 Found 152 hits with Last Name = 'zhang' and Initial = 'yz'
Found 152 hits with Last Name = 'zhang' and Initial = 'yz' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Polycomb protein EED
(Homo sapiens (Human)) | BDBM50594944
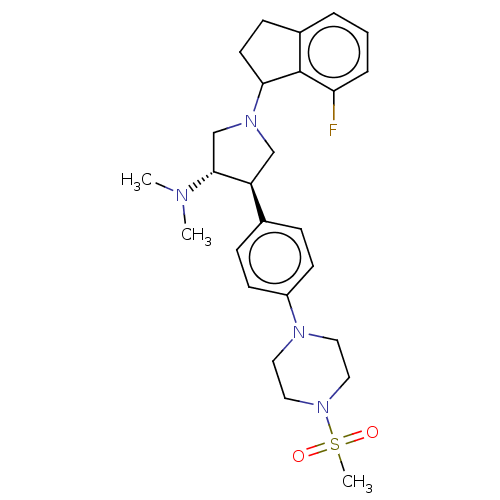
(CHEMBL5181703)Show SMILES CN(C)[C@@H]1CN(C[C@H]1c1ccc(cc1)N1CCN(CC1)S(C)(=O)=O)C1CCc2cccc(F)c12 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114144
BindingDB Entry DOI: 10.7270/Q2DN4921 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50095155

((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C34H38N6O5/c35-26(17-22-12-14-24(41)15-13-22)34(45)40-16-6-11-30(40)33(44)39-29(19-23-20-37-27-10-5-4-9-25(23)27)32(43)38-28(31(36)42)18-21-7-2-1-3-8-21/h1-5,7-10,12-15,20,26,28-30,37,41H,6,11,16-19,35H2,(H2,36,42)(H,38,43)(H,39,44)/t26-,28-,29-,30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DAMGO from MOR in Wistar rat brain membranes measured after 1 hr by scintillation counting method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01631
BindingDB Entry DOI: 10.7270/Q2988BVJ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50579952
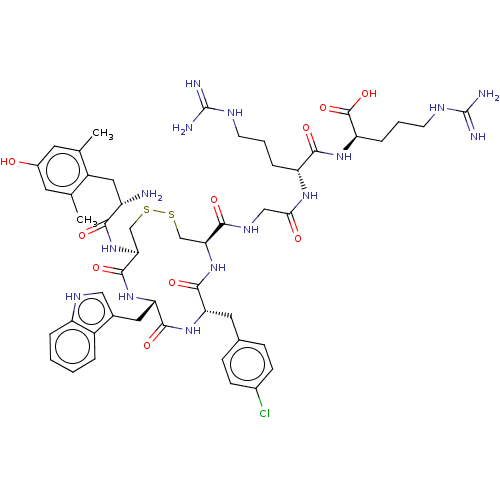
(CHEMBL5076581)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](Cc2ccc(Cl)cc2)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC1=O)C(=O)NCC(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(O)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DAMGO from MOR in Wistar rat brain membranes measured after 1 hr by scintillation counting method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01631
BindingDB Entry DOI: 10.7270/Q2988BVJ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50579953
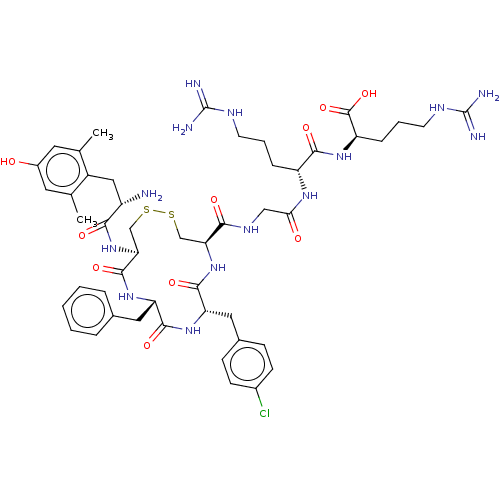
(CHEMBL5085104)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](Cc2ccc(Cl)cc2)NC(=O)[C@H](Cc2ccccc2)NC1=O)C(=O)NCC(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(O)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DAMGO from MOR in Wistar rat brain membranes measured after 1 hr by scintillation counting method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01631
BindingDB Entry DOI: 10.7270/Q2988BVJ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50139013

