 Found 221 hits with Last Name = 'madhavi' and Initial = 'yv'
Found 221 hits with Last Name = 'madhavi' and Initial = 'yv' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Enoyl-[acyl-carrier-protein] reductase [NADH] FabI
(Escherichia coli) | BDBM8726

(5-chloro-2-(2,4-dichlorophenoxy)phenol | CHEMBL849...)Show InChI InChI=1S/C12H7Cl3O2/c13-7-1-3-11(9(15)5-7)17-12-4-2-8(14)6-10(12)16/h1-6,16H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
| 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Enoyl-[acyl-carrier-protein] reductase [NADH] FabI
(Escherichia coli) | BDBM36537

(2-phenoxylphenol | PT51 | US10071965, Compound PT5...)Show InChI InChI=1S/C12H10O2/c13-11-8-4-5-9-12(11)14-10-6-2-1-3-7-10/h1-9,13H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADH] FabI
(Escherichia coli) | BDBM36538
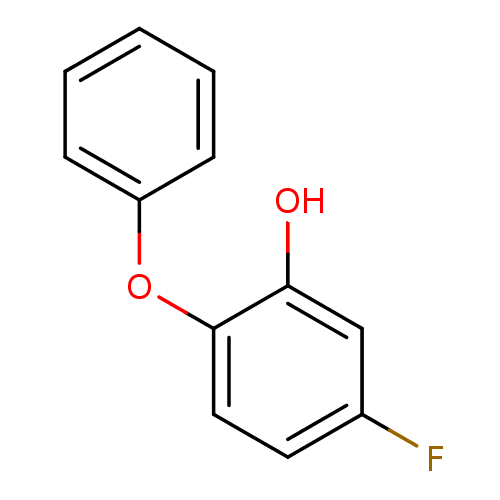
(5-fluoro-2-phenoxylphenol | PT55 | US10071965, Com...)Show InChI InChI=1S/C12H9FO2/c13-9-6-7-12(11(14)8-9)15-10-4-2-1-3-5-10/h1-8,14H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
| | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADH] FabI
(Escherichia coli) | BDBM36539
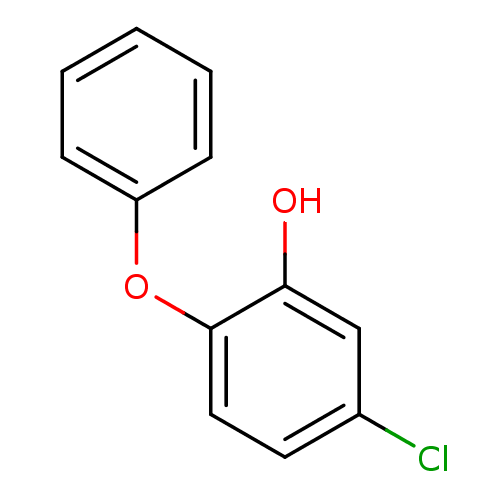
(5-chloro-2-phenoxylphenol | PT52 | US10071965, Com...)Show InChI InChI=1S/C12H9ClO2/c13-9-6-7-12(11(14)8-9)15-10-4-2-1-3-5-10/h1-8,14H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI
(Staphylococcus aureus) | BDBM8726

(5-chloro-2-(2,4-dichlorophenoxy)phenol | CHEMBL849...)Show InChI InChI=1S/C12H7Cl3O2/c13-7-1-3-11(9(15)5-7)17-12-4-2-8(14)6-10(12)16/h1-6,16H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Enoyl-[acyl-carrier-protein] reductase [NADH] FabI
(Escherichia coli) | BDBM36542
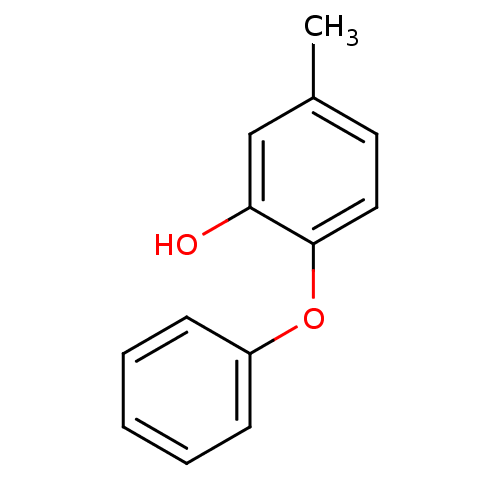
(5-methyl-2-phenoxylphenol | PT53 | US10071965, Com...)Show InChI InChI=1S/C13H12O2/c1-10-7-8-13(12(14)9-10)15-11-5-3-2-4-6-11/h2-9,14H,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADH]
(Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM8726

(5-chloro-2-(2,4-dichlorophenoxy)phenol | CHEMBL849...)Show InChI InChI=1S/C12H7Cl3O2/c13-7-1-3-11(9(15)5-7)17-12-4-2-8(14)6-10(12)16/h1-6,16H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Enoyl-[acyl-carrier-protein] reductase [NADH]
(Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50443008

(Pyridomycin)Show SMILES CC\C(C)=C1/OC(=O)[C@H](C)[C@H](O)[C@H](Cc2cccnc2)NC(=O)[C@@H](NC(=O)c2ncccc2O)[C@@H](C)OC1=O |r| Show InChI InChI=1S/C27H32N4O8/c1-5-14(2)23-27(37)38-16(4)20(31-25(35)21-19(32)9-7-11-29-21)24(34)30-18(12-17-8-6-10-28-13-17)22(33)15(3)26(36)39-23/h6-11,13,15-16,18,20,22,32-33H,5,12H2,1-4H3,(H,30,34)(H,31,35)/b23-14-/t15-,16-,18+,20+,22+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
| 4.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Enoyl-[acyl-carrier-protein] reductase [NADH]
(Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50443008

