

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50584094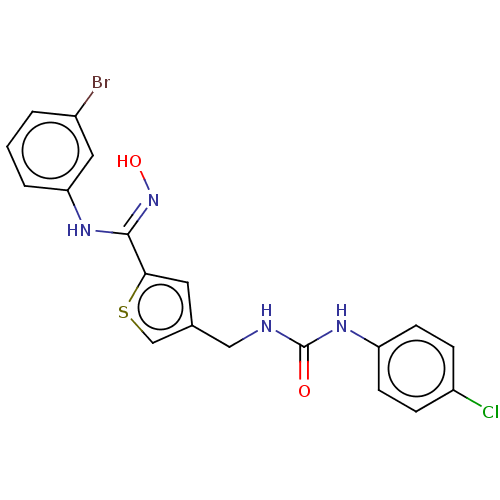 (CHEMBL5077002) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human IDO1 protein assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate by methylene blue reagent base... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.114043 BindingDB Entry DOI: 10.7270/Q2SX6J39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50584075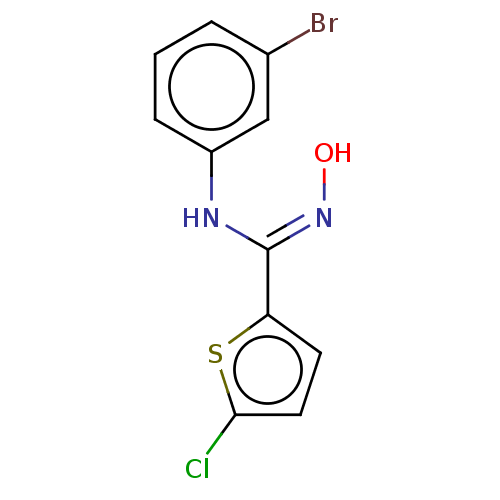 (CHEMBL5076745) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human IDO1 protein assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate by methylene blue reagent base... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.114043 BindingDB Entry DOI: 10.7270/Q2SX6J39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50584093 (CHEMBL5087302) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human IDO1 protein assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate by methylene blue reagent base... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.114043 BindingDB Entry DOI: 10.7270/Q2SX6J39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50584087 (CHEMBL5093619) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human IDO1 protein assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate by methylene blue reagent base... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.114043 BindingDB Entry DOI: 10.7270/Q2SX6J39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50584085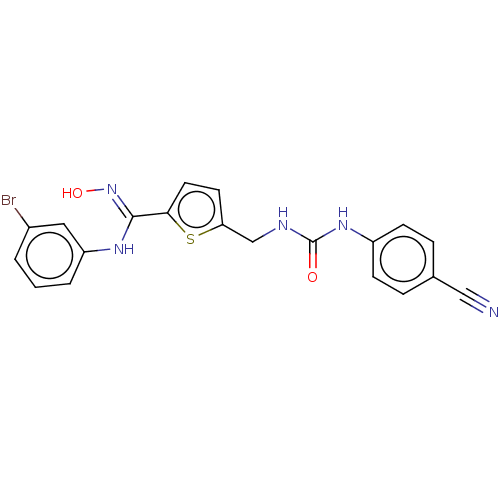 (CHEMBL5074542) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human IDO1 protein assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate by methylene blue reagent base... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.114043 BindingDB Entry DOI: 10.7270/Q2SX6J39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50584073 (CHEMBL5081219) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human IDO1 protein assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate by methylene blue reagent base... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.114043 BindingDB Entry DOI: 10.7270/Q2SX6J39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50584074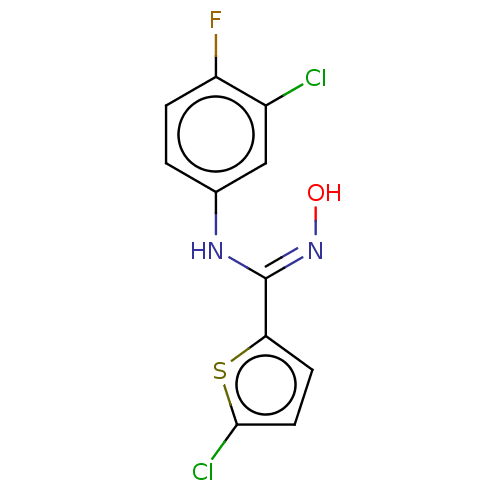 (CHEMBL5075428) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human IDO1 protein assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate by methylene blue reagent base... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.114043 BindingDB Entry DOI: 10.7270/Q2SX6J39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50584076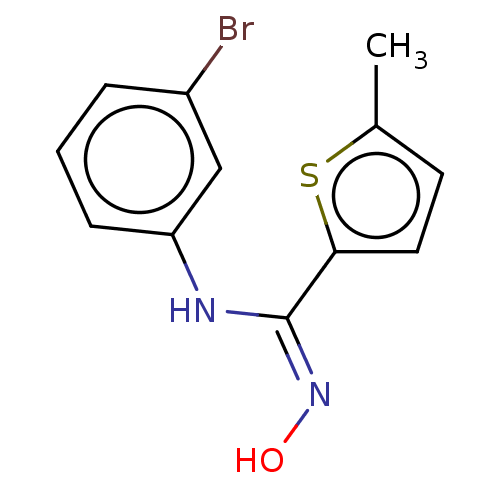 (CHEMBL5083059) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human IDO1 protein assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate by methylene blue reagent base... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.114043 BindingDB Entry DOI: 10.7270/Q2SX6J39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50584086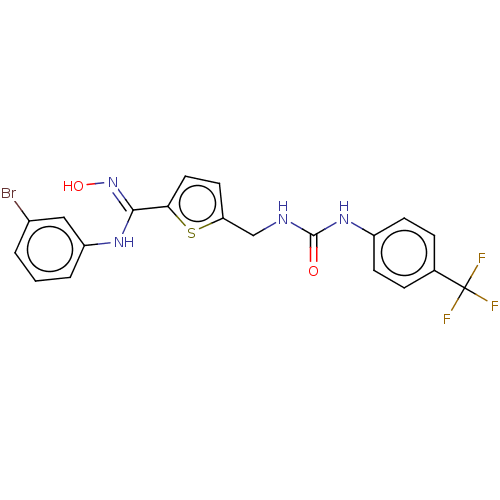 (CHEMBL5094947) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human IDO1 protein assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate by methylene blue reagent base... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.114043 BindingDB Entry DOI: 10.7270/Q2SX6J39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50584077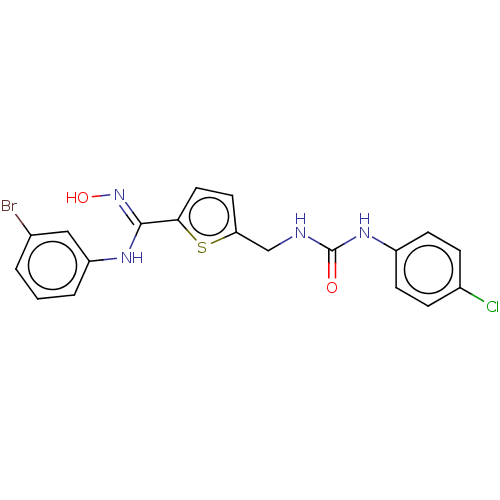 (CHEMBL5091205) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human IDO1 protein assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate by methylene blue reagent base... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.114043 BindingDB Entry DOI: 10.7270/Q2SX6J39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50584084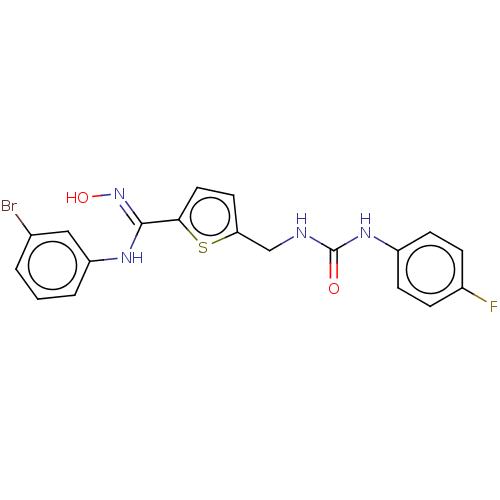 (CHEMBL5080101) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human IDO1 protein assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate by methylene blue reagent base... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.114043 BindingDB Entry DOI: 10.7270/Q2SX6J39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50584090 (CHEMBL5094353) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 103 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human IDO1 protein assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate by methylene blue reagent base... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.114043 BindingDB Entry DOI: 10.7270/Q2SX6J39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50584078 (CHEMBL5093621) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 106 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human IDO1 protein assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate by methylene blue reagent base... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.114043 BindingDB Entry DOI: 10.7270/Q2SX6J39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50584080 (CHEMBL5078610) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 106 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human IDO1 protein assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate by methylene blue reagent base... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.114043 BindingDB Entry DOI: 10.7270/Q2SX6J39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50584081 (CHEMBL5070676) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 128 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human IDO1 protein assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate by methylene blue reagent base... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.114043 BindingDB Entry DOI: 10.7270/Q2SX6J39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50584089 (CHEMBL5078852) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 129 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human IDO1 protein assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate by methylene blue reagent base... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.114043 BindingDB Entry DOI: 10.7270/Q2SX6J39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50584079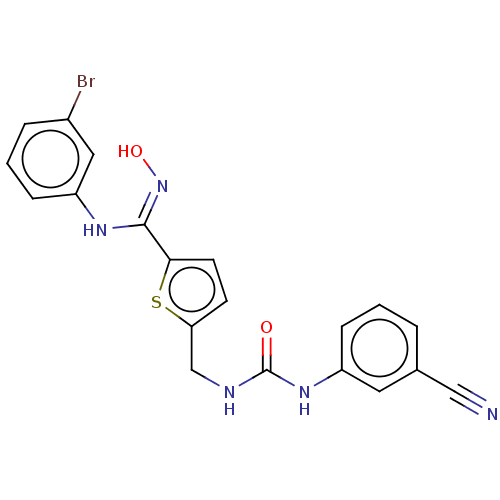 (CHEMBL5080294) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 138 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human IDO1 protein assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate by methylene blue reagent base... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.114043 BindingDB Entry DOI: 10.7270/Q2SX6J39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50584082 (CHEMBL5094486) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 148 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human IDO1 protein assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate by methylene blue reagent base... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.114043 BindingDB Entry DOI: 10.7270/Q2SX6J39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50584091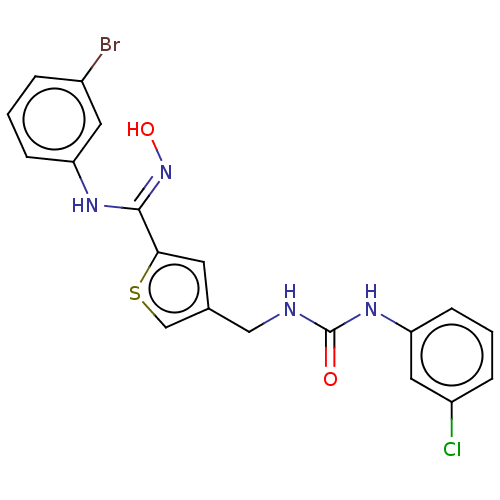 (CHEMBL5087813) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 159 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human IDO1 protein assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate by methylene blue reagent base... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.114043 BindingDB Entry DOI: 10.7270/Q2SX6J39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50584088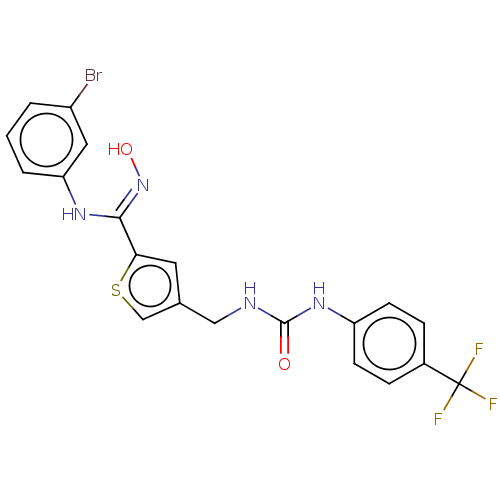 (CHEMBL5082408) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 162 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human IDO1 protein assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate by methylene blue reagent base... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.114043 BindingDB Entry DOI: 10.7270/Q2SX6J39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50584072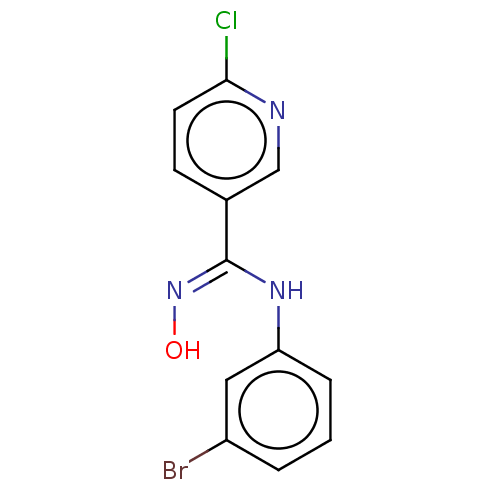 (CHEMBL5071322) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 172 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human IDO1 protein assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate by methylene blue reagent base... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.114043 BindingDB Entry DOI: 10.7270/Q2SX6J39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50584092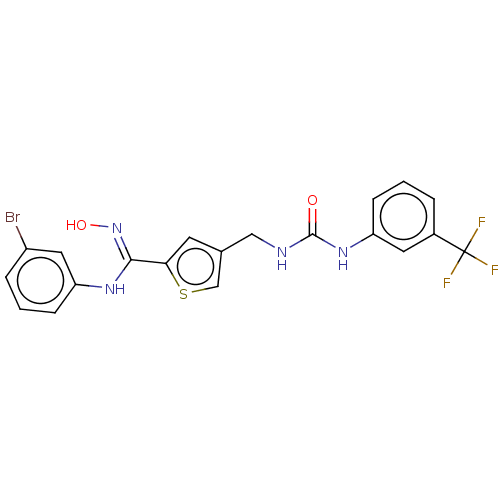 (CHEMBL5089919) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human IDO1 protein assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate by methylene blue reagent base... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.114043 BindingDB Entry DOI: 10.7270/Q2SX6J39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50584083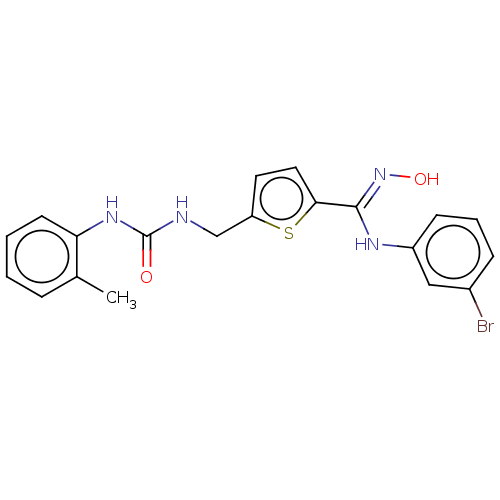 (CHEMBL5078069) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human IDO1 protein assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate by methylene blue reagent base... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.114043 BindingDB Entry DOI: 10.7270/Q2SX6J39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50584071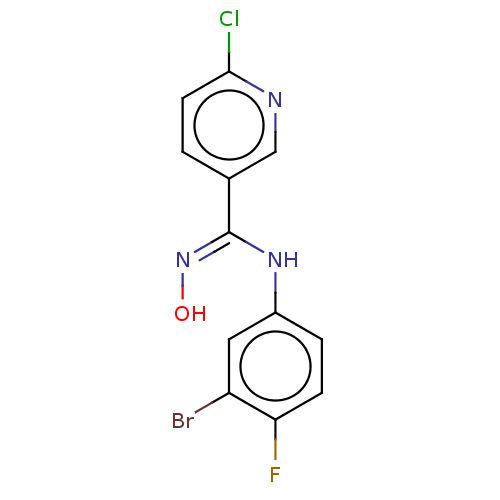 (CHEMBL5074528) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 742 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human IDO1 protein assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate by methylene blue reagent base... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.114043 BindingDB Entry DOI: 10.7270/Q2SX6J39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50584069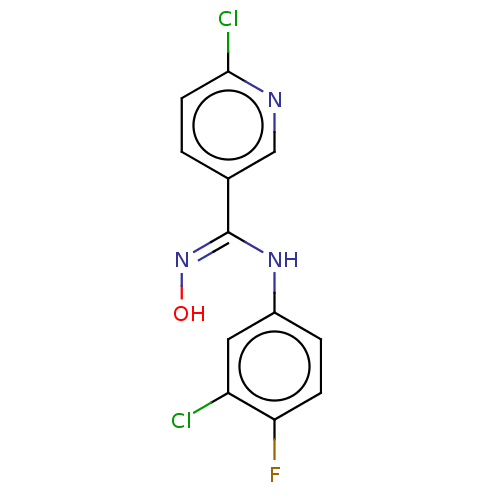 (CHEMBL5074663) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human IDO1 protein assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate by methylene blue reagent base... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.114043 BindingDB Entry DOI: 10.7270/Q2SX6J39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50584072 (CHEMBL5071322) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human TDO2 protein assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate incubated for 1 hr... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.114043 BindingDB Entry DOI: 10.7270/Q2SX6J39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50584070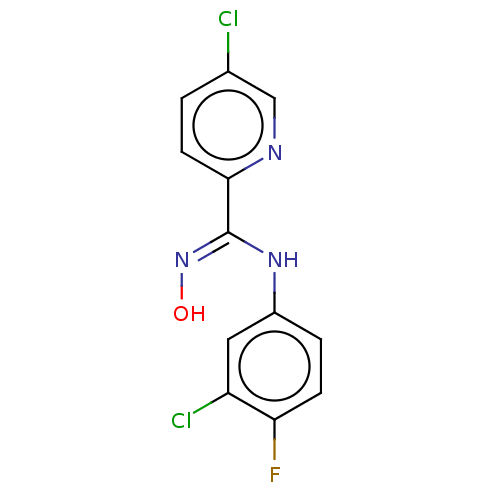 (CHEMBL5080559) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human IDO1 protein assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate by methylene blue reagent base... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.114043 BindingDB Entry DOI: 10.7270/Q2SX6J39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50584073 (CHEMBL5081219) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human TDO2 protein assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate incubated for 1 hr... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.114043 BindingDB Entry DOI: 10.7270/Q2SX6J39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50584075 (CHEMBL5076745) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human TDO2 protein assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate incubated for 1 hr... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.114043 BindingDB Entry DOI: 10.7270/Q2SX6J39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50584093 (CHEMBL5087302) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human TDO2 protein assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate incubated for 1 hr... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.114043 BindingDB Entry DOI: 10.7270/Q2SX6J39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50584076 (CHEMBL5083059) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human TDO2 protein assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate incubated for 1 hr... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.114043 BindingDB Entry DOI: 10.7270/Q2SX6J39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50523205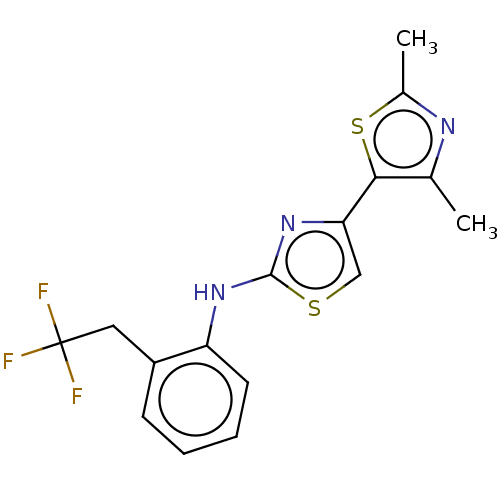 (CHEMBL4463839) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHOK1 cells coexpressing Galpha15 assessed as increase in intracellular calcium flux in pre... | Eur J Med Chem 