((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C32H37N5O5/c33-25(18-23-13-15-24(38)16-14-23)32(42)37-17-7-12-28(37)31(41)36-27(20-22-10-5-2-6-11-22)30(40)35-26(29(34)39)19-21-8-3-1-4-9-21/h1-6,8-11,13-16,25-28,38H,7,12,17-20,33H2,(H2,34,39)(H,35,40)(H,36,41)/t25-,26-,27-,28-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DAMGO from MOR in Wistar rat brain membranes measured after 1 hr by scintillation counting method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01631
BindingDB Entry DOI: 10.7270/Q2988BVJ |
More data for this
Ligand-Target Pair | |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM223986
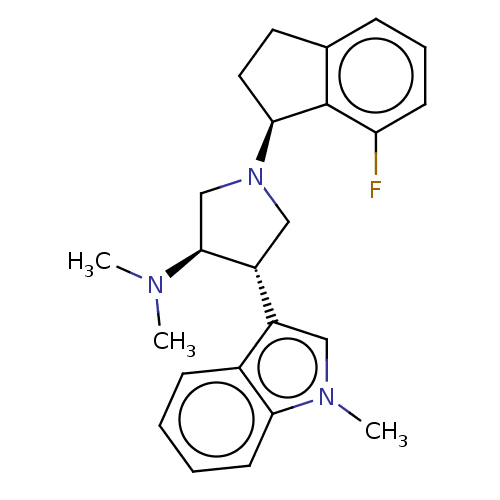
((3R,4S)-1-[(1S)-7-fluoroindan-1-yl]-N,N-dimethyl-4...)Show SMILES CN(C)[C@H]1CN(C[C@@H]1c1cn(C)c2ccccc12)[C@H]1CCc2cccc(F)c12 |r| Show InChI InChI=1S/C24H28FN3/c1-26(2)23-15-28(22-12-11-16-7-6-9-20(25)24(16)22)14-19(23)18-13-27(3)21-10-5-4-8-17(18)21/h4-10,13,19,22-23H,11-12,14-15H2,1-3H3/t19-,22+,23+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114144
BindingDB Entry DOI: 10.7270/Q2DN4921 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50184807

((R)-1-(4-(4-fluoro-2-methyl-1H-indol-5-yloxy)-5-me...)Show SMILES C[C@@H](O)COc1cn2ncnc(Oc3ccc4[nH]c(C)cc4c3F)c2c1C Show InChI InChI=1S/C19H19FN4O3/c1-10-6-13-14(23-10)4-5-15(17(13)20)27-19-18-12(3)16(26-8-11(2)25)7-24(18)22-9-21-19/h4-7,9,11,23,25H,8H2,1-3H3/t11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against VEGFR2 |
J Med Chem 49: 2143-6 (2006)
Article DOI: 10.1021/jm051106d
BindingDB Entry DOI: 10.7270/Q2WW7H7N |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50184807

((R)-1-(4-(4-fluoro-2-methyl-1H-indol-5-yloxy)-5-me...)Show SMILES C[C@@H](O)COc1cn2ncnc(Oc3ccc4[nH]c(C)cc4c3F)c2c1C Show InChI InChI=1S/C19H19FN4O3/c1-10-6-13-14(23-10)4-5-15(17(13)20)27-19-18-12(3)16(26-8-11(2)25)7-24(18)22-9-21-19/h4-7,9,11,23,25H,8H2,1-3H3/t11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against VEGFR2 |
J Med Chem 49: 2143-6 (2006)
Article DOI: 10.1021/jm051106d
BindingDB Entry DOI: 10.7270/Q2WW7H7N |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Mus musculus) | BDBM50184807

((R)-1-(4-(4-fluoro-2-methyl-1H-indol-5-yloxy)-5-me...)Show SMILES C[C@@H](O)COc1cn2ncnc(Oc3ccc4[nH]c(C)cc4c3F)c2c1C Show InChI InChI=1S/C19H19FN4O3/c1-10-6-13-14(23-10)4-5-15(17(13)20)27-19-18-12(3)16(26-8-11(2)25)7-24(18)22-9-21-19/h4-7,9,11,23,25H,8H2,1-3H3/t11-/m1/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against Flk1 |
J Med Chem 49: 2143-6 (2006)
Article DOI: 10.1021/jm051106d
BindingDB Entry DOI: 10.7270/Q2WW7H7N |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50579948

(CHEMBL5080666)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC1=O)C(=O)NCC(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(O)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DAMGO from MOR in Wistar rat brain membranes measured after 1 hr by scintillation counting method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01631
BindingDB Entry DOI: 10.7270/Q2988BVJ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50579949

(CHEMBL5084034)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC1=O)C(=O)NCC(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(O)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DAMGO from MOR in Wistar rat brain membranes measured after 1 hr by scintillation counting method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01631
BindingDB Entry DOI: 10.7270/Q2988BVJ |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 1
(Homo sapiens (Human)) | BDBM50184807