(Pyridomycin)Show SMILES CC\C(C)=C1/OC(=O)[C@H](C)[C@H](O)[C@H](Cc2cccnc2)NC(=O)[C@@H](NC(=O)c2ncccc2O)[C@@H](C)OC1=O |r| Show InChI InChI=1S/C27H32N4O8/c1-5-14(2)23-27(37)38-16(4)20(31-25(35)21-19(32)9-7-11-29-21)24(34)30-18(12-17-8-6-10-28-13-17)22(33)15(3)26(36)39-23/h6-11,13,15-16,18,20,22,32-33H,5,12H2,1-4H3,(H,30,34)(H,31,35)/b23-14-/t15-,16-,18+,20+,22+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
| 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Platelet-derived growth factor receptor alpha/beta
(Mus musculus (mouse)) | BDBM50408387
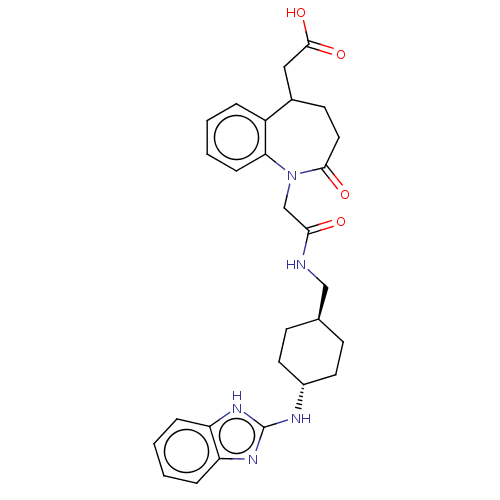
(CHEMBL5268482)Show SMILES CC(O)C(NC(C)=O)C(=O)N[C@H](Cc1cc2ccccc2n1C(C)=O)C(=O)N1C2CCCCC2CC1C(=O)N(C)Cc1ccccc1 Show InChI InChI=1S/C36H45N5O6/c1-22(42)33(37-23(2)43)34(45)38-29(20-28-18-26-14-8-10-16-30(26)40(28)24(3)44)35(46)41-31-17-11-9-15-27(31)19-32(41)36(47)39(4)21-25-12-6-5-7-13-25/h5-8,10,12-14,16,18,22,27,29,31-33,42H,9,11,15,17,19-21H2,1-4H3,(H,37,43)(H,38,45)/t22?,27?,29-,31?,32?,33?/m1/s1 | MMDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human P2X7 receptor transfected in THP1 cells assessed as inhibition of benzoyl-ATP-induced changes in plasma membrane pore fo... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Mus musculus (mouse)) | BDBM50408386
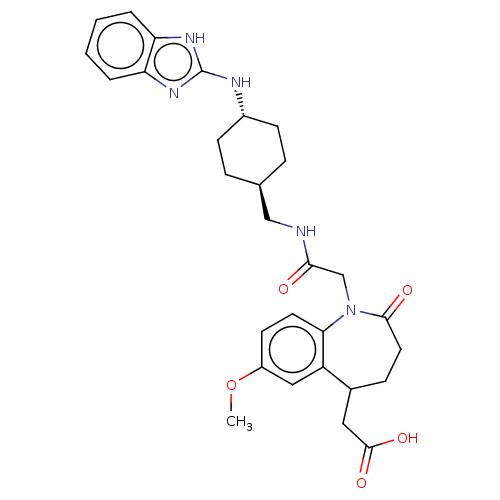
(CHEMBL5287343)Show SMILES CC(O)C(NC(C)=O)C(=O)N[C@H](Cc1cc2ccccc2n1C(C)=O)C(=O)NC(Cc1ccccc1)C(=O)N(C)C(C)c1ccccc1 Show InChI InChI=1S/C37H43N5O6/c1-23(28-16-10-7-11-17-28)41(5)37(48)32(20-27-14-8-6-9-15-27)40-35(46)31(39-36(47)34(24(2)43)38-25(3)44)22-30-21-29-18-12-13-19-33(29)42(30)26(4)45/h6-19,21,23-24,31-32,34,43H,20,22H2,1-5H3,(H,38,44)(H,39,47)(H,40,46)/t23?,24?,31-,32?,34?/m1/s1 | MMDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human P2X7 receptor transfected in THP1 cells assessed as inhibition of benzoyl-ATP-induced changes in plasma membrane pore fo... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
B2 bradykinin receptor
(Homo sapiens (Human)) | BDBM50142954
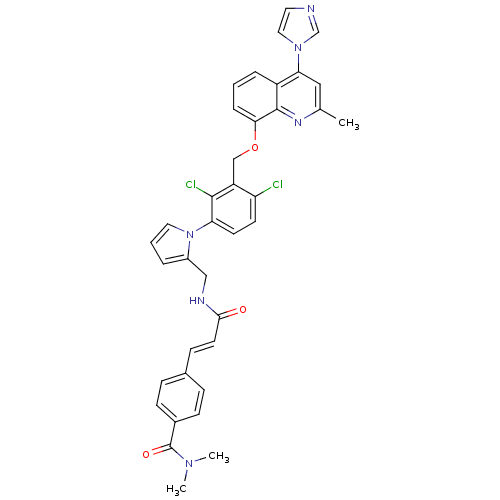
(4-[(E)-2-({1-[2,4-Dichloro-3-(4-imidazol-1-yl-2-me...)Show SMILES CN(C)C(=O)c1ccc(\C=C\C(=O)NCc2cccn2-c2ccc(Cl)c(COc3cccc4c(cc(C)nc34)-n3ccnc3)c2Cl)cc1 Show InChI InChI=1S/C37H32Cl2N6O3/c1-24-20-32(44-19-17-40-23-44)28-7-4-8-33(36(28)42-24)48-22-29-30(38)14-15-31(35(29)39)45-18-5-6-27(45)21-41-34(46)16-11-25-9-12-26(13-10-25)37(47)43(2)3/h4-20,23H,21-22H2,1-3H3,(H,41,46)/b16-11+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Displacement of [3H]bradykinin from human recombinant bradykinin B2 receptor expressed in CHO cells incubated for 2 hrs by liquid scintillation count... |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.07.064
BindingDB Entry DOI: 10.7270/Q2V69NXT |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI
(Staphylococcus aureus) | BDBM50481806
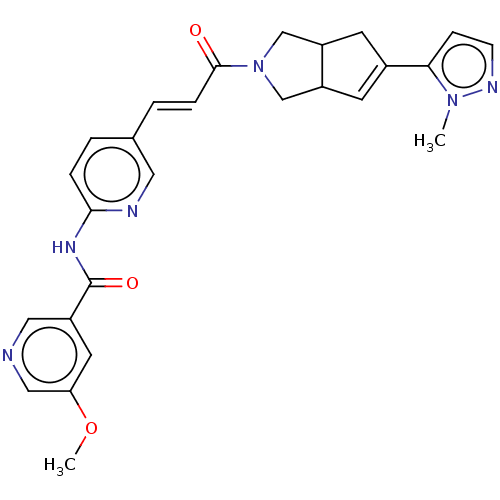
(CHEMBL5269135)Show SMILES NS(=O)(=O)C1C=CC(NC(=O)CCCN2CCOCC2)C=C1 |c:5,21,(4.09,-27.1,;3.32,-25.77,;2.92,-24.27,;4.81,-25.36,;1.78,-25.76,;1.02,-27.09,;-.52,-27.09,;-1.3,-25.76,;-2.84,-25.77,;-3.61,-24.43,;-2.84,-23.1,;-5.15,-24.44,;-5.92,-23.1,;-7.46,-23.1,;-8.23,-21.77,;-7.45,-20.44,;-8.22,-19.11,;-9.76,-19.11,;-10.53,-20.44,;-9.76,-21.78,;-.52,-24.43,;1.02,-24.43,)| Show InChI InChI=1S/C14H23N3O4S/c15-22(19,20)13-5-3-12(4-6-13)16-14(18)2-1-7-17-8-10-21-11-9-17/h3-6,12-13H,1-2,7-11H2,(H,16,18)(H2,15,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI
(Staphylococcus aureus) | BDBM50481809
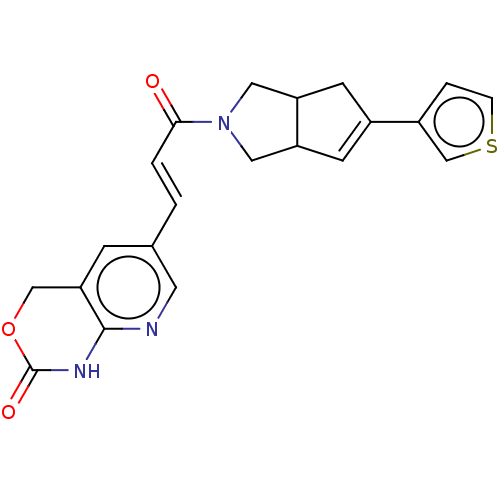
(CHEMBL5268904)Show SMILES COc1cc(\C=N\c2ccc(cc2)S(N)(=O)=O)ccc1C(C)=O Show InChI InChI=1S/C16H16N2O4S/c1-11(19)15-8-3-12(9-16(15)22-2)10-18-13-4-6-14(7-5-13)23(17,20)21/h3-10H,1-2H3,(H2,17,20,21)/b18-10+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI
(Staphylococcus aureus) | BDBM50481808
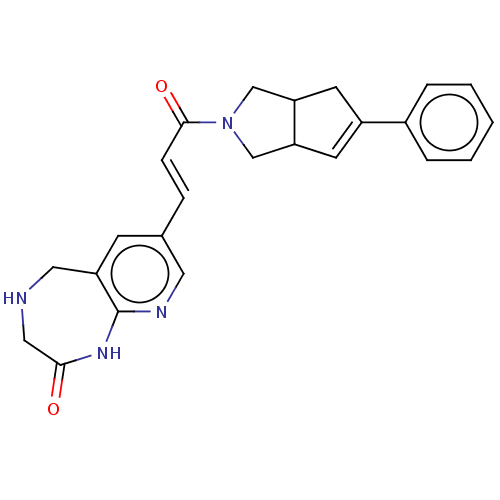
(CHEMBL5273284)Show SMILES NS(=O)(=O)c1ccc(cc1)\N=C\C1=C(Cl)CCC=C1 |c:13,18| Show InChI InChI=1S/C13H13ClN2O2S/c14-13-4-2-1-3-10(13)9-16-11-5-7-12(8-6-11)19(15,17)18/h1,3,5-9H,2,4H2,(H2,15,17,18)/b16-9+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI
(Staphylococcus aureus) | BDBM50481807
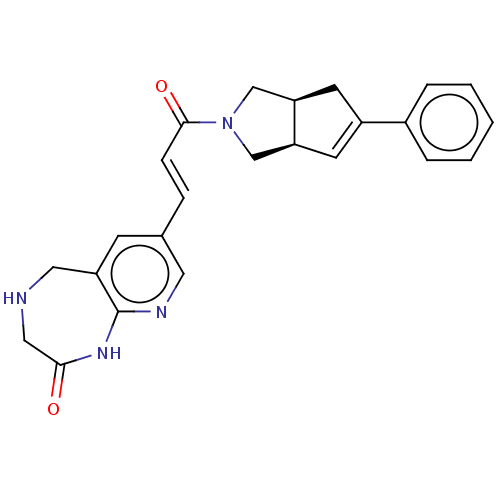
(CHEMBL5291446)Show SMILES NS(=O)(=O)C1C=CC(NC(=O)CCCCN2CCOCC2)C=C1 |c:5,22,(23.8,-27.71,;23.03,-26.38,;22.63,-24.88,;24.52,-25.97,;21.49,-26.38,;20.73,-27.7,;19.19,-27.7,;18.41,-26.38,;16.87,-26.38,;16.1,-25.05,;16.87,-23.71,;14.56,-25.05,;13.79,-23.72,;12.25,-23.72,;11.48,-22.39,;9.94,-22.39,;9.18,-21.05,;7.64,-21.05,;6.87,-22.38,;7.64,-23.72,;9.18,-23.72,;19.19,-25.04,;20.73,-25.04,)| Show InChI InChI=1S/C15H25N3O4S/c16-23(20,21)14-6-4-13(5-7-14)17-15(19)3-1-2-8-18-9-11-22-12-10-18/h4-7,13-14H,1-3,8-12H2,(H,17,19)(H2,16,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
B2 bradykinin receptor
(Cavia porcellus) | BDBM50067290