167: 312-323 (2019) Article DOI: 10.1016/j.ejmech.2019.01.063 BindingDB Entry DOI: 10.7270/Q22B92FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50523206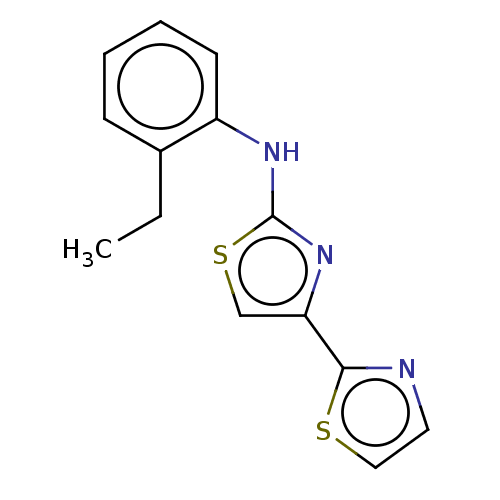 (CHEMBL4558860) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHOK1 cells coexpressing Galpha15 assessed as increase in intracellular calcium flux in pre... | Eur J Med Chem 167: 312-323 (2019) Article DOI: 10.1016/j.ejmech.2019.01.063 BindingDB Entry DOI: 10.7270/Q22B92FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50523207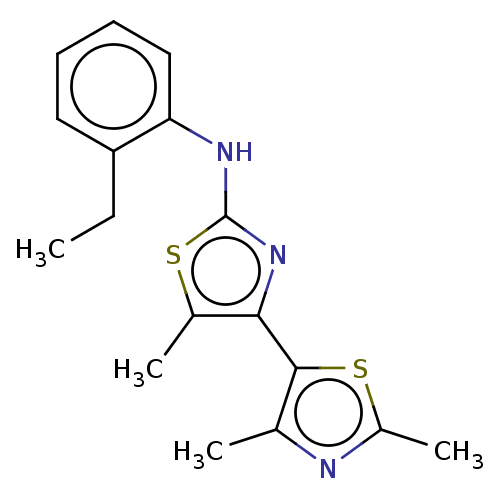 (CHEMBL4445379) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHOK1 cells coexpressing Galpha15 assessed as increase in intracellular calcium flux in pre... | Eur J Med Chem 167: 312-323 (2019) Article DOI: 10.1016/j.ejmech.2019.01.063 BindingDB Entry DOI: 10.7270/Q22B92FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50523208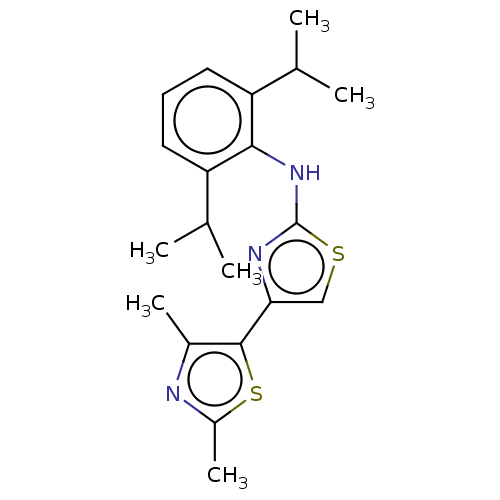 (CHEMBL4435948) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHOK1 cells coexpressing Galpha15 assessed as increase in intracellular calcium flux in pre... | Eur J Med Chem 167: 312-323 (2019) Article DOI: 10.1016/j.ejmech.2019.01.063 BindingDB Entry DOI: 10.7270/Q22B92FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50523209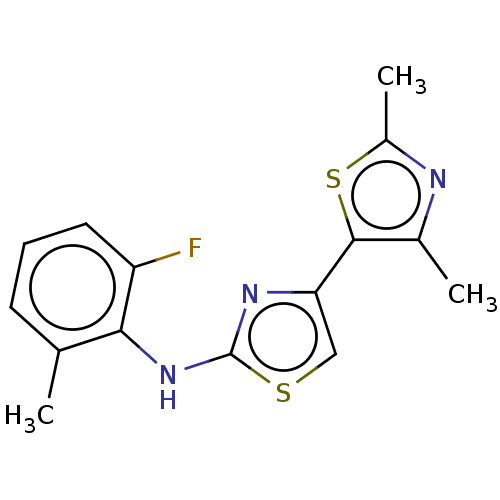 (CHEMBL4455986) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHOK1 cells coexpressing Galpha15 assessed as increase in intracellular calcium flux in pre... | Eur J Med Chem 167: 312-323 (2019) Article DOI: 10.1016/j.ejmech.2019.01.063 BindingDB Entry DOI: 10.7270/Q22B92FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50523210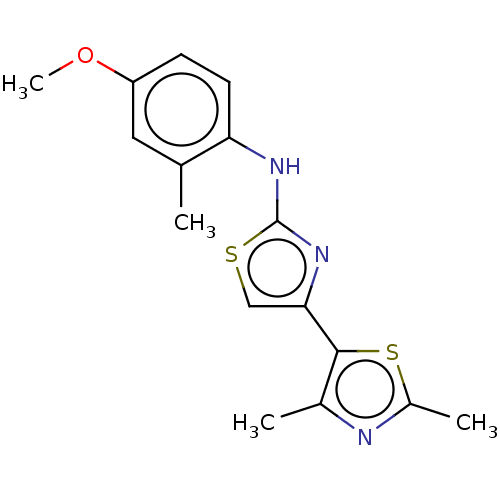 (CHEMBL4454409) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHOK1 cells coexpressing Galpha15 assessed as increase in intracellular calcium flux in pre... | Eur J Med Chem 167: 312-323 (2019) Article DOI: 10.1016/j.ejmech.2019.01.063 BindingDB Entry DOI: 10.7270/Q22B92FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50523211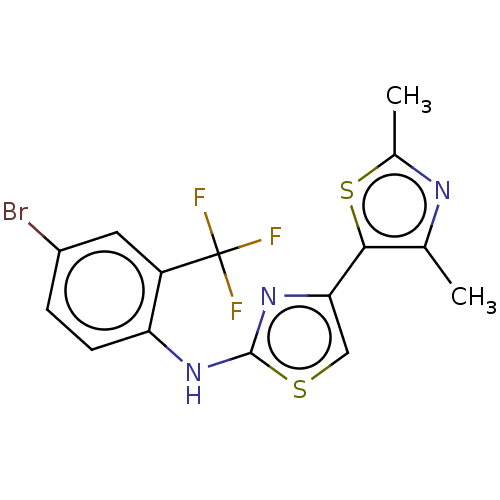 (CHEMBL4472177) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.38E+4 | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHOK1 cells coexpressing Galpha15 assessed as increase in intracellular calcium flux in pre... | Eur J Med Chem 167: 312-323 (2019) Article DOI: 10.1016/j.ejmech.2019.01.063 BindingDB Entry DOI: 