((R)-1-(4-(4-fluoro-2-methyl-1H-indol-5-yloxy)-5-me...)Show SMILES C[C@@H](O)COc1cn2ncnc(Oc3ccc4[nH]c(C)cc4c3F)c2c1C Show InChI InChI=1S/C19H19FN4O3/c1-10-6-13-14(23-10)4-5-15(17(13)20)27-19-18-12(3)16(26-8-11(2)25)7-24(18)22-9-21-19/h4-7,9,11,23,25H,8H2,1-3H3/t11-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against VEGFR1 |
J Med Chem 49: 2143-6 (2006)
Article DOI: 10.1021/jm051106d
BindingDB Entry DOI: 10.7270/Q2WW7H7N |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50579951
(CHEMBL5078349)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](Cc2ccc(Cl)cc2)NC(=O)[C@H](Cc2ccccc2)NC1=O)C(=O)NCC(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(O)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DAMGO from MOR in Wistar rat brain membranes measured after 1 hr by scintillation counting method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01631
BindingDB Entry DOI: 10.7270/Q2988BVJ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50579950

(CHEMBL5080233)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](Cc2ccc(Cl)cc2)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC1=O)C(=O)NCC(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(O)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DAMGO from MOR in Wistar rat brain membranes measured after 1 hr by scintillation counting method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01631
BindingDB Entry DOI: 10.7270/Q2988BVJ |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50579952

(CHEMBL5076581)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](Cc2ccc(Cl)cc2)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC1=O)C(=O)NCC(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(O)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DPDPE from DOR in Wistar rat brain membranes measured after 3 hrs by scintillation counting method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01631
BindingDB Entry DOI: 10.7270/Q2988BVJ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50579952

(CHEMBL5076581)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](Cc2ccc(Cl)cc2)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC1=O)C(=O)NCC(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(O)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-U69593 from KOR in Wistar rat brain membranes measured after 1 hr by scintillation counting method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01631
BindingDB Entry DOI: 10.7270/Q2988BVJ |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50579953

(CHEMBL5085104)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](Cc2ccc(Cl)cc2)NC(=O)[C@H](Cc2ccccc2)NC1=O)C(=O)NCC(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(O)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DPDPE from DOR in Wistar rat brain membranes measured after 3 hrs by scintillation counting method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01631
BindingDB Entry DOI: 10.7270/Q2988BVJ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50579953

(CHEMBL5085104)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](Cc2ccc(Cl)cc2)NC(=O)[C@H](Cc2ccccc2)NC1=O)C(=O)NCC(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(O)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-U69593 from KOR in Wistar rat brain membranes measured after 1 hr by scintillation counting method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01631
BindingDB Entry DOI: 10.7270/Q2988BVJ |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50095155

((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C34H38N6O5/c35-26(17-22-12-14-24(41)15-13-22)34(45)40-16-6-11-30(40)33(44)39-29(19-23-20-37-27-10-5-4-9-25(23)27)32(43)38-28(31(36)42)18-21-7-2-1-3-8-21/h1-5,7-10,12-15,20,26,28-30,37,41H,6,11,16-19,35H2,(H2,36,42)(H,38,43)(H,39,44)/t26-,28-,29-,30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DPDPE from DOR in Wistar rat brain membranes measured after 3 hrs by scintillation counting method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01631
BindingDB Entry DOI: 10.7270/Q2988BVJ |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50139013

((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C32H37N5O5/c33-25(18-23-13-15-24(38)16-14-23)32(42)37-17-7-12-28(37)31(41)36-27(20-22-10-5-2-6-11-22)30(40)35-26(29(34)39)19-21-8-3-1-4-9-21/h1-6,8-11,13-16,25-28,38H,7,12,17-20,33H2,(H2,34,39)(H,35,40)(H,36,41)/t25-,26-,27-,28-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DPDPE from DOR in Wistar rat brain membranes measured after 3 hrs by scintillation counting method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01631
BindingDB Entry DOI: 10.7270/Q2988BVJ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50579948

(CHEMBL5080666)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC1=O)C(=O)NCC(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(O)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-U69593 from KOR in Wistar rat brain membranes measured after 1 hr by scintillation counting method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01631
BindingDB Entry DOI: 10.7270/Q2988BVJ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50579949

(CHEMBL5084034)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC1=O)C(=O)NCC(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(O)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-U69593 from KOR in Wistar rat brain membranes measured after 1 hr by scintillation counting method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01631
BindingDB Entry DOI: 10.7270/Q2988BVJ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50139013