(5-{2-[({[2,4-Dichloro-3-(2-methyl-quinolin-8-yloxy...)Show SMILES CNC(=O)c1ccc(\C=C\C(=O)NCC(=O)N(C)c2ccc(Cl)c(COc3cccc4ccc(C)nc34)c2Cl)cn1 Show InChI InChI=1S/C30H27Cl2N5O4/c1-18-7-10-20-5-4-6-25(29(20)36-18)41-17-21-22(31)11-13-24(28(21)32)37(3)27(39)16-35-26(38)14-9-19-8-12-23(34-15-19)30(40)33-2/h4-15H,16-17H2,1-3H3,(H,33,40)(H,35,38)/b14-9+ | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Displacement of [3H]BK from Bradykinin B2 Receptor in guinea pig ileum by liquid scintillation counter |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.07.064
BindingDB Entry DOI: 10.7270/Q2V69NXT |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Mus musculus (mouse)) | BDBM50411638
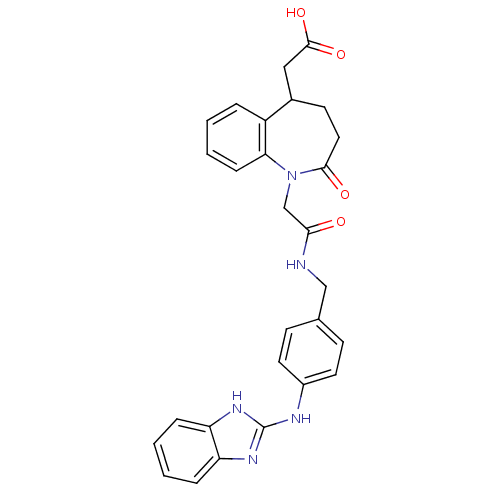
(CHEMBL404371)Show SMILES OC(=O)CC1CCC(=O)N(CC(=O)NCc2ccc(Nc3nc4ccccc4[nH]3)cc2)c2ccccc12 Show InChI InChI=1S/C28H27N5O4/c34-25(17-33-24-8-4-1-5-21(24)19(15-27(36)37)11-14-26(33)35)29-16-18-9-12-20(13-10-18)30-28-31-22-6-2-3-7-23(22)32-28/h1-10,12-13,19H,11,14-17H2,(H,29,34)(H,36,37)(H2,30,31,32) | MMDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human P2X7 receptor transfected in THP1 cells assessed as inhibition of benzoyl-ATP-induced changes in plasma membrane pore fo... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 2
(Homo sapiens (Human)) | BDBM50446130

