436869 (CHEMBL2403846) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of NPM-fused ALK (unknown origin) expressed in mouse BAF3 cells after 2 to 3 days by luciferase reporter gene assay | J Med Chem 56: 5675-90 (2014) Article DOI: 10.1021/jm400402q BindingDB Entry DOI: 10.7270/Q2G1627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50436850 (CERITINIB | CHEMBL2403108 | LDK378 | US10053458, C...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of TEL-fused ALK (unknown origin) by cell-based assay | J Med Chem 56: 5675-90 (2014) Article DOI: 10.1021/jm400402q BindingDB Entry DOI: 10.7270/Q2G1627J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 3/Nuclear receptor corepressor 2 (HDAC3/NCoR2) (Homo sapiens (Human)) | BDBM50354086 (FK-228 | Istodax | ROMIDEPSIN) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC3/N-CoR2 using Boc-L-Lys(epsilon-trifluoroacetyl)-AMC as substrate by two-step fluorogenic assay in presence of 1... | J Nat Prod 74: 2031-8 (2011) Article DOI: 10.1021/np200324x BindingDB Entry DOI: 10.7270/Q2MW2HJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM482158 (BDBM50242742 | TAE684) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of TEL-fused insulin receptor (unknown origin) expressed in mouse BAF3 cells after 2 to 3 days by luciferase reporter gene assay | J Med Chem 56: 5675-90 (2014) Article DOI: 10.1021/jm400402q BindingDB Entry DOI: 10.7270/Q2G1627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50436870 (CHEMBL2403829) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of NPM-fused ALK (unknown origin) expressed in mouse BAF3 cells after 2 to 3 days by luciferase reporter gene assay | J Med Chem 56: 5675-90 (2014) Article DOI: 10.1021/jm400402q BindingDB Entry DOI: 10.7270/Q2G1627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50436871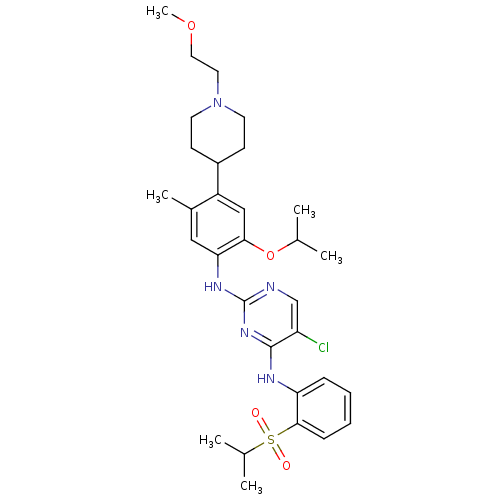 (CHEMBL2403837) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of NPM-fused ALK (unknown origin) expressed in mouse BAF3 cells after 2 to 3 days by luciferase reporter gene assay | J Med Chem 56: 5675-90 (2014) Article DOI: 10.1021/jm400402q BindingDB Entry DOI: 10.7270/Q2G1627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50436868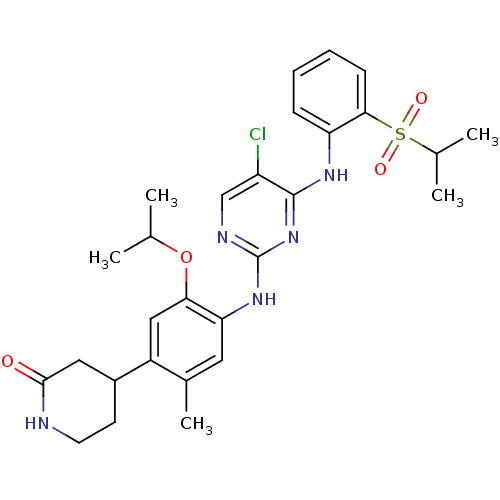 (CHEMBL2403830) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of NPM-fused ALK (unknown origin) expressed in mouse BAF3 cells after 2 to 3 days by luciferase reporter gene assay | J Med Chem 56: 5675-90 (2014) Article DOI: 10.1021/jm400402q BindingDB Entry DOI: 10.7270/Q2G1627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50436850 (CERITINIB | CHEMBL2403108 | LDK378 | US10053458, C...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of FLT3 (unknown origin) after 60 mins | J Med Chem 56: 5675-90 (2014) Article DOI: 10.1021/jm400402q BindingDB Entry DOI: 10.7270/Q2G1627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50436865 (CHEMBL2403828) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of NPM-fused ALK (unknown origin) expressed in mouse BAF3 cells after 2 to 3 days by luciferase reporter gene assay | J Med Chem 56: 5675-90 (2014) Article DOI: 10.1021/jm400402q BindingDB Entry DOI: 10.7270/Q2G1627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3/Nuclear receptor corepressor 2 (HDAC3/NCoR2) (Homo sapiens (Human)) | BDBM50354088 (CHEMBL1836142) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC3/NCOR2 using Boc-L-Lys(epsilon-trifluoroacetyl)-AMC as substrate by two-step fluorogenic assay | J Nat Prod 74: 2031-8 (2011) Article DOI: 10.1021/np200324x BindingDB Entry DOI: 10.7270/Q2MW2HJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50436867 (CHEMBL2403840) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of NPM-fused ALK (unknown origin) expressed in mouse BAF3 cells after 2 to 3 days by luciferase reporter gene assay | J Med Chem 56: 5675-90 (2014) Article DOI: 10.1021/jm400402q BindingDB Entry DOI: 10.7270/Q2G1627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 159 total ) | Next | Last >> |