((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C32H37N5O5/c33-25(18-23-13-15-24(38)16-14-23)32(42)37-17-7-12-28(37)31(41)36-27(20-22-10-5-2-6-11-22)30(40)35-26(29(34)39)19-21-8-3-1-4-9-21/h1-6,8-11,13-16,25-28,38H,7,12,17-20,33H2,(H2,34,39)(H,35,40)(H,36,41)/t25-,26-,27-,28-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-U69593 from KOR in Wistar rat brain membranes measured after 1 hr by scintillation counting method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01631
BindingDB Entry DOI: 10.7270/Q2988BVJ |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50579951

(CHEMBL5078349)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](Cc2ccc(Cl)cc2)NC(=O)[C@H](Cc2ccccc2)NC1=O)C(=O)NCC(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(O)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DPDPE from DOR in Wistar rat brain membranes measured after 3 hrs by scintillation counting method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01631
BindingDB Entry DOI: 10.7270/Q2988BVJ |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50579950

(CHEMBL5080233)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](Cc2ccc(Cl)cc2)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC1=O)C(=O)NCC(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(O)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DPDPE from DOR in Wistar rat brain membranes measured after 3 hrs by scintillation counting method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01631
BindingDB Entry DOI: 10.7270/Q2988BVJ |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50579949

(CHEMBL5084034)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC1=O)C(=O)NCC(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(O)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DPDPE from DOR in Wistar rat brain membranes measured after 3 hrs by scintillation counting method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01631
BindingDB Entry DOI: 10.7270/Q2988BVJ |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50579948

(CHEMBL5080666)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC1=O)C(=O)NCC(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(O)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DPDPE from DOR in Wistar rat brain membranes measured after 3 hrs by scintillation counting method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01631
BindingDB Entry DOI: 10.7270/Q2988BVJ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50095155

((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C34H38N6O5/c35-26(17-22-12-14-24(41)15-13-22)34(45)40-16-6-11-30(40)33(44)39-29(19-23-20-37-27-10-5-4-9-25(23)27)32(43)38-28(31(36)42)18-21-7-2-1-3-8-21/h1-5,7-10,12-15,20,26,28-30,37,41H,6,11,16-19,35H2,(H2,36,42)(H,38,43)(H,39,44)/t26-,28-,29-,30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-U69593 from KOR in Wistar rat brain membranes measured after 1 hr by scintillation counting method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01631
BindingDB Entry DOI: 10.7270/Q2988BVJ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50579951

(CHEMBL5078349)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](Cc2ccc(Cl)cc2)NC(=O)[C@H](Cc2ccccc2)NC1=O)C(=O)NCC(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(O)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-U69593 from KOR in Wistar rat brain membranes measured after 1 hr by scintillation counting method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01631
BindingDB Entry DOI: 10.7270/Q2988BVJ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50579950

(CHEMBL5080233)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](Cc2ccc(Cl)cc2)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC1=O)C(=O)NCC(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(O)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-U69593 from KOR in Wistar rat brain membranes measured after 1 hr by scintillation counting method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01631
BindingDB Entry DOI: 10.7270/Q2988BVJ |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Oryctolagus cuniculus (rabbit)) | BDBM50351156

(CHEMBL1233215)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c1cc(O)ccc1O |r| Show InChI InChI=1S/C12H16O7/c13-4-8-9(16)10(17)11(18)12(19-8)6-3-5(14)1-2-7(6)15/h1-3,8-18H,4H2/t8-,9-,10+,11-,12+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 9.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 6.8 | n/a |
National Hellenic Research Foundation
Curated by ChEMBL
| Assay Description
Competitive inhibition of rabbit muscle glycogen phosphorylase b assessed as inorganic phosphate release using alphaD glucose-1-phosphate as substrat... |
Bioorg Med Chem 19: 5125-36 (2011)
Article DOI: 10.1016/j.bmc.2011.07.024
BindingDB Entry DOI: 10.7270/Q2GX4BX1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glycogen phosphorylase, muscle form
(Oryctolagus cuniculus (rabbit)) | BDBM50351159
(CHEMBL1817918)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)C1=CC(=O)C=CC1=O |r,c:16,t:12| Show InChI InChI=1S/C12H14O7/c13-4-8-9(16)10(17)11(18)12(19-8)6-3-5(14)1-2-7(6)15/h1-3,8-13,16-18H,4H2/t8-,9-,10+,11-,12+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30E+6 | n/a | n/a | n/a | n/a | n/a | n/a | 6.8 | n/a |
National Hellenic Research Foundation
Curated by ChEMBL
| Assay Description
Competitive inhibition of rabbit muscle glycogen phosphorylase b assessed as inorganic phosphate release using alphaD glucose-1-phosphate as substrat... |
Bioorg Med Chem 19: 5125-36 (2011)
Article DOI: 10.1016/j.bmc.2011.07.024
BindingDB Entry DOI: 10.7270/Q2GX4BX1 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Oryctolagus cuniculus (rabbit)) | BDBM50351158
(CHEMBL423707)Show SMILES OC[C@H]1O[C@H](O)[C@H](O)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C6H12O6/c7-1-2-3(8)4(9)5(10)6(11)12-2/h2-11H,1H2/t2-,3-,4+,5-,6+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.70E+6 | n/a | n/a | n/a | n/a | n/a | n/a | 6.8 | n/a |
National Hellenic Research Foundation
Curated by ChEMBL
| Assay Description
Competitive inhibition of rabbit muscle glycogen phosphorylase b assessed as inorganic phosphate release using alphaD glucose-1-phosphate as substrat... |
Bioorg Med Chem 19: 5125-36 (2011)
Article DOI: 10.1016/j.bmc.2011.07.024
BindingDB Entry DOI: 10.7270/Q2GX4BX1 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM50594938
(CHEMBL5201204)Show SMILES [2H]C([2H])(Nc1ncc(-c2cccnc2C)c(=O)n1C)c1c2CCOc2ccc1F | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114144
BindingDB Entry DOI: 10.7270/Q2DN4921 |
More data for this
Ligand-Target Pair | |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM50594938