(AG-014699 | AG-14447 | RUCAPARIB CAMSYLATE | Rucap...)Show SMILES CNCc1ccc(cc1)-c1[nH]c2cc(F)cc3C(=O)NCCc1c23 Show InChI InChI=1S/C19H18FN3O/c1-21-10-11-2-4-12(5-3-11)18-14-6-7-22-19(24)15-8-13(20)9-16(23-18)17(14)15/h2-5,8-9,21,23H,6-7,10H2,1H3,(H,22,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human P2X7 receptor transfected in THP1 cells assessed as inhibition of benzoyl-ATP-induced changes in plasma membrane pore fo... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50446130

(AG-014699 | AG-14447 | RUCAPARIB CAMSYLATE | Rucap...)Show SMILES CNCc1ccc(cc1)-c1[nH]c2cc(F)cc3C(=O)NCCc1c23 Show InChI InChI=1S/C19H18FN3O/c1-21-10-11-2-4-12(5-3-11)18-14-6-7-22-19(24)15-8-13(20)9-16(23-18)17(14)15/h2-5,8-9,21,23H,6-7,10H2,1H3,(H,22,24) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human P2X7 receptor transfected in THP1 cells assessed as inhibition of benzoyl-ATP-induced changes in plasma membrane pore fo... |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50515481