10.7270/Q22B92FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50523187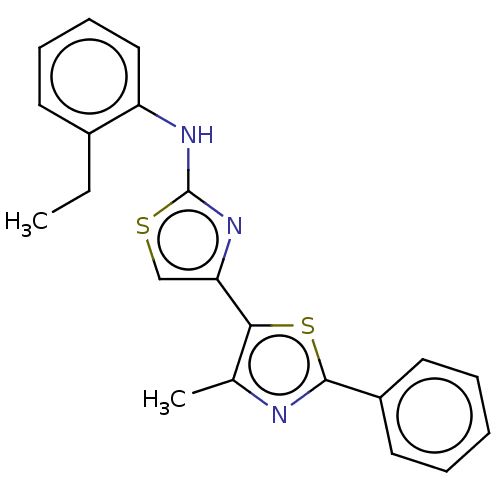 (CHEMBL4582748) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHOK1 cells coexpressing Galpha15 assessed as increase in intracellular calcium flux in pre... | Eur J Med Chem 167: 312-323 (2019) Article DOI: 10.1016/j.ejmech.2019.01.063 BindingDB Entry DOI: 10.7270/Q22B92FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50523188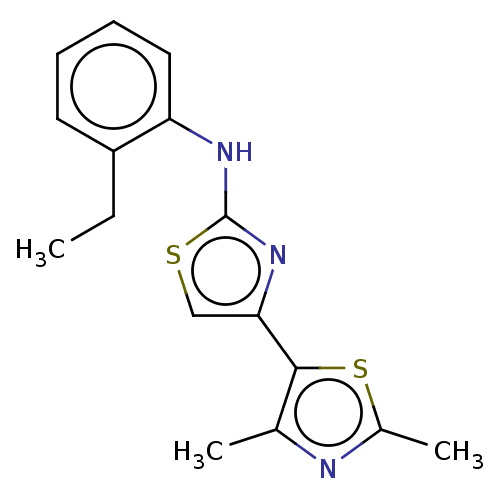 (CHEMBL4457219) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHOK1 cells coexpressing Galpha15 assessed as increase in intracellular calcium flux in pre... | Eur J Med Chem 167: 312-323 (2019) Article DOI: 10.1016/j.ejmech.2019.01.063 BindingDB Entry DOI: 10.7270/Q22B92FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50523189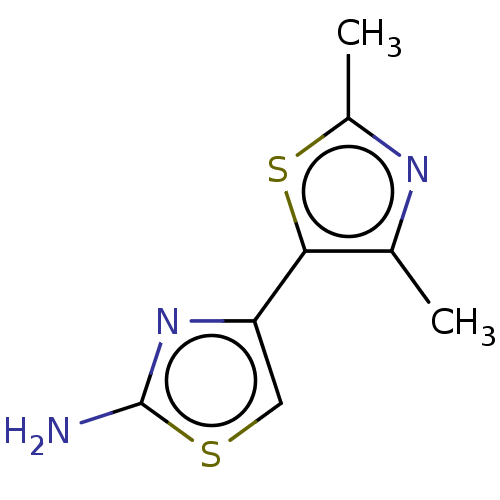 (CHEMBL1528675) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHOK1 cells coexpressing Galpha15 assessed as increase in intracellular calcium flux in pre... | Eur J Med Chem 167: 312-323 (2019) Article DOI: 10.1016/j.ejmech.2019.01.063 BindingDB Entry DOI: 10.7270/Q22B92FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50523190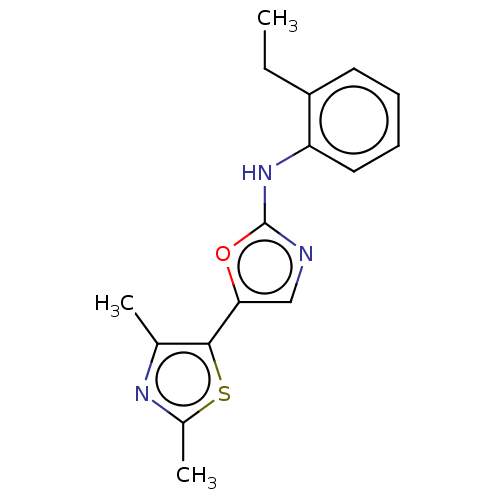 (CHEMBL4587559) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.33E+4 | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHOK1 cells coexpressing Galpha15 assessed as increase in intracellular calcium flux in pre... | Eur J Med Chem 167: 312-323 (2019) Article DOI: 10.1016/j.ejmech.2019.01.063 BindingDB Entry DOI: 10.7270/Q22B92FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50523191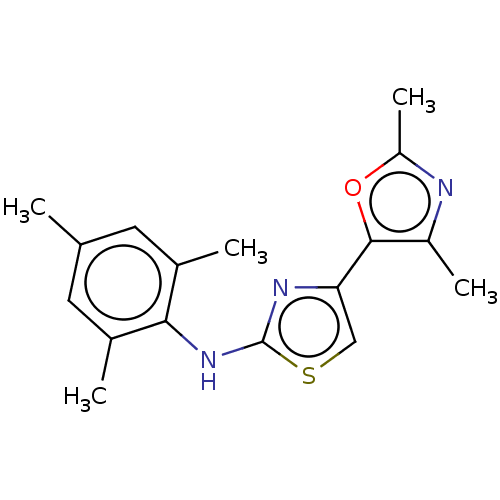 (CHEMBL4541902) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHOK1 cells coexpressing Galpha15 assessed as increase in intracellular calcium flux in pre... | Eur J Med Chem 167: 312-323 (2019) Article DOI: 10.1016/j.ejmech.2019.01.063 BindingDB Entry DOI: 10.7270/Q22B92FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50523192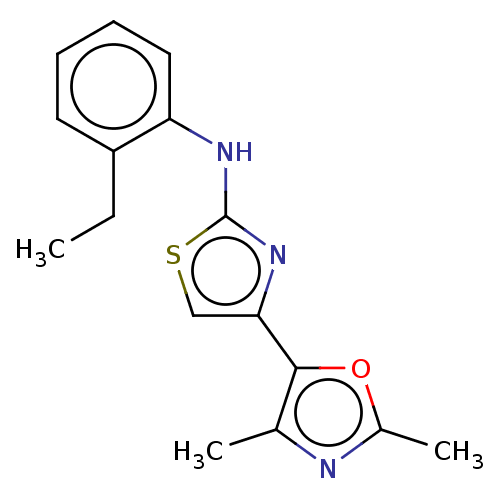 (CHEMBL4518522) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHOK1 cells coexpressing Galpha15 assessed as increase in intracellular calcium flux in pre... | Eur J Med Chem 167: 312-323 (2019) Article DOI: 10.1016/j.ejmech.2019.01.063 BindingDB Entry DOI: 10.7270/Q22B92FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50523193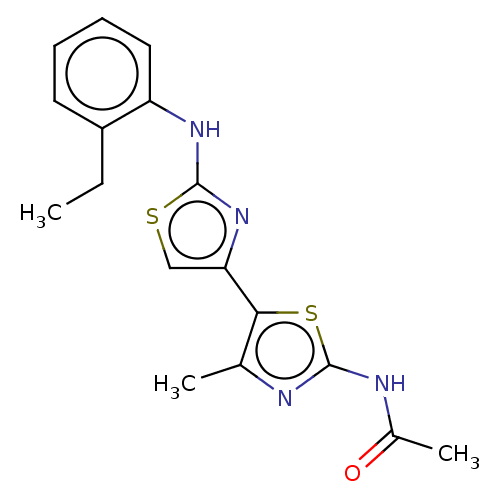 (CHEMBL4569902) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHOK1 cells coexpressing Galpha15 assessed as increase in intracellular calcium flux in pre... | Eur J Med Chem 167: 312-323 (2019) Article DOI: 10.1016/j.ejmech.2019.01.063 BindingDB Entry DOI: 10.7270/Q22B92FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50523194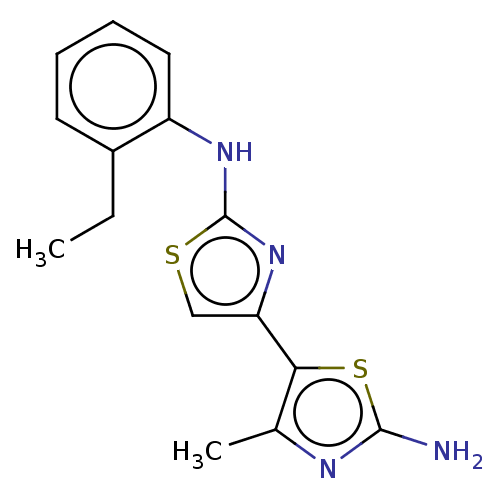 (CHEMBL4576051) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHOK1 cells coexpressing Galpha15 assessed as increase in intracellular calcium flux in pre... | Eur J Med Chem 167: 312-323 (2019) Article DOI: 10.1016/j.ejmech.2019.01.063 BindingDB Entry DOI: 10.7270/Q22B92FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50523195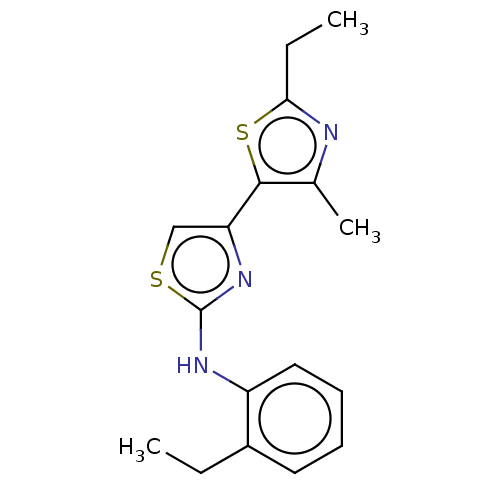 (CHEMBL4465237) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 8.60E+3 | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHOK1 cells coexpressing Galpha15 assessed as increase in intracellular calcium flux in pre... | Eur J Med Chem 167: 312-323 (2019) Article DOI: 10.1016/j.ejmech.2019.01.063 BindingDB Entry DOI: 10.7270/Q22B92FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50523196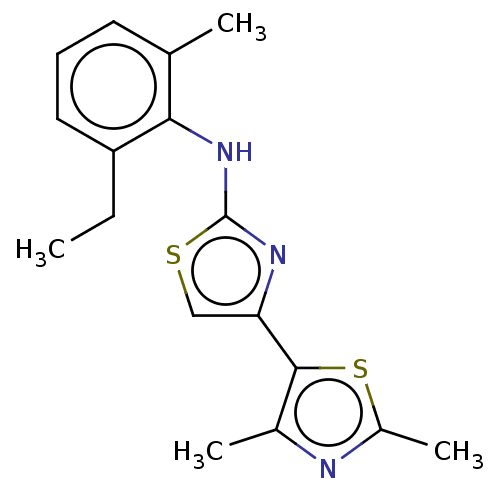 (CHEMBL4456958) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHOK1 cells coexpressing Galpha15 assessed as increase in intracellular calcium flux in pre... | Eur J Med Chem 167: 312-323 (2019) Article DOI: 10.1016/j.ejmech.2019.01.063 BindingDB Entry DOI: 10.7270/Q22B92FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50523197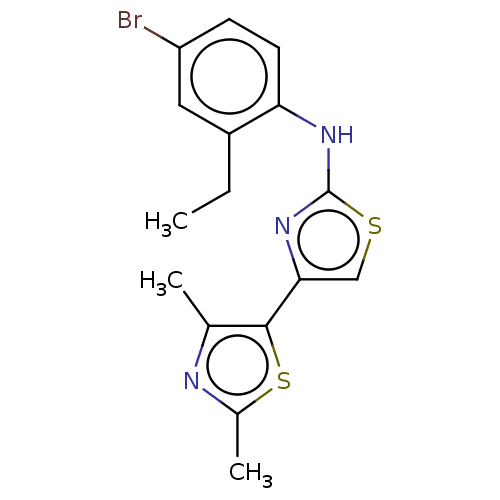 (CHEMBL4586970) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHOK1 cells coexpressing Galpha15 assessed as increase in intracellular calcium flux in pre... | Eur J Med Chem 167: 312-323 (2019) Article DOI: 10.1016/j.ejmech.2019.01.063 BindingDB Entry DOI: 10.7270/Q22B92FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50523198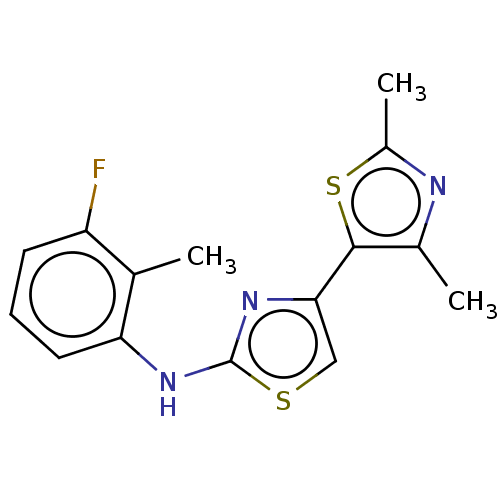 (CHEMBL4561162) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.01E+4 | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHOK1 cells coexpressing Galpha15 assessed as increase in intracellular calcium flux in pre... | Eur J Med Chem 167: 312-323 (2019) Article DOI: 10.1016/j.ejmech.2019.01.063 BindingDB Entry DOI: 10.7270/Q22B92FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 82 total ) | Next | Last >> |