(CHEMBL5201204)Show SMILES [2H]C([2H])(Nc1ncc(-c2cccnc2C)c(=O)n1C)c1c2CCOc2ccc1F | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114144
BindingDB Entry DOI: 10.7270/Q2DN4921 |
More data for this
Ligand-Target Pair | |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM291687
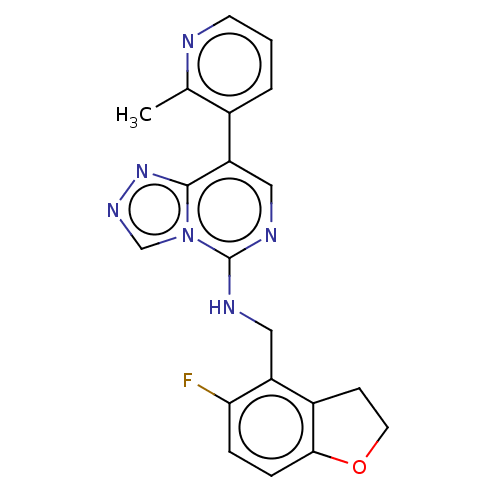
(N-((5-Fluoro-2,3-dihydrobenzofuran-4-yl)methyl)-8-...)Show InChI InChI=1S/C20H17FN6O/c1-12-13(3-2-7-22-12)16-10-24-20(27-11-25-26-19(16)27)23-9-15-14-6-8-28-18(14)5-4-17(15)21/h2-5,7,10-11H,6,8-9H2,1H3,(H,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114144
BindingDB Entry DOI: 10.7270/Q2DN4921 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM50594945
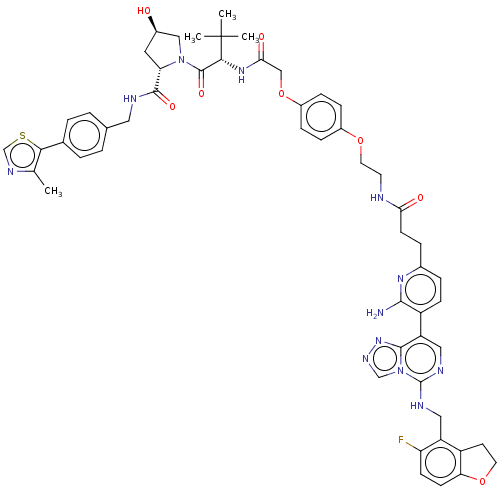
(CHEMBL5180485)Show SMILES Cc1ncsc1-c1ccc(CNC(=O)[C@@H]2C[C@@H](O)CN2C(=O)[C@@H](NC(=O)COc2ccc(OCCNC(=O)CCc3ccc(c(N)n3)-c3cnc(NCc4c5CCOc5ccc4F)n4cnnc34)cc2)C(C)(C)C)cc1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114144
BindingDB Entry DOI: 10.7270/Q2DN4921 |
More data for this
Ligand-Target Pair | |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM50562610