(CHEMBL4439680)Show SMILES COc1ccc(\C=C\C(=O)Nc2cc3c(Nc4ccc(F)c(Cl)c4)ncnc3cc2O[C@@H]2CCOC2)cc1 |r| Show InChI InChI=1S/C28H24ClFN4O4/c1-36-19-6-2-17(3-7-19)4-9-27(35)34-25-13-21-24(14-26(25)38-20-10-11-37-15-20)31-16-32-28(21)33-18-5-8-23(30)22(29)12-18/h2-9,12-14,16,20H,10-11,15H2,1H3,(H,34,35)(H,31,32,33)/b9-4+/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR (unknown origin) |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.07.064
BindingDB Entry DOI: 10.7270/Q2V69NXT |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50515483
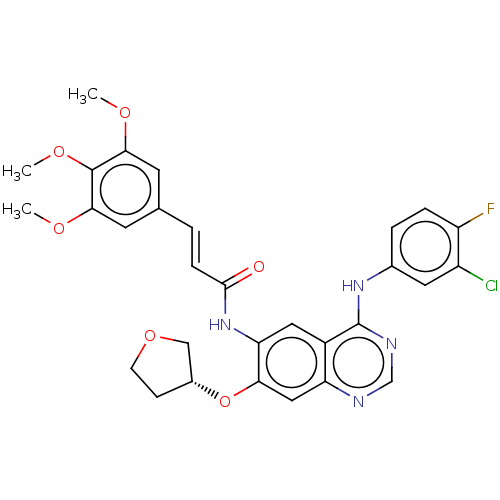
(CHEMBL4466350)Show SMILES COc1cc(\C=C\C(=O)Nc2cc3c(Nc4ccc(F)c(Cl)c4)ncnc3cc2O[C@@H]2CCOC2)cc(OC)c1OC |r| Show InChI InChI=1S/C30H28ClFN4O6/c1-38-26-10-17(11-27(39-2)29(26)40-3)4-7-28(37)36-24-13-20-23(14-25(24)42-19-8-9-41-15-19)33-16-34-30(20)35-18-5-6-22(32)21(31)12-18/h4-7,10-14,16,19H,8-9,15H2,1-3H3,(H,36,37)(H,33,34,35)/b7-4+/t19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR (unknown origin) |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.07.064
BindingDB Entry DOI: 10.7270/Q2V69NXT |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Rattus norvegicus (rat)) | BDBM7262
(14-nitro-8,18-diazatetracyclo[9.7.0.0^{2,7}.0^{12,...)Show SMILES [O-][N+](=O)c1ccc2[nH]c-3c(CC(=O)Nc4ccccc-34)c2c1 Show InChI InChI=1S/C16H11N3O3/c20-15-8-12-11-7-9(19(21)22)5-6-14(11)18-16(12)10-3-1-2-4-13(10)17-15/h1-7,18H,8H2,(H,17,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity in phenoxybenzamine-treated rat by Pressor assay |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Enoyl-[acyl-carrier-protein] reductase [NADH]
(Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50443004
(CHEMBL3088173)Show SMILES Cc1csc(n1)C(C)(O)c1nnc(Nc2ccn(Cc3c(F)cccc3F)n2)s1 Show InChI InChI=1S/C18H16F2N6OS2/c1-10-9-28-15(21-10)18(2,27)16-23-24-17(29-16)22-14-6-7-26(25-14)8-11-12(19)4-3-5-13(11)20/h3-7,9,27H,8H2,1-2H3,(H,22,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM7262

(14-nitro-8,18-diazatetracyclo[9.7.0.0^{2,7}.0^{12,...)Show SMILES [O-][N+](=O)c1ccc2[nH]c-3c(CC(=O)Nc4ccccc-34)c2c1 Show InChI InChI=1S/C16H11N3O3/c20-15-8-12-11-7-9(19(21)22)5-6-14(11)18-16(12)10-3-1-2-4-13(10)17-15/h1-7,18H,8H2,(H,17,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity in rat uterus by uterotonic assay |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM7262

(14-nitro-8,18-diazatetracyclo[9.7.0.0^{2,7}.0^{12,...)Show SMILES [O-][N+](=O)c1ccc2[nH]c-3c(CC(=O)Nc4ccccc-34)c2c1 Show InChI InChI=1S/C16H11N3O3/c20-15-8-12-11-7-9(19(21)22)5-6-14(11)18-16(12)10-3-1-2-4-13(10)17-15/h1-7,18H,8H2,(H,17,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist potency at human bradykinin B2 receptor assessed as effect on inositol monophosphate accumulation in CHOdhfr- cells |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADH]
(Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50481812
(CHEMBL5284077)Show InChI InChI=1S/C14H14N2O3S/c15-20(18,19)13-7-5-11(6-8-13)9-16-10-12-3-1-2-4-14(12)17/h1-8,10,17H,9H2,(H2,15,18,19)/b16-10+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM50259155
((Z)-Hymenialdisine | Hymenialdisine)Show SMILES NC1=NC(=O)\C(N1)=C1/CCNC(=O)c2[nH]c(Br)cc12 |t:1| Show InChI InChI=1S/C11H10BrN5O2/c12-6-3-5-4(7-10(19)17-11(13)16-7)1-2-14-9(18)8(5)15-6/h3,15H,1-2H2,(H,14,18)(H3,13,16,17,19)/b7-4- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist potency on BK-induced contraction of guinea pig ileum longitudinal smooth muscle |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk2
(Homo sapiens (Human)) | BDBM50256044
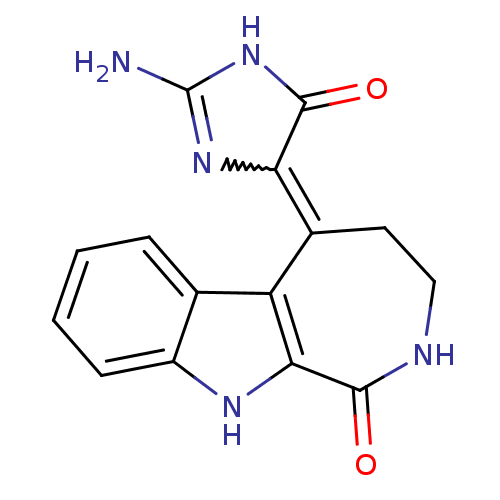
(5-(2-amino-4-oxo-1H-imidazol-5(4H)-ylidene)-2,3,4,...)Show SMILES NC1=NC(C(=O)N1)=C1CCNC(=O)c2[nH]c3ccccc3c12 |w:3.2,t:1| Show InChI InChI=1S/C15H13N5O2/c16-15-19-11(14(22)20-15)8-5-6-17-13(21)12-10(8)7-3-1-2-4-9(7)18-12/h1-4,18H,5-6H2,(H,17,21)(H3,16,19,20,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity in rat uterus by uterotonic assay |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50515483

(CHEMBL4466350)Show SMILES COc1cc(\C=C\C(=O)Nc2cc3c(Nc4ccc(F)c(Cl)c4)ncnc3cc2O[C@@H]2CCOC2)cc(OC)c1OC |r| Show InChI InChI=1S/C30H28ClFN4O6/c1-38-26-10-17(11-27(39-2)29(26)40-3)4-7-28(37)36-24-13-20-23(14-25(24)42-19-8-9-41-15-19)33-16-34-30(20)35-18-5-6-22(32)21(31)12-18/h4-7,10-14,16,19H,8-9,15H2,1-3H3,(H,36,37)(H,33,34,35)/b7-4+/t19-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of EGFR (unknown origin) |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.07.064
BindingDB Entry DOI: 10.7270/Q2V69NXT |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50515481

(CHEMBL4439680)Show SMILES COc1ccc(\C=C\C(=O)Nc2cc3c(Nc4ccc(F)c(Cl)c4)ncnc3cc2O[C@@H]2CCOC2)cc1 |r| Show InChI InChI=1S/C28H24ClFN4O4/c1-36-19-6-2-17(3-7-19)4-9-27(35)34-25-13-21-24(14-26(25)38-20-10-11-37-15-20)31-16-32-28(21)33-18-5-8-23(30)22(29)12-18/h2-9,12-14,16,20H,10-11,15H2,1H3,(H,34,35)(H,31,32,33)/b9-4+/t20-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of EGFR (unknown origin) |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.07.064
BindingDB Entry DOI: 10.7270/Q2V69NXT |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM7266

(14-bromo-8,18-diazatetracyclo[9.7.0.0^{2,7}.0^{12,...)Show InChI InChI=1S/C16H11BrN2O/c17-9-5-6-14-11(7-9)12-8-15(20)18-13-4-2-1-3-10(13)16(12)19-14/h1-7,19H,8H2,(H,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist potency at human bradykinin B2 receptor assessed as effect on inositol monophosphate accumulation in CHOdhfr- cells |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Rattus norvegicus (rat)) | BDBM50259155

((Z)-Hymenialdisine | Hymenialdisine)Show SMILES NC1=NC(=O)\C(N1)=C1/CCNC(=O)c2[nH]c(Br)cc12 |t:1| Show InChI InChI=1S/C11H10BrN5O2/c12-6-3-5-4(7-10(19)17-11(13)16-7)1-2-14-9(18)8(5)15-6/h3,15H,1-2H2,(H,14,18)(H3,13,16,17,19)/b7-4- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity in phenoxybenzamine-treated rat by Pressor assay |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI
(Staphylococcus aureus) | BDBM50399407
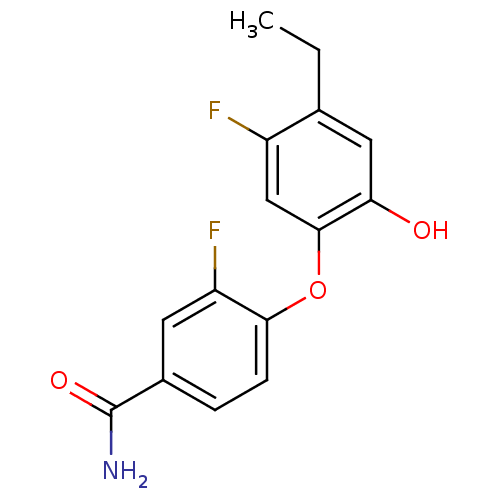
(CHEMBL2178284 | MUT056399)Show InChI InChI=1S/C15H13F2NO3/c1-2-8-6-12(19)14(7-10(8)16)21-13-4-3-9(15(18)20)5-11(13)17/h3-7,19H,2H2,1H3,(H2,18,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI
(Staphylococcus aureus) | BDBM50399407