(CHEMBL4779022)Show SMILES Cc1ncsc1-c1ccc(CNC(=O)[C@@H]2C[C@@H](O)CN2C(=O)[C@@H](NC(=O)CCCCNC(=O)CCc2ccc(c(C)n2)-c2cnc(NCc3c4CCOc4ccc3F)n3cnnc23)C(C)(C)C)cc1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114144
BindingDB Entry DOI: 10.7270/Q2DN4921 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50184818
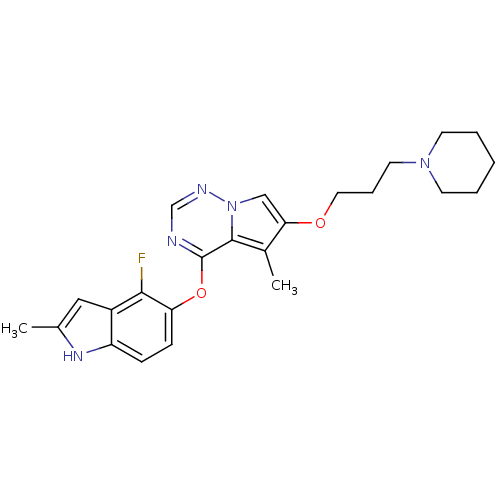
(4-(4-fluoro-2-methyl-1H-indol-5-yloxy)-5-methyl-6-...)Show SMILES Cc1cc2c(F)c(Oc3ncnn4cc(OCCCN5CCCCC5)c(C)c34)ccc2[nH]1 Show InChI InChI=1S/C24H28FN5O2/c1-16-13-18-19(28-16)7-8-20(22(18)25)32-24-23-17(2)21(14-30(23)27-15-26-24)31-12-6-11-29-9-4-3-5-10-29/h7-8,13-15,28H,3-6,9-12H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against VEGFR2 |
J Med Chem 49: 2143-6 (2006)
Article DOI: 10.1021/jm051106d
BindingDB Entry DOI: 10.7270/Q2WW7H7N |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50184822
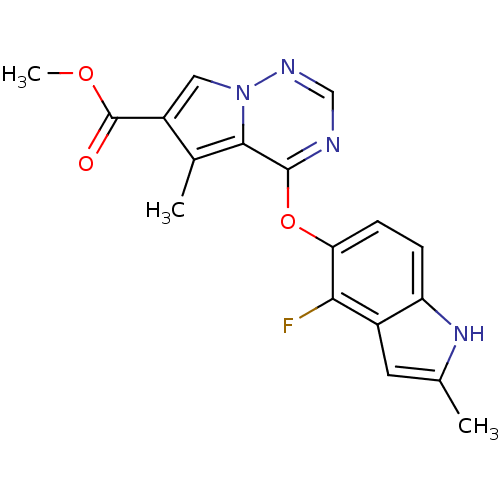
(CHEMBL383671 | methyl 4-(4-fluoro-2-methyl-1H-indo...)Show SMILES COC(=O)c1cn2ncnc(Oc3ccc4[nH]c(C)cc4c3F)c2c1C Show InChI InChI=1S/C18H15FN4O3/c1-9-6-11-13(22-9)4-5-14(15(11)19)26-17-16-10(2)12(18(24)25-3)7-23(16)21-8-20-17/h4-8,22H,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against VEGFR2 |
J Med Chem 49: 2143-6 (2006)
Article DOI: 10.1021/jm051106d
BindingDB Entry DOI: 10.7270/Q2WW7H7N |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2/Polycomb protein EED/Polycomb protein SUZ12
(Homo sapiens (Human)) | BDBM50594944

(CHEMBL5181703)Show SMILES CN(C)[C@@H]1CN(C[C@H]1c1ccc(cc1)N1CCN(CC1)S(C)(=O)=O)C1CCc2cccc(F)c12 |r| | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114144
BindingDB Entry DOI: 10.7270/Q2DN4921 |
More data for this
Ligand-Target Pair | |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM225230

(EED226 | US11013745, Compound EED226)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cnc(NCc2ccco2)n2cnnc12 Show InChI InChI=1S/C17H15N5O3S/c1-26(23,24)14-6-4-12(5-7-14)15-10-19-17(22-11-20-21-16(15)22)18-9-13-3-2-8-25-13/h2-8,10-11H,9H2,1H3,(H,18,19) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114144
BindingDB Entry DOI: 10.7270/Q2DN4921 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM50594931
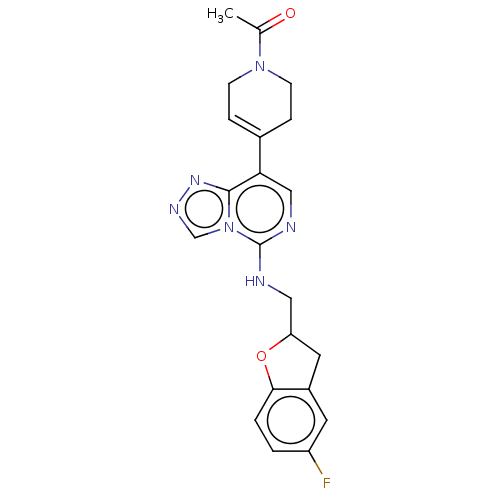
(CHEMBL5169768)Show SMILES CC(=O)N1CCC(=CC1)c1cnc(NCC2Cc3cc(F)ccc3O2)n2cnnc12 |c:6| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114144
BindingDB Entry DOI: 10.7270/Q2DN4921 |
More data for this
Ligand-Target Pair | |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM50594930