(CHEMBL2178284 | MUT056399)Show InChI InChI=1S/C15H13F2NO3/c1-2-8-6-12(19)14(7-10(8)16)21-13-4-3-9(15(18)20)5-11(13)17/h3-7,19H,2H2,1H3,(H2,18,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Mus musculus (mouse)) | BDBM50408185
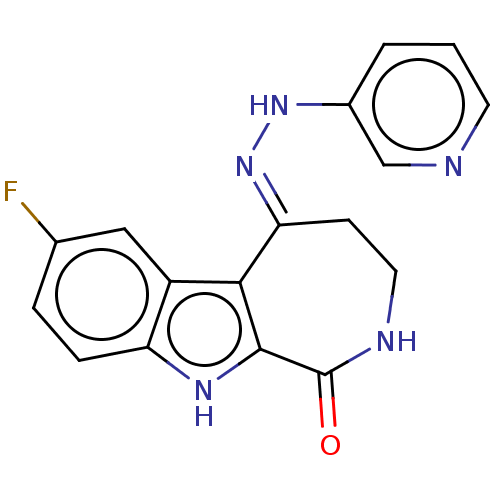
(CHEMBL5265856)Show InChI InChI=1S/C15H15N5OS/c16-14(21)11-3-1-10(2-4-11)13-8-18-15(20-13)22-6-5-12-7-17-9-19-12/h1-4,7-9H,5-6H2,(H2,16,21)(H,17,19)(H,18,20) | MMDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at rat UT receptor |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Mus musculus (mouse)) | BDBM50408186
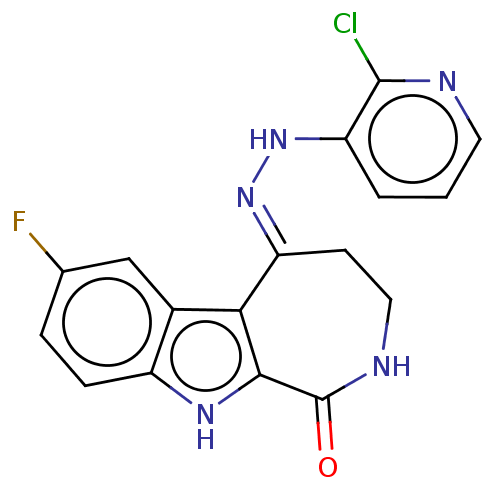
(CHEMBL5272006)Show SMILES [O-][N+](=O)c1ccc(cc1)-c1c[nH]c(SCCc2cnc[nH]2)n1 Show InChI InChI=1S/C14H13N5O2S/c20-19(21)12-3-1-10(2-4-12)13-8-16-14(18-13)22-6-5-11-7-15-9-17-11/h1-4,7-9H,5-6H2,(H,15,17)(H,16,18) | MMDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at rat UT receptor |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI
(Staphylococcus aureus) | BDBM50052244

(CHEMBL1652621)Show SMILES CN(Cc1oc2ccccc2c1C)C(=O)\C=C\c1cnc2NC(=O)CCc2c1 Show InChI InChI=1S/C22H21N3O3/c1-14-17-5-3-4-6-18(17)28-19(14)13-25(2)21(27)10-7-15-11-16-8-9-20(26)24-22(16)23-12-15/h3-7,10-12H,8-9,13H2,1-2H3,(H,23,24,26)/b10-7+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50256003
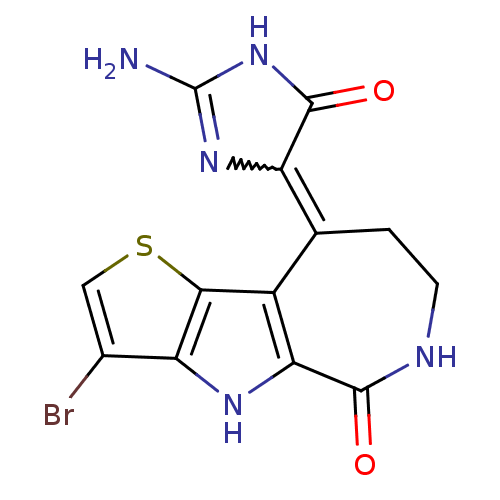
(4-[2-Amino-5-oxo-3,5-dihydro-imidazol-(4)-ylidene]...)Show SMILES NC1=NC(C(=O)N1)=C1CCNC(=O)c2[nH]c3c(Br)csc3c12 |w:3.2,t:1| Show InChI InChI=1S/C13H10BrN5O2S/c14-5-3-22-10-6-4(7-12(21)19-13(15)18-7)1-2-16-11(20)9(6)17-8(5)10/h3,17H,1-2H2,(H,16,20)(H3,15,18,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity in phenoxybenzamine-treated rat by Pressor assay |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk2
(Homo sapiens (Human)) | BDBM50363648
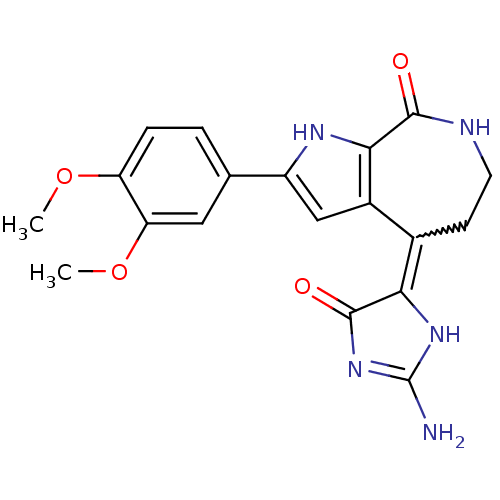
(CHEMBL1947252)Show SMILES COc1ccc(cc1OC)-c1cc2c([nH]1)C(=O)NCCC2=C1NC(N)=NC1=O |w:20.21,c:27| Show InChI InChI=1S/C19H19N5O4/c1-27-13-4-3-9(7-14(13)28-2)12-8-11-10(15-18(26)24-19(20)23-15)5-6-21-17(25)16(11)22-12/h3-4,7-8,22H,5-6H2,1-2H3,(H,21,25)(H3,20,23,24,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity in phenoxybenzamine-treated rat by Pressor assay |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Mus musculus (mouse)) | BDBM7262

(14-nitro-8,18-diazatetracyclo[9.7.0.0^{2,7}.0^{12,...)Show SMILES [O-][N+](=O)c1ccc2[nH]c-3c(CC(=O)Nc4ccccc-34)c2c1 Show InChI InChI=1S/C16H11N3O3/c20-15-8-12-11-7-9(19(21)22)5-6-14(11)18-16(12)10-3-1-2-4-13(10)17-15/h1-7,18H,8H2,(H,17,20) | MMDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Activity at human B2 receptor transfected in CHO cells assessed as ability to antagonize BK-induced inositol-phosphate accumulation |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Rattus norvegicus (rat)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity in rat uterus by uterotonic assay |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase Chk2
(Homo sapiens (Human)) | BDBM50363646

(CHEMBL1947250)Show SMILES NC1=NC(=O)C(N1)=C1CCNC(=O)c2[nH]c(cc12)-c1ccccc1 |w:7.8,t:1| Show InChI InChI=1S/C17H15N5O2/c18-17-21-13(16(24)22-17)10-6-7-19-15(23)14-11(10)8-12(20-14)9-4-2-1-3-5-9/h1-5,8,20H,6-7H2,(H,19,23)(H3,18,21,22,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity in rat uterus by uterotonic assay |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 3
(Homo sapiens (Human)) | BDBM7266

(14-bromo-8,18-diazatetracyclo[9.7.0.0^{2,7}.0^{12,...)Show InChI InChI=1S/C16H11BrN2O/c17-9-5-6-14-11(7-9)12-8-15(20)18-13-4-2-1-3-10(13)16(12)19-14/h1-7,19H,8H2,(H,18,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist potency at human bradykinin B2 receptor assessed as effect on inositol monophosphate accumulation in CHOdhfr- cells |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 3
(Homo sapiens (Human)) | BDBM7262

(14-nitro-8,18-diazatetracyclo[9.7.0.0^{2,7}.0^{12,...)Show SMILES [O-][N+](=O)c1ccc2[nH]c-3c(CC(=O)Nc4ccccc-34)c2c1 Show InChI InChI=1S/C16H11N3O3/c20-15-8-12-11-7-9(19(21)22)5-6-14(11)18-16(12)10-3-1-2-4-13(10)17-15/h1-7,18H,8H2,(H,17,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist potency at human bradykinin B2 receptor assessed as effect on inositol monophosphate accumulation in CHOdhfr- cells |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Rattus norvegicus (rat)) | BDBM50166289
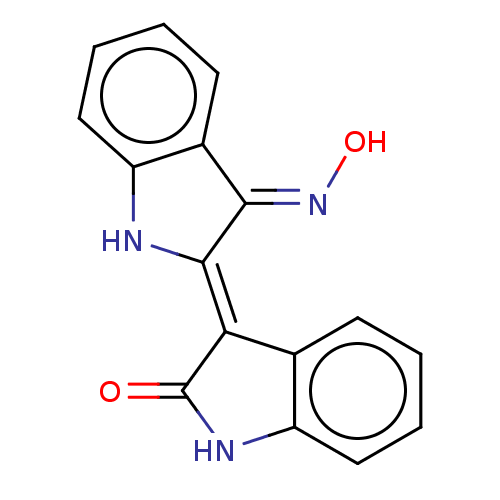
(CHEBI:43645 | CHEMBL216543)Show InChI InChI=1S/C16H11N3O2/c20-16-13(9-5-1-3-7-11(9)18-16)15-14(19-21)10-6-2-4-8-12(10)17-15/h1-8,17,21H,(H,18,20)/b15-13-,19-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity in phenoxybenzamine-treated rat by Pressor assay |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Platelet-derived growth factor receptor alpha/beta
(Mus musculus (mouse)) | BDBM50259155

((Z)-Hymenialdisine | Hymenialdisine)Show SMILES NC1=NC(=O)\C(N1)=C1/CCNC(=O)c2[nH]c(Br)cc12 |t:1| Show InChI InChI=1S/C11H10BrN5O2/c12-6-3-5-4(7-10(19)17-11(13)16-7)1-2-14-9(18)8(5)15-6/h3,15H,1-2H2,(H,14,18)(H3,13,16,17,19)/b7-4- | MMDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist effect against 2-methyl-5-HT activity at 5-HT3 receptor in longitudinal muscle myenteric plexus from guinea pig ileum |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Rattus norvegicus (rat)) | BDBM7266

(14-bromo-8,18-diazatetracyclo[9.7.0.0^{2,7}.0^{12,...)Show InChI InChI=1S/C16H11BrN2O/c17-9-5-6-14-11(7-9)12-8-15(20)18-13-4-2-1-3-10(13)16(12)19-14/h1-7,19H,8H2,(H,18,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity in rat uterus by uterotonic assay |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Mus musculus (mouse)) | BDBM7263

(9-cyanopaullone | 9-oxo-8,18-diazatetracyclo[9.7.0...)Show InChI InChI=1S/C17H11N3O/c18-9-10-5-6-15-12(7-10)13-8-16(21)19-14-4-2-1-3-11(14)17(13)20-15/h1-7,20H,8H2,(H,19,21) | MMDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human M3 receptor assessed as inhibition of carbachol binding |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Mus musculus (mouse)) | BDBM7263

(9-cyanopaullone | 9-oxo-8,18-diazatetracyclo[9.7.0...)Show InChI InChI=1S/C17H11N3O/c18-9-10-5-6-15-12(7-10)13-8-16(21)19-14-4-2-1-3-11(14)17(13)20-15/h1-7,20H,8H2,(H,19,21) | MMDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Activity at human B2 receptor transfected in CHO cells assessed as ability to antagonize BK-induced inositol-phosphate accumulation |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data