(CHEMBL5188142)Show SMILES CN1CCC(=CC1)c1cnc(NCC2Cc3cc(F)ccc3O2)n2cnnc12 |c:4| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114144
BindingDB Entry DOI: 10.7270/Q2DN4921 |
More data for this
Ligand-Target Pair | |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM50594929
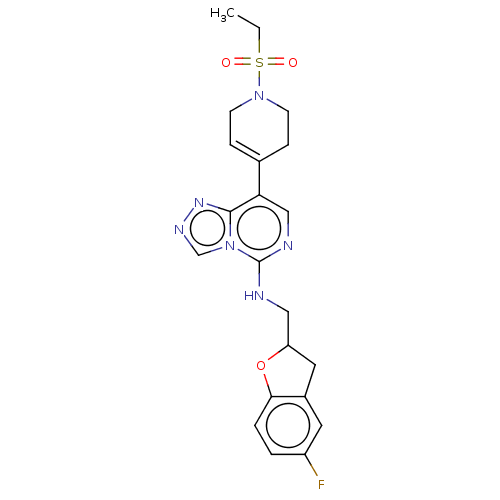
(CHEMBL5173010)Show SMILES CCS(=O)(=O)N1CCC(=CC1)c1cnc(NCC2Cc3cc(F)ccc3O2)n2cnnc12 |c:8| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114144
BindingDB Entry DOI: 10.7270/Q2DN4921 |
More data for this
Ligand-Target Pair | |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM50594928

(CHEMBL5171195)Show SMILES CS(=O)(=O)N1CCC(=CC1)c1cnc(NCC2Cc3cc(F)ccc3O2)n2cnnc12 |c:7| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114144
BindingDB Entry DOI: 10.7270/Q2DN4921 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50184812

(4-(4-fluoro-2-methyl-1H-indol-5-yloxy)-5-methyl-6-...)Show SMILES CN1CCC(CCOc2cn3ncnc(Oc4ccc5[nH]c(C)cc5c4F)c3c2C)CC1 Show InChI InChI=1S/C24H28FN5O2/c1-15-12-18-19(28-15)4-5-20(22(18)25)32-24-23-16(2)21(13-30(23)27-14-26-24)31-11-8-17-6-9-29(3)10-7-17/h4-5,12-14,17,28H,6-11H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against VEGFR2 |
J Med Chem 49: 2143-6 (2006)
Article DOI: 10.1021/jm051106d
BindingDB Entry DOI: 10.7270/Q2WW7H7N |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50184817
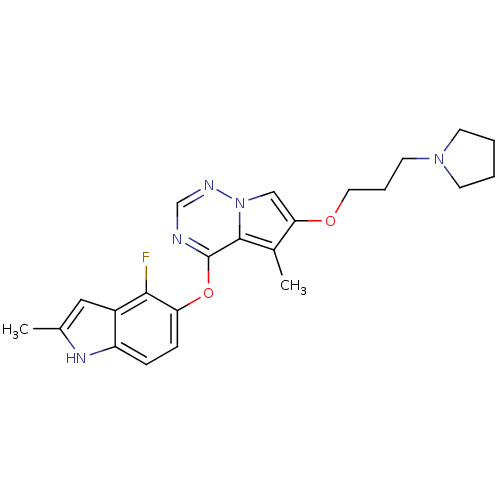
(4-(4-fluoro-2-methyl-1H-indol-5-yloxy)-5-methyl-6-...)Show SMILES Cc1cc2c(F)c(Oc3ncnn4cc(OCCCN5CCCC5)c(C)c34)ccc2[nH]1 Show InChI InChI=1S/C23H26FN5O2/c1-15-12-17-18(27-15)6-7-19(21(17)24)31-23-22-16(2)20(13-29(22)26-14-25-23)30-11-5-10-28-8-3-4-9-28/h6-7,12-14,27H,3-5,8-11H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against VEGFR2 |
J Med Chem 49: 2143-6 (2006)
Article DOI: 10.1021/jm051106d
BindingDB Entry DOI: 10.7270/Q2WW7H7N |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50611346
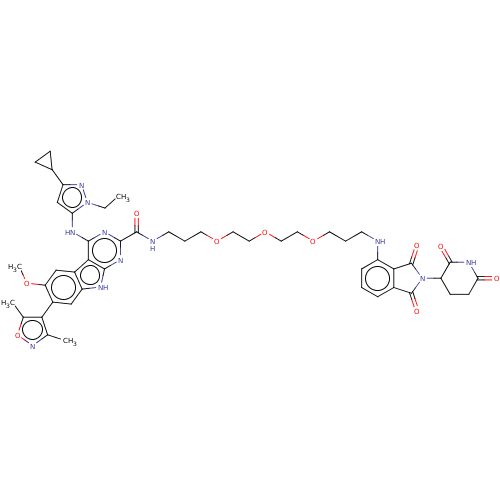
(CHEMBL5266070)Show SMILES CCn1nc(cc1Nc1nc(nc2[nH]c3cc(-c4c(C)noc4C)c(OC)cc3c12)C(=O)NCCCOCCOCCOCCCNc1cccc2C(=O)N(C3CCC(=O)NC3=O)C(=O)c12)C1CC1 |(-11.52,3.67,;-10.07,4.21,;-8.89,3.22,;-7.39,3.6,;-6.57,2.3,;-7.56,1.11,;-8.99,1.69,;-10.29,.86,;-10.23,-.67,;-8.87,-1.39,;-8.81,-2.93,;-10.11,-3.75,;-11.48,-3.03,;-12.92,-3.57,;-13.87,-2.36,;-15.41,-2.26,;-16.09,-.87,;-17.63,-.77,;-18.61,-1.96,;-18.23,-3.45,;-20.04,-1.38,;-19.94,.15,;-18.45,.53,;-17.88,1.96,;-15.23,.41,;-15.91,1.79,;-15.06,3.07,;-13.7,.3,;-13.02,-1.08,;-11.53,-1.5,;-7.45,-3.65,;-7.39,-5.19,;-6.14,-2.83,;-4.78,-3.55,;-3.48,-2.72,;-2.12,-3.44,;-.81,-2.62,;.55,-3.34,;1.85,-2.52,;3.21,-3.24,;4.52,-2.41,;5.88,-3.13,;7.18,-2.31,;8.55,-3.03,;9.85,-2.21,;11.21,-2.92,;12.51,-2.1,;13.88,-2.82,;13.94,-4.36,;15.3,-5.08,;16.6,-4.26,;16.54,-2.72,;17.65,-1.64,;19.16,-1.91,;16.96,-.26,;17.68,1.1,;16.86,2.4,;17.58,3.77,;19.12,3.83,;19.84,5.19,;19.94,2.52,;19.22,1.16,;20.04,-.14,;15.44,-.48,;14.37,.62,;15.18,-2,;-5.04,2.2,;-3.76,1.34,;-3.66,2.88,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50184811
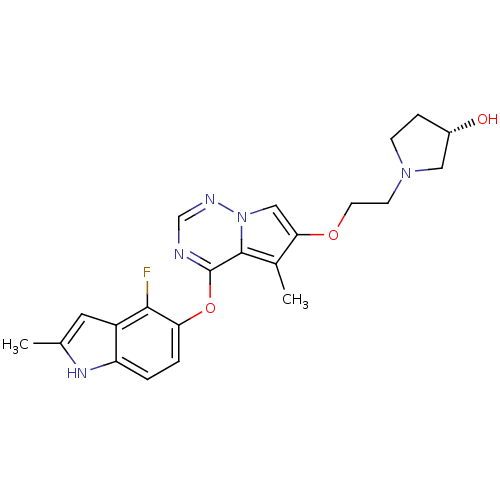
((R)-1-(2-(4-(4-fluoro-2-methyl-1H-indol-5-yloxy)-5...)Show SMILES Cc1cc2c(F)c(Oc3ncnn4cc(OCCN5CC[C@H](O)C5)c(C)c34)ccc2[nH]1 Show InChI InChI=1S/C22H24FN5O3/c1-13-9-16-17(26-13)3-4-18(20(16)23)31-22-21-14(2)19(11-28(21)25-12-24-22)30-8-7-27-6-5-15(29)10-27/h3-4,9,11-12,15,26,29H,5-8,10H2,1-2H3/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against VEGFR2 |
J Med Chem 49: 2143-6 (2006)
Article DOI: 10.1021/jm051106d
BindingDB Entry DOI: 10.7270/Q2WW7H